ABSTRACT
Senna alata leaf extract demonstrates antimicrobial properties that promise utility for treatment of topical infections. Its combination with similarly bioactive Eugenia uniflora leaf extract in soap formulation could enhance anti-infective efficacy. The objective of this study was to develop potent antiseptic herbal soap formulations (HSFs) with the combined leaf extracts of the two plants. A soap base having suitable physicochemical properties (emolliency, foaming potential, and pH) was selected from a series of trial formulations produced from basic soap ingredients. Into this was incorporated three different preparations (namely, the methanolic fresh leaf extract (FLE), methanolic dry leaf extract (DLE), and the pulverized dry leaf sample (DLP) of S. alata and E. uniflora, respectively, singly or combined in 1:1 (w/w) ratio; to produce HSFs containing 5, 9, or 11%w/w concentrations of the leaf preparations. The physicochemical properties of the HSFs were determined as well as their antimicrobial activities by hole-in-plate agar diffusion assay against Staphylococcus aureus, Bacillus subtilis and Candida albicans. The selected soap base exhibited highest-rank emolliency, satisfactory stable froth production, and pH value. The physicochemical properties of the resulting HSFs were similar. The HSFs containing combinations of the DLEs at 9 and 11% concentrations demonstrated antimicrobial activities against S. aureus and C. albicans comparable (p>0.05) to those of the comparator commercial antiseptic soap containing 0.30% triclosan. B. subtilis was less sensitive (p<0.05) to the HSFs. On the other hand, when used singly, the DLEs as well as the FLEs and DLPs were significantly less potent (p<0.05) than the DLEs combined in the soap formulations. In conclusion, the HSFs containing S. alata and E. uniflora DLEs combined (1:1 w/w) at 9 and 11% concentrations exhibited satisfactory physicochemical properties and potent antimicrobial activities similar to the comparator commercial antiseptic soap employed in the study.
Key words: Senna alata, Eugenia uniflora, leaf extracts, herbal soap formulation.
Plants have always contributed largely to medicines and healthcare preparations by providing lead compounds for drug development or as refined herbal remedies (Iwu, 1993). Different plant parts have been used in traditional medicines around the world for treatment of human diseases and infections (Vineela and Elizabeth, 2005; Ekpo and Etim, 2009). Plants containing bioactive (antimicrobial) principles demonstrate potential for use as anti-infective agents and could be formulated as topical herbal remedies (as ointment, cream, lotion, gel, soap or crude/solvent extract) for the care and treatment of skin infections, as alternative to using synthetic antimicrobial agents. Senna alata (L.) Roxb (Caesalpiniaceae), synonym Cassia alata, is a shrub widely distributed in tropical countries and popularly known as ringworm plant due to the utilization of its fresh leaves for treatment of skin diseases such as ringworm, eczema, pruritis, scabies, and ulcers (Burkill, 1995; Reezal et al., 2002).
Phytochemical screening of alcoholic extract of Senna leaves has revealed the presence of anthraquinone glycosides, phenolic compounds and saponins, which could account for some of its biological activities, including antimicrobial and antioxidant effects (Sharma et al., 2010). The leaf extract of S. alata prepared in different solvents and by various techniques has been reported to demonstrate antimicrobial activity. When the fresh leaves were extracted with different solvents, only the extracts derived from polar solvents (water, methanol) exhibited antibacterial activity against Staphylococcus aureus, while the extracts by non-polar solvents (n-hexane, acetone) were inactive (Faruq et al., 2010). Whereas the freeze-dried aqueous extract of the fresh leaves showed antifungal activities comparable to that of acriflavine (6 mg/ml) against Epidermophyton floccosum and Candida pseudotropicalis (Akinde et al., 2002), the air-dried powdered leaf ethanolic and aqueous extracts demonstrated much broader spectrum of antimicrobial activities (Ogunjobi and Abiala, 2013).
Air-dried S. alata leaves formulated as soap exhibited antifungal activity against the fungus, Saccharomyces cerevisiae, but showed no inhibitory activity against bacterial organisms: S. aureus, E. coli and P. aeruginosa (Aminuddin et al., 2016). Thus, preparation and formulation factors were shown to influence the antimicrobial properties of S. alata crude preparations. Antimicrobial activities of dried, powdered leaf ethanolic extract and leaf essential oil of Eugenia uniflora Linn (Myrtaceae) have also been reported against several bacterial and fungal species (Fiuza et al., 2008; Victoria et al., 2012), while other biological activities and potentials of its various parts and constituents are also reported, which support the ethnomedicinal uses of the plant in treating bronchitis, influenza and intestinal problems (Souza et al., 2004; Fortes et al., 2015; da Cunha et al., 2016).
