ABSTRACT
Vaccium meridionale Swartz, commonly Andean Berry, has a high content of several phytochemicals, such as anthocyanins, phenolic acids, and other flavonoids. However, in spite of its antioxidant capacity, there is little information about its anticarcinogenic properties. This study evaluated the antiproliferative and pro-apoptotic activity of Andean Berry Juice (ABJ) on human colon adenocarcinoma SW480 cells. The antiproliferative activity of ABJ was evaluated on SW480 cells using the Sulphorodamine B assay.The effect on cell viability, cytotoxicity and activation of caspase-3 was analyzed using The ApoTox-Glo™ Triplex Assay. Specific apoptotic biomarkers cleaved PARP, total Bcl-2-associated death promote (BAD), phosphorylated BAD, total p53, and phosphorylated p53 were also analyzed. To determine the intracellular redox-state, the Glutathione Assay Kit and 2′-7′-Dichlorodihydrofluorescein diacetate (DCFH-DA) were used, respectively. The antiproliferative assay showed a IC50 value of 8% v/v ABJ, the caspase 3 activity was increased in time-dependent manner in SW480 treated cells, the proapoptotic proteins (cleaved caspase 3, cleaved PARP, P53 and total BAD) were increased by 1.6 to 2.0 fold. In addition, the ABJ-treated SW480 cells increased significantly the production of intracellular reactive oxygen species (ROS), parallel with reduction in the intracellular content of glutathione (GSH) and consequently a decrease of GSH/ oxidized glutathione (GSSG) ratio. In conclusion, the ABJ was able to inhibit SW480 cells proliferation involving apoptotic mechanisms through the perturbation of intracellular oxidative state.
Key words: Vaccinum, colon cancer, anthocyanins, apoptosis, oxidative stress.
According to the International Agency for Research on Cancer (IARC), approximately 663.904 new cases of Colorrectal Cancer (CRC) were diagnosed and 320.397 people died worldwide during 2008 (Boyle and Levin, 2008). The countries with the highest incidence rates include Australia, New Zealand, Canada, USA and some parts of Europe (Forman et al., 2014). According to epidemiological findings of Doll and Peto (1981), 35% of all deaths from CRC are associated with nutritional factors, either by the presence of dietary carcinogens, or by the absence in the diet of foods with preventive properties. The World Cancer Research Fund (WCRF) and the American Institute for Cancer Research (AICR) concluded that, the dietary factors associated with the increase of risk for CRC are: (i) the consumption of red and processed meat, (2) low intake of foods containing fiber, milk and calcium, and (3) high intake of alcohol supported with convincing and probable evidence (Boyle and Levin, 2008).
At the same time, the IARC reported that eating foods containing fiber such as fruits, whole grain, cereals, legumes and vegetables in three servings/days, reduced the risk of CRC by 21.5% (Forman et al., 2014). In addition, it has promoted the consumption of certain types of fruits and vegetables whose antioxidant, antigenotoxic, anti-inflammatory and antiproliferative properties are able to prevent initiation of colorectal carcinogenesis, or retard this process towards to promotion stages.
There is strong evidence that the antioxidants present in fruits and vegetables protect lipids, proteins and nucleic acids against the oxidative damage initiated by free radicals (Ferguson et al., 2004). Fruit extracts of Vaccinium species including slowbush blueberry, bilberry, cranberry, and lingonberry inhibit multiple stages of carcinogenesis and stimulate the apoptosis of cancer cells (Kraft et al., 2005; Ferguson et al., 2016; Safrin et al., 2016). These effects may be partly attributable to polyphenolic compounds such as flavonols, anthocyanins, and proanthocyanidins contained in this genus.
Vaccinium meridionale Swartz (Andean Berry) is a Colombian native plant that belongs to the Ericacea family. This fruit has been considered as a potential functional food because of its high content of phenolic compounds and anthocyanins that attribute an antioxidant capacity similar to or higher than the values reported for other species of Vaccinium (Gaviria et al., 2009; Gaviria et al., 2012). The total antioxidant capacity and phenolic composition of Andean Berry was investigated by (Gaviria et al., 2009). They reported that, the content of anthocyanins and total phenols with values of 201 ± 10 mg eq/100 g of fruit and 609 ± 39 mg eq/100 g of fruit, respectively. The antioxidant activity was studied by the methodologies 1,1-diphenyl-2-picryl-hydrazyl (DPPH: 2404 ± 120 values of mM de trolox®/100 g of fruit), ABTS (8694 ± 435 values of mg de ac. Asc/100 g of fruit) and FRAP (581 ± 29 values of mg de ac. Asc/100 g of fruit), all these results are comparable or superior to other Vaccinium species published in other researches (Capocasa et al., 2008; Çelik et al., 2008).
Recently, our group reported that (Maldonado et al., 2014) the aqueous extracts of Andean Berry contain total anthocyanins: 150.7 mg of cyanidin-3-glucoside equivalents/100 g of lyophilized; total phenols: 2546 mg of gallic acid equivalents/100 g of lyophilized. Phenolic acids such as chlorogenic: 126 mg/100 g of lyophilized; ferulic: 108 mg/100 g of lyophilized, coumaric: 63/100 g of lyophilized. In addition, this extract presented a high trapping capacity of reactive oxygen species (ROS), reactive nitrogen species (RNS) and hydroxyl radical scavenging capacity: 36147.5 ± 6274.7 (μmol DMSO/100 g lyophilized), total scavenger capacity to ROS and RNS: 29255.9 ± 6531.27 μmol Trolox/100 g lyophilized), 41775.2 ± 6168.2 μmol Trolox/100 g lyophilized), respectively, and ORAC value: 41775.2 ± 6168.2 μmol Trolox/100 g lyophilized). These properties could be partially explained by the presence of the high content of anthocyanins and phenolic acids (Maldonado et al., 2014).
In this study, it was reported for the first time the proapoptotic effects of an aqueous extract (juice) of Andean Berry on colon adenocarcinoma SW480 cells. Evidence was presented that a juice of Andean Berry was able to inhibit the growth of SW480 cells by triggering apoptosis and involving the oxidative stress which favours death of SW480 cancer cells.
Plant
Fresh ripe berries of V. meridionale (Andean Berry) were harvested from the Municipality of Retiro (Antioquia, Colombia), at 2175 m altitude and 16°C in May 2015. Berries were washed, selected, disinfected (sodium hypochlorite 100 ppm), dried and processed for 2 min at 2500 rpm and freeze-dried in a vacuum chamber under pressure 0.427 + 0.5 mm Hg, at a temperature of -50°C, after lyophilization the powder was stored at room temperatura (RT) and protected from light in PET packaging aluminium, to be used as an ingredient in the subsequent preparation of the juice.
Preparation of Andean berry juice
Andean Berry Juice (ABJ) was prepared as described previously by Franco-Tobón et al. (2016). In brief, freeze-dried powder of Andean Berry was dissolved in sterile water and sucrose to obtain a juice of 11.1° Brix, acidity 4.33 mg citric acid/ml, pH 3.06. Firstly, the juice was prepared. Further, the homogenized samples (juice) were kept in a 2.0 ml micro centrifuge tubes and sonicated at different time intervals of 15, 30, 45 and 60 min at room temperature (25 ± 1°C). The ultrasonic treatment was performed using an ultrasonic cleaner (42 kHz, 135 W; Branson ultrasonic corporation, USA). The sonicated product was aliquoted and stored at -70°C, protected from light until it was used for cell treatments.
Cell culture
SW480 cells were cultured as described by Maldonado et al. (2014). In Brief, a 15 cm2 Falcon flask with Dulbecco’s modified eagle’s medium was supplemented with 25 mM glucose, 2 mM L-glutamine, 10% heat (56°C)-inactivated horse serum, 100 U/ml penicillin, 100 µg/ml streptomycin, and 1% no-essential amino acids. The cells were cultured at 37°C in a humidified atmosphere with 5% CO2. For all experiments the ITS medium (10 µg/ml insulin, 5 µg/ml transferrin and 5 ng/ml selenium) was used and the horse serum was reduced to 3%.