Furthermore, combinations of E. uniflora with other plant extracts (Bernardo et al., 2015) or chemical agent (metronidazole) (Santos et al., 2013) have demonstrated enhanced antimicrobial activity of the plant, while the activity of formulations of E. uniflora extracts as soaps and ointments has also been reported (Alalor et al., 2012; Aminuddin et al., 2016). Studies on triclosan, an antimicrobial agent popularly used in antiseptic toiletries, have raised questions on its possible hazard to human health (Deliaert et al., 2008; Zorrilla et al., 2009) and its contribution to development of antibiotic-resistant germs in the environment (Chalew and Halden, 2009). In the United States of America, the Food and Drug Administration (FDA) has announced the prohibition of sale of “consumer antiseptic washes” containing triclosan effective September, 2017 (FDA, 2016). The need for safer antiseptic ingredients has, therefore, become more apt. This present study aimed to develop an effective anti-infective herbal soap formulation with a combined leaf extracts of Senna alata and E. uniflora using soap ingredients that would enhance emolliency on the skin.
Good quality grade palm kernel oil, coconut oil and shea butter were procured locally at the Main Market, Ile-Ife Nigeria. The shea butter was purified by melting and filtering through a filter paper No. 100 (24 cm diameter, Rundfilter MN713 Macherey-Nagel D-5160 Duren, Germany) in a funnel into a flask placed in an oven (60°C). The filtrate was poured into a clean glass container and left for seven days at room temperature (30±2°C) to solidify. Standard grades of other formulation ingredients namely, sodium hydroxide (pellets), sodium lauryl sulphate, stearic acid, and oleic acid (Evans Medical Ltd., Liverpool) were also used.
Collection of S. alata and E. uniflora leaves
Fresh leaves of S. alata and E. uniflora were collected from Adagun Abiri Road Ile-Ife, and at New Buka, Obafemi Awolowo University (OAU) Ile-Ife, respectively, within the period from July to August, 2013. The leaves were authenticated at the herbarium of the Faculty of Pharmacy, OAU Ile-Ife, Nigeria.
Preparation of S. alata and E. uniflora leaves
Approximately, 200 g of the freshly collected leaves of each plant was macerated in neat methanol (solvent) on the same day of collection, extracted using a Soxhlet extractor (Scientific Glass Laboratories (SGL) Ltd. Staffordshire) at 40°C, and subsequently concentrated using a rotary evaporator (Rotavapor RII, Buchi Labortechnik, UK) at 40°C. The concentrate was oven-dried at 35°C for 2 h to produce the methanolic extract of the fresh leaves (that is, the fresh leaf extracted, FLE). The dried, pulverized (dry leaf powdered, DLP) forms of the S. alata and E. uniflora leaves were prepared by air-drying (for 50 to 60 days; approximately 400 g) of the collected leaves at the ambient temperature (30±3°C), and then grinding the dry leaves with a laboratory mill (Christy and Morris Ltd., Chelmsford Essex, NJ USA) into fine powder. A 150 g portion of the dry, powdered leaves of each plant was macerated and extracted with methanol using the Soxhlet extractor, concentrated with the rotary evaporator at 40°C, and oven-dried at 35°C for 2 h to produce the methanolic extract of the dried, pulverized leaves (that is, the dry leaf extracted, DLE).
Preparation of soap base formulations
Three formulations of soap base (A, B, and C; 130 g each) were initially prepared in duplicates by the cold and hot processes using the basic soap ingredients: palm kernel oil (PKO), sodium hydroxide (NaOH) and distilled water, in concentrations shown in Table 1. In the cold process, the required weight of NaOH pellets was dissolved in the required quantity of water and approximately 2 min was allowed for the exothermic dissolution of the pellets. The PKO was heated on a water-bath to about the same temperature (≈ 60°C) as the NaOH solution. The NaOH solution was then slowly poured into the oil (PKO) while stirring continuously with a plastic spatula until a slurry was formed (that is, the slurry stage). The slurry was then poured into plastic moulds to produce soap tablets, approximately 25 g each, and allowed to stand undisturbed for 48 h at the ambient temperature (29±2°C), to solidify. The soap preparation was removed from the moulds, wrapped in cellophane and kept for four weeks, to allow for curing.
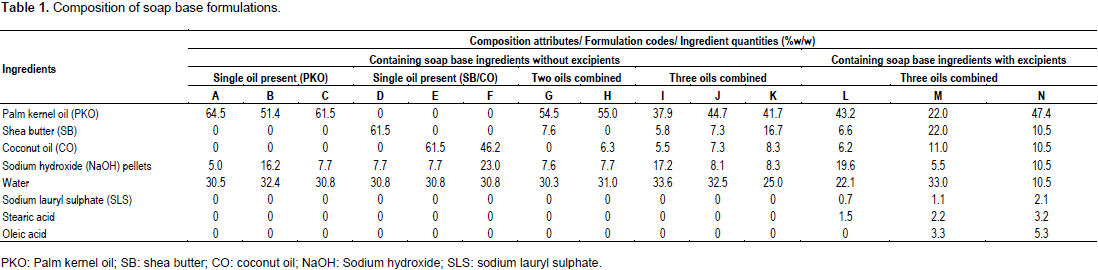
The hot process was similar in procedure at its initial steps to the cold process. After mixing the warm aqueous NaOH solution with the heated oil (PKO), the hot slurry was further heated on a water-bath until a suitable endpoint for the required heating process was reached, indicated by whitish coagulates appearing in the hot slurry. The slurry was then poured into moulds and allowed to set, and subsequently cured over the next four weeks. Other soap base formulations (D, E, F, G, H, I, J, and K; 130 g each; Table 1) were also prepared by the hot process using shea butter and/or coconut oil, in varied proportions of the soap base ingredients as well as other soap base formulations (L, M, and N; 130 g each; Table 1) with inclusion of excipients, such as sodium lauryl sulphate (a surfactant), stearic acid or/and oleic acid (fatty acids) intended to enhance performance and stability of the soap.
Determination of physicochemical properties of soap base formulations
All the soap base formulations prepared were tested for their physicochemical properties.
Foaming propensity testing
To determine the foaming propensity, a 1 g portion of each soap formulation was dissolved in 10 ml of water (distilled and tap water) by minimum heat (≤60°C) and 5 ml of the resultant solution was transferred into a 10-ml test tube. The test tube was shaken for 1 min using a vortex test tube mixer (Salford Scientific Supplies Ltd, Henderson Biomedical, UK) and then left to stand undisturbed. The time taken for the soap solution to defoam, in triplicate tests, was recorded.
pH determination
The pH value of 1 g sample of each soap formulation dissolved in 10 ml of distilled water was determined in triplicates with a digital pH meter (HM Digital Inc. Culver City, USA) at preset time intervals after production of the soap, namely, 24 h (Day 1), Day 7 (Week 1), Week 4, Week 12, and Week 18.
Emolliency test
The emolliency test was designed to evaluate occlusiveness of the formulations. A 2 g portion of each soap formulation was smeared onto the surface of white sheets of paper over approximately 5 cm2 surface area and left to stand on the laboratory shelf for 24 h (temperature 29±1°C; humidity 78±2%, determined with wet/dry bulb hygrometer); after which the degree of translucency was graded into a three-level ranking: mild, moderate, or strong translucency.
Preparation and determination of physicochemical properties of herbal soap formulations
The FLE, DLE, and DLP preparations of S. alata and E. uniflora, as well as equal quantity combinations (1:1 w/w ratio mixing) of the preparations, namely: S. alata FLE mixed with E. uniflora FLE; S. alata DLE mixed with E. uniflora DLE; and S. alata DLP mixed with E. uniflora DLP; were each incorporated into the selected soap base formulation (coded K) at the slurry stage of the preparation process before pouring into moulds. The different test preparations were incorporated at concentrations of 5, 9, or 11%w/w into the soap base formula K (Table 1). Foaming propensity test and pH determination at preset intervals over 12 weeks were carried out on the resulting herbal soap formulations. Similar tests were carried out on the comparator soap, Septol® antiseptic soap (Bush W.J. & Co. (Nig.) Ltd.), a commercial antiseptic soap product containing 0.30% Triclosan as the active (antimicrobial) principle.
Antimicrobial activity testing of herbal soap formulations
The antimicrobial activities of the herbal soap formulations, the soap base (K) (negative control), and of Septol® antiseptic soap (positive control), were determined using the hole-in-plate agar diffusion assay against S. aureus (NCTC 6571), Bacillus subtilis (NCTC 8236) and Candida albicans (a clinical isolate obtained at Microbiology Department, OAU Ile-Ife, Nigeria). A pure distinct colony of each bacterial strain inoculated in 10 mL Mueller Hinton broth (Oxoid, UK) aliquots and incubated at 37°C for 18 h was used. 0.2 mL of the culture of each organism was then seeded into 20 mL aliquots of molten Mueller Hinton agar (Oxoid) (MHA) in sterile Petri dishes and allowed to set. Antifungal activity against Candida was tested using a 48 h surface culture of C. albicans on Sabouraud dextrose agar (SDA; Oxoid) slopes, which after being washed off was diluted to an inoculum size of 107 cfu/mL and used to seed 20 mL aliquots of molten SDA in sterile Petri dishes and allowed to set. Wells (9 mm diameter) were cut into the seeded agar plates with a sterile cork borer and approximately 150 mg of each herbal soap sample was introduced into the holes in quadruplicate experiments. The plates were left at room temperature (29±1°C) for 1 h to allow for diffusion and then incubated at 37°C for 24 h for bacteria and 25°C for 48 h for fungi, after which the diameters of inhibition zones were measured.
Statistical analysis
The data obtained were evaluated by two-way analysis of variance (ANOVA) followed by the F test, and Student’s t-test for paired mean comparisons, to determine statistical significance of differences in computed mean values. In all cases, differences were considered significant at the p≤0.05 level. The data were presented as mean ± standard error of mean (SEM).
Foam stability and pH profile of soap base formulations
The time taken for foam disappearance or complete foam collapse of the aqueous solution of different soap base formulations varied. The foams persisted longer (indicating higher foaming capacity and foam stability) in distilled water than in tap water (community pipe-borne supply). Formulation J gave the most stable foam produced in distilled water, lasting 69 min (Table 2). Soap base formulations C, D and E contained the same quantities (61.5%) of PKO, SB and CO and NaOH (7.7%). However, E demonstrated the longest foam stability in distilled water while D had the shortest stability in tap water. Soap base K had the least foam stability and pH lower than the other two soap bases in the three oil-combination soap bases (I, J, K). All the soap base formulations expectedly produced alkaline pH solutions (Tokosh and Baig, 1995), values of which decreased gradually over 18 weeks of study (Table 2). The use of relatively high concentrations of NaOH with low oil concentrations resulted in higher pH of the soap base solutions (B, F).