Antiproliferative sulforhodamine B (SRB) assay
This assay is based on staining of total cellular protein from cells with SRB dye and performed as described Gossé et al. (2005). Briefly, SW480 cells were seeded in 96-well culture plates at a concentration of 2×103 cells per well and cultured at 37°C in 5% CO2. After 24 h seeding, the cells were exposed to different concentrations of the ABJ (3, 6, 8, and 10% v/v per well) and incubated for different times (24, 48, and 72 h). Cell layers were fixed to the well bottoms by adding 50 µl of 10% trichloroacetic acid (TCA) in each well, and the plates were incubated at RT for 1 h. The wells were then drained, rinsed twice with distilled water, and air dried. SRB (0.4% w/v in 1% glacial acetic acid) was then added (100 µl/well), and the plates were incubated for 30 min. Unbound dye was drained and removed by washing 3 times with 1% glacial acetic acid. After air-drying the plate overnight, the dye was solubilized by adding 100 µl/well of 10 mM Tris base and stirling for 10 min. Absorbance at 520 nm was measured in a GloMax®-Multi+ Microplate Multimode Reade (Molecular Devices; Sunnyvale, CA, USA). All experiments were performed in triplicate. The concentration able to kill 50% of cells (IC50) was calculated using GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA). The absorbance of control group (non-treated cells) was considered as 100% viability. The percent inhibition was calculated using the following equation:
Inhibition (%) = [1 - (ODt / ODc)] × 100
ODt is the optical density (OD) of treated cells and ODc for control (non-treated cells).
Cell viability, cytotoxicity and caspase 3 activity
The SW480 cells were plated in 96-well micro plates (2×103 cells/well). After 24 h, the cells were incubated during 48 h, at 37°C, 5% CO2 at different concentrations of ABJ (3, 6, 8 and 10% v/v). The effect of juice on SW480 cell viability, cytotoxicity and activation of caspase-3 was analyzed using The ApoTox-Glo™ Triplex Assay kit (Promega, Madison, WI, USA) following the manufacturer’s instructions. Briefly, 10 µl of viability/cytotoxicity reagent containing both glycylphenylalanyl-aminofluorocoumarin (GF-AFC) substrate and bis-alanylalanyl-phenylalanyl-rhodamine 110 (bis-AAF-R110) substrates were added to all wells. After incubation for 1 h at 37°C, the Relative Fluorescence Units (RFU) was determined at 505 nm excitation/400 nm emission for cell viability and at 520 nm excitation/485 nm emission for cytotoxicity. To determine the effect on caspase-3 activity of SW480 cells treated or not with the Andean berry juice, 10 µl of caspase- substrate DEVD-sequence complexed with luciferase, which will be hydrolyzed by caspase-3 activated to generate a luminic signal produced by luciferase, thus the luminance is proportional to the amount of Andean Berry juice-activated caspase 3 in SW480 cells, the Relative Luminiscence Units (RLU) from the caspase-3 activated was measured after 30 min of incubation. The fluorescence and luminiscence was detected using a GloMax®-Multi+ Microplate Multimode Reader (Molecular Devices; Sunnyvale, CA, USA).
Apoptosis analysis
To determine the apoptotic effect of ABJ on SW480 cells, the PathScan® Apoptosis Multi-Target Sandwich ELISA Kit (Cell Signaling Technology, Masachusset, USA) was used. Briefly, antibodies for detect cleaved caspase 3, cleaved PARP, total BAD, phosphorylated BAD, total p53 and phosphorylated p53, had been coated onto microwells by the manufacturer. After incubation with lysates from treated SW480 cells during 48 h, with 8% v/v of Andean berry juice, the target protein was captured by the coated antibodies. Following extensive washing, a detection antibody was added to detect the captured target protein. An Horseradish Peroxidase (HRP)-linked secondary antibody was then used to recognize the bound detection antibody, after TMB HRP-substrate was finally added for blue color development which was proportional to the quantity of bound target protein, color reaction was stopped by an acidic stop solution and optical density was analyzed at 450 nm in GloMax®-Multi+ Microplate Multimode Reader (Molecular Devices; Sunnyvale, CA, USA).
Glutathione assay
The SW480 cells were cultured as describe earlier and treated with the ABJ at 3, 6, 8 and 10% v/v final concentrations per well for 48 h, 37°C and 5% CO2. To determine the antioxidant cell status after treatment, the GSH Glo® Glutathione Assay Kit