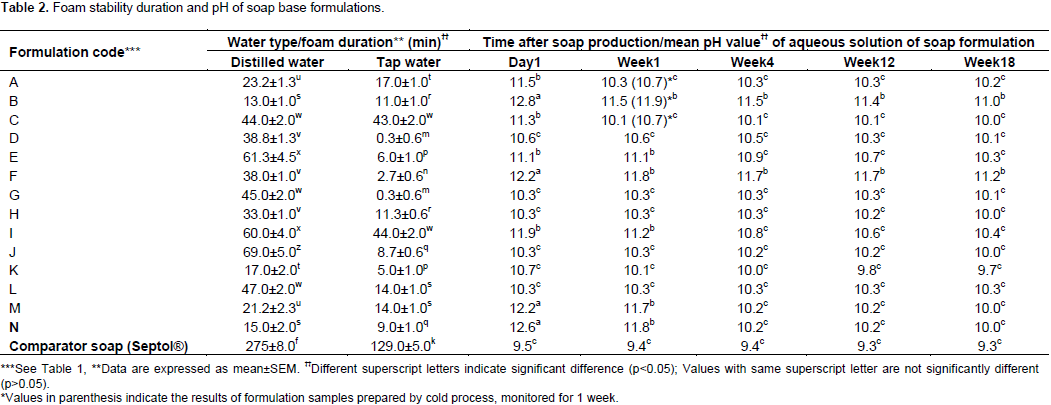
Emolliency of soap base formulations
The ranked emolliency results of soap base formulations (Figure 1) revealed a trend. The relative translucency produced by the formulations showed general correlation with overall concentrations of oil present in the soap formulations (rounded, in Figure 1, to nearest integers). Thus, most of the formulations that produced strong translucency (A, C, D, E, and K) contained very high (62 to 67% w/w) total oil concentrations (Figure 1). Formulations G and H (55% oil content), which also gave strong translucency on white paper, contained two oils combined in their formulae (Table 1). Of the three soap bases prepared with combinations of the three oils (I, J, K), soap base K which contained a relatively higher proportion of SB demonstrated the highest emolliency (Figure 1). The fact that formulation F, having only one oil component at 46% concentration in its formula (Table 1), demonstrated strong emolliency (Figure 1), suggests that its coconut oil component possesses greater oleaginous (lipophilic) property than does PKO (the oil component of formulation B; Table 1); formulation B being a similar (single oil, PKO) composition soap product with higher (51%) oil concentration level (Table 1), but showing only mild occlusive character (Figure 1). Formulation B had the lowest oil concentration (51%) among the formulations containing PKO as sole oil ingredient (Table 1) but contained the highest NaOH of the three indicating more effective saponification of the oil which would have lesser unsaponified oil to give emolliency. The soap base formulation K was finally selected as the most suitable for incorporation of the S. alata and E. uniflora leaf preparations, since it demonstrated the highest emolliency (Figure 1) and consistently showed the lowest pH values throughout the 18 weeks of study (Table 2).
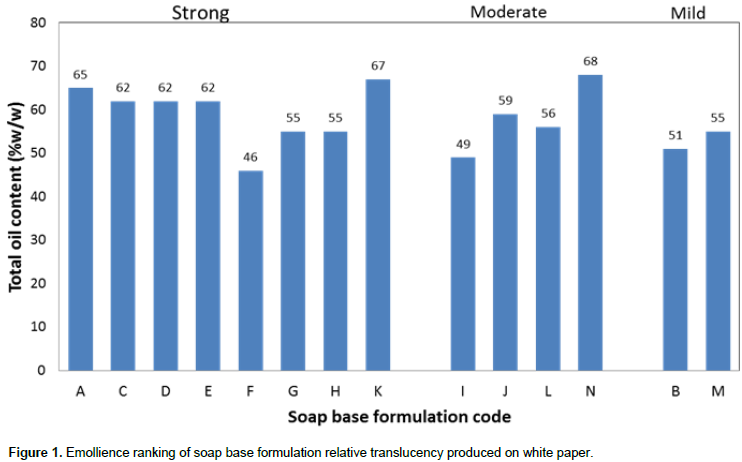
Physicochemical properties of herbal soap formulations
Foaming propensities of the herbal soap formulations in tap and distilled water (Table 3) were in similar range to those of the soap base K (Table 2), but were much lower than those of the comparator soap, Septol®, the froths of which lasted 129 ± 5 and 275 ± 8 min (approximately 2 and 4½ h), respectively, in tap and distilled water. On the other hand, pH values of the herbal soap solutions (Table 3) were also similar to those of the plain soap base K (Table 2), indicating that incorporation of S. alata and E. uniflora leaf preparations did not alter the physicochemical properties of the soap base considerably. Septol® aqueous solutions demonstrated a lower pH value (9.34 ± 0.12) than soap base K (9.7; Table 2) but the values were not significantly different (p>0.05), and remained virtually unchanged over the study period, as found also for the herbal soap counterparts (Table 3).
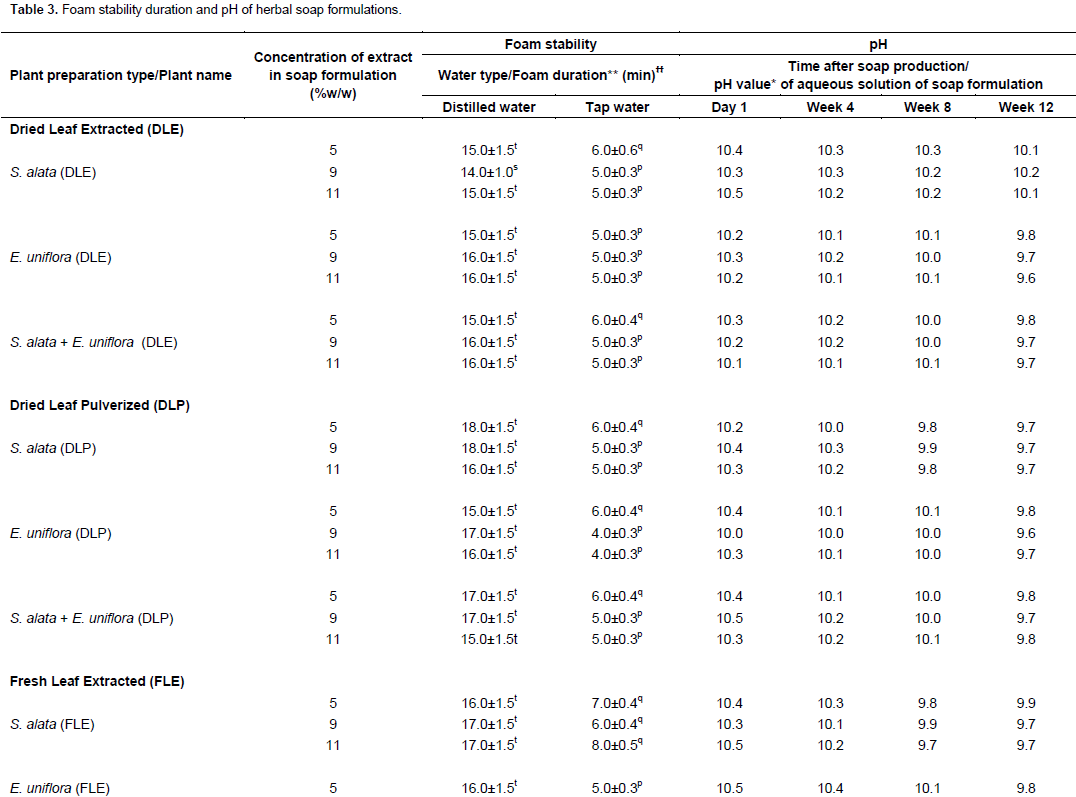
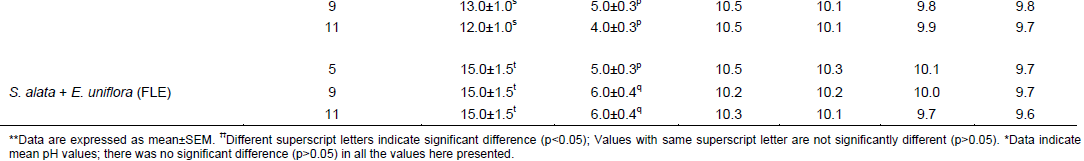
Antimicrobial activities of herbal soap formulations
The soap base (K) demonstrated antibacterial activities, giving inhibition zone diameters of 15.5±1.5 and 18.5±1.5 mm (mean±SEM), respectively, against S. aureus and B. subtilis; but no activity against C. albicans. However, incorporation of the plant preparations made the resulting herbal soap formulations active against C. albicans and also more active against the bacteria innocula (Table 4). DLE forms of S. alata and E. uniflora combined (at 9 and 11% w/w concentrations) in soap preparations resulted into herbal soap formulations that demonstrated antimicrobial activities similar to those of Septol® against S. aureus and C. albicans (p>0.05) (Table 4). They were, however, significantly lower in activity than Septol® against B. subtilis (p<0.05). B. subtilis was also insensitive to some of the herbal soap formulations produced with the dry leaf powdered (DLP) or FLE forms of both plants combined. On the other hand, the activities of S. alata and E. uniflora leaf preparations singly used in soap formulations were significantly lower than those of Septol® (p<0.05) (Table 4).
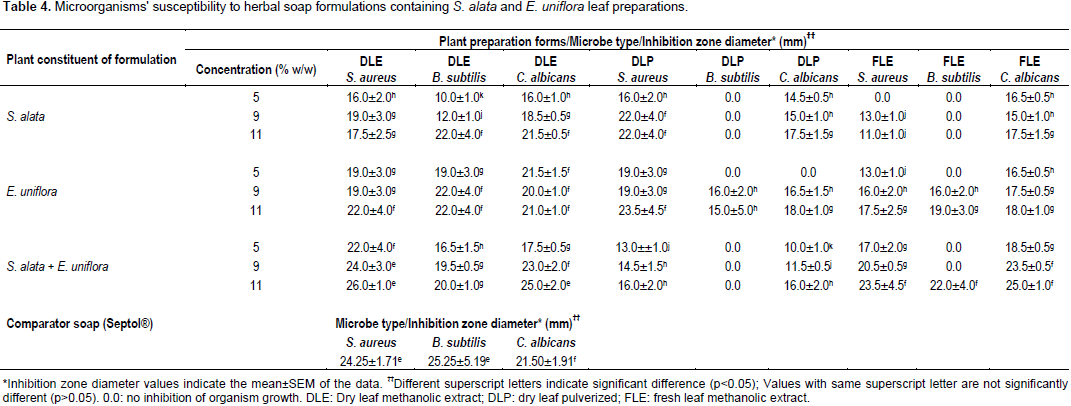
DLE forms of S. alata and E. uniflora used in the soap formulations (whether singly or combined) were active against all the test organisms (Table 4), and so proved superior in their antimicrobial activities to similar formulations produced with the other leaf preparations, against which some organisms demonstrated no apparent sensitivity (Table 4). The E. uniflora DLE used alone in the soap formulations generally demonstrated greater antimicrobial activities than the S. alata DLE used alone, particularly at the lower (5 and 9%) concentrations (Table 4). The 9 and 11% concentrations of both S. alata and E. uniflora leaf extractives in the soap formulations generally produced higher antimicrobial activities than the lower concentration (5%) (Table 4). Higher than 11% w/w concentrations of the plant preparations were, however, not used in the study because preliminary experiments had shown that foaming propensity of the soap formulations was lost at such concentrations.
The importance of an anti-infective soap formulation is to keep the user’s skin both clean and healthy through its cleansing and antimicrobial actions, removing skin-surface hydrophobic dirt and microbes, which can clog and infect dermal pores. It is the combination of these functions that makes an antiseptic soap formulation superior and often preferred above the use of ordinary soap preparations for the prevention or treatment of inflammatory skin conditions, such as acne or impetigo. Soaps belong to the anionic group of surfactants. The anionic and cationic surfactants are known to generally enhance penetration of antimicrobial agents through the cell wall of microorganisms; and this constitutes a possible mechanism by which they usually enhance the activities of such agents against infection-causing organisms (Hugo and Russell, 1998; Aiello et al., 2007). The soap bases prepared in this study demonstrated some degree of antibacterial activity. Inclusion of leaf extracts increased the antibacterial activity and extended antifungal activity.
Fresh leaf juice of S. alata has been reported to exhibit reduced activity on storage beyond 48 h at ambient temperature, due probably to hydrolysis of the active constituents (Akinde et al., 2002). The presence of water in the fresh leaves that would reduce its relative weight used for the extraction in comparison to the weight of the dry leaves would affect the overall quantity of active components of the FLE. These factors may account for the higher antimicrobial activity of S. alata DLE compared to the FLE. On the whole, the results of this study have established the potency of S. alata and E. uniflora DLE forms combined in soap formulation against susceptible organisms (S. aureus and C. albicans) as being comparable to the activities of the comparator antiseptic soap, Septol®. These organisms are known to be commonly associated with human skin (Chiller et al., 2001) or as opportunistic pathogens in man (Gow and Yadav, 2017).
Evaluation of emolliency may include the use of ranked indices (Parente et al., 2008). The emolliency test used in the present study was designed to evaluate occlusiveness of the soap formulations. Occlusive agents produce translucency on white paper due to the presence of residual oils in the formulation. The extent of translucence should therefore indicate the relative amount of residual oils present in the soap sample after the saponification process. This is demonstrated by the results in Figure 1 where the highest emolliency was observed with soaps containing high concentrations of oils, singly or combined. By mechanism of action, emollients are occlusive, humectant and/or restorative. Occlusive agents form a thin film on skin surface preventing moisture loss, mostly due to the presence of natural oils (Choi and Maibach, 2005; Bouwstra and Ponec, 2006).
The use of emollients in topical products corrects problems in skin scaling disorders and emollients may also have suppressive effects on epidermal thickening, in addition to anti-inflammatory activity and transient relief from irritation (Nola et al., 2003). The glycerol (end-product of saponification reactions) in all the soap formulations of this study was not separated from the soap, for the possible benefit of contributing its moisturizing quality to the user’s skin (Tucker, 2011) from the soap products when used. Coconut oil is reputed for producing good quality suds when used in the preparation of soaps (Gervajio, 2005), hence, the quality of the foam produced with the oil compared to those of PKO and SB. Shea butter contains a higher proportion of unsaponifiable matter than the other two oils (Moharram et al., 2006).
This might be responsible for its low foaming ability but caused its soap base to be more emollient and with lower pH. The presence of excessive NaOH in a soap preparation will increase the pH of such soap, as observed with soap bases B and F. Tap water is likely to contain divalent and trivalent metals which may reduce foaming and foam stability of the monovalent sodium soaps in water by forming water immiscible divalent soaps. The skin has a pH range of 4 to 6. To reduce irritation, skin products are expected to have pH as close to this range as much as possible. The pH of the comparator soap product is similar to that of the formulated herbal soap, even though the values are not within the pH range for the skin. The comparator soap is popularly used with no reported adverse effect on the skin due to pH.
This study has shown that S. alata and E. uniflora dry leaf methanolic extracts combined in 1:1 w/w ratio and formulated into soap at 9 or 11% w/w concentration exhibit antimicrobial activities against S. aureus and C. albicans comparable to those of a comparator soap, Septol®, containing 0.30% triclosan. The resultant herbal soap formulations also demonstrated suitable pH and foam stability properties, and could therefore serve as a substitute for soaps containing synthetic antiseptic agents especially triclosan, which has become controversial because of its untoward effects in humans (Deliaert et al., 2008) and the environment (Chalew and Halden, 2009).
The authors have not declared any conflict of interests.
REFERENCES
|
Aiello AE, Larson EL, Levy SB (2007). Consumer antibacterial soaps: effective or just risky? Clin. Infect. Dis. 45(Suppl)2:S137-S147.
|
|
|
|
Akinde BE, Orafidiya OO, Oyedele AO (2002). The effect of time of collection and storage on the antifungal activity of Cassia alata Linn (Caesalpinaceae) aqueous leaf extracts from old and young leaves. Niger. J. Pharm. Res. 1(1):64-96.
|
|
|
|
|
Alalor CA, Igwilo CI, Azubuike CP (2012). Evaluation of the antibacterial activity of herbal ointments formulated with methanolic extract of Cassia alata. Asian J. Biomed. Pharm. Sci. 2(13):15-19.
|
|
|
|
|
Aminuddin MF, Basri AM, Taha H, Adlan AM, Ahmad N (2016). Antimicrobial activities of soaps containing Senna alata leaf extract. Sci. Brunei. (Biol.) Special Issue 1:44-47.
|
|
|
|
|
Bernardo THL, Veríssimo RCSS, Alvino V, Araujo MGS, Pires dos Santos RFE, Viana MDM, Bastos ML, Alexandre-Moreira MS, Xavier de Araújo-Júnior J (2015). Antimicrobial analysis of an antiseptic made from ethanol crude extracts of P. granatum and E. uniflora in Wistar rats against Staphylococcus aureus and Staphylococcus epidermidis. Sci. World J. 2015:751791.
Crossref
|
|
|
|
|
Bouwstra JA, Ponec M (2006). The skin barrier in healthy and diseased state. Biochim. Biophys. Acta 1758(12):2080-2095.
Crossref
|
|
|
|
|
Burkill HM (1995). The Useful Plants of West Tropical Africa, Volume 3: Families J-L. Royal Botanic Gardens, Kew. pp. 151-153.
|
|
|
|
|
Chalew TE, Halden RU (2009). Environmental exposure of aquatic and terrestrial biota to triclosan and triclocarban. J. Am. Water Works Assoc. 45(1):4-13.
Crossref
|
|
|
|
|
Chiller K, Selkin BA, Murakawa GJ (2001). Skin microflora and bacterial infections of the skin. J. Invest. Dermatol. Symp. P. 6(3):170-174.
Crossref
|
|
|
|
|
Choi MJ, Maibach HI (2005). Role of ceramides in barrier function of healthy and diseased skin. Am. J. Clin. Dermatol. 6(4):215-223.
Crossref
|
|
|
|
|
da Cunha FAB, Waczuk EP, Duarte AE, Barros LM, Elekofehinti OO, Matias EFF, da Costa JGM, Sanmi AA, Boligon AA, da Rocha JBT, Souza DO, Posser T, Coutinho HDM, Franco JL, Kamdem JP (2016). Cytotoxic and antioxidative potentials of ethanolic extract of Eugenia uniflora L. (Myrtaceae) leaves on human blood cells. Biomed Pharmacother. 84:614-621.
Crossref
|
|
|
|
|
Deliaert E, den Kerckhove EV, Tuinder S, Fieuws S, Sawor JH, Meesters-Caberg MA, van der Hulst RR (2008). The effect of triclosan-coated sutures in wound healing. A double blind randomised prospective pilot study. J. Plast. Reconstr. Aes. 20:1-3.
|
|
|
|
|
Ekpo MA, Etim PC (2009). Antimicrobial activity of ethanolic and aqueous extracts of Sida acuta on microorganisms from skin infections. J. Med. Plants Res. 3(9):621-624.
|
|
|
|
|
Faruq ZU, Rahman UA, Bello M, Obianke M, Atiku FA (2010). Antibacterial activity of the active component of Cassia alata (Linn) leaves. Niger. J. Basic Applied Sci. 18(1):97-100.
|
|
|
|
|
Fiuza TS, Saboia-Morais SMT, Paula JR, Tresvenzol LMF, Pimenta FC (2008). Evaluation of antimicrobial activity of the crude ethanol extract of Eugenia uniflora L. leaves. Rev Cienc Farmaceut. Basica. Aplic. 29(3):245-250.
|
|
|
|
|
Food and Drug Administration (FDA) USA News Release (2016). "FDA issues final rule on safety and effectiveness of antibacterial soaps". U.S. Food and Drug Administration, September 2, 2016. Rule removes triclosan and triclocarban from over-the-counter antibacterial hand and body washes. Available at:
View
|
|
|
|
|
Fortes GAC, Carvalho AG, Ramalho RRF, da Silva AJR, Ferri PH, Santos SC (2015). Antioxidant activities of hydrolysable tannins and flavonoid glycosides isolated from Eugenia uniflora L. Rec. Nat. Prod. 9(2):251-256.
|
|
|
|
|
Gervajio GC (2005) Fatty acids and derivatives from coconut oil; In: Bailey's Industrial oil and fat products, 6th ed. Editor: Shahidi F. John Wiley & Sons Inc. pp. 1-56.
Crossref
|
|
|
|
|
Gow NAR, Yadav B (2017). Microbe profile: Candida albicans: A shape-changing, opportunistic pathogenic fungus of humans. Microbiology 163:1145-1147.
Crossref
|
|
|
|
|
Hugo WB, Russell AD (1998). Chemical disinfectants, antiseptics and preservatives, In "Pharmaceutical Microbiology" 6th ed. Blackwell Science Ltd, Oxford. pp. 224-225.
|
|
|
|
|
Iwu MM (1993). Handbook of African medicinal plants, CRC Press. ISBN 0-8493-4266-X
|
|
|
|
|
Moharram H, Ray J, Ozbas S, Juliani H, Simon J (2006). Shea butter chemistry, quality, and new market potentials. In: Herbs-Challenges in chemistry and biology: Chap. 25. New use agriculture and natural plant products program, Department of Plant Biology and Pathology, Rutgers, The State University of New Jersey, New Brunswick, NJ 08901. pp. 326-340.
Crossref
|
|
|
|
|
Nola I, Kostovic K, Kotrulja L, Lugovic L (2003). The use of emollients as sophisticated therapy in dermatology. Acta Dermatovenerol. Croat. 11(2):80-87.
|
|
|
|
|
Ogunjobi AA, Abiala MA (2013). Antimicrobial activity of Senna alata and Phyllanthus amarus. Global J. Pharmacol. 7(2):198-202.
|
|
|
|
|
Parente MA, Gambaro A, Ares G (2008). Sensory characterization of emollients. J. Sensory Stud. 23(2):149-161.
Crossref
|
|
|
|
|
Reezal I, Somchif MN, Abdul RM (2002). In vitro antifungal properties of Cassia alata. Proceedings of the 'Regional symposium on Environment and Natural Resources,' Hotel Renaissance Kuala Lumpur Malaysia. pp. 654-659.
|
|
|
|
|
Santos KKA, Matias EFF, Tintino SR, Souza CES, Braga MFBM, Guedes GMM, Costa JGM, Menezes IRA, Coutinho HDM (2013). Enhancement of the antifungal activity of antimicrobial drugs by Eugenia uniflora L. J. Med. Food 16(7):669-671.
Crossref
|
|
|
|
|
Sharma S, Dangi MS, Wadhwa S, Daniel V, Tiwari A (2010). Antibacterial activity of Cassia tora leaves. Int. J. Pharm. Biol. Arch. 1:84-86.
|
|
|
|
|
Souza GC, Haas AP, von Poser GL, Schapoval EE, Elisabetsky E (2004). Ethnopharmacological studies of antimicrobial remedies in the south of Brazil. J. Ethnopharmacol. 90:135-143.
Crossref
|
|
|
|
|
Tokosh R, Baig MA (1995). Transparent soap formulations and methods of making same, US Patent 5529714; Available at:
View
|
|
|
|
|
Tucker R (2011). What evidence is there for moisturizers? Pharm. J. Online 1:1-4.
|
|
|
|
|
Victoria FN, Lenardão EJ, Savegnago L, Perin G, Jacob RG, Alves D, da Silva WP, da Motta A, Nascente P (2012). Essential oil of the leaves of Eugenia uniflora L.: Antioxidant and antimicrobial properties. Food Chem. Toxicol. 50:2668-2674.
Crossref
|
|
|
|
|
Vineela CH, Elizabeth KM (2005). Antimicrobial activity of marine algae of Visakhapatnam City, Andhra Pradesh. Asian J. Microbiol. Biotechnol. Environ. Sci. 7:209-212.
|
|
|
|
|
Zorrilla LM, Gibson EK, Jeffay SC, Crofton KM, Setzer WR, Cooper RL, Stoker TE (2009) The effects of triclosan on puberty and thyroid hormones in male Wistar Rats. Toxicol. Sci. 107(1):56-64.
Crossref
|
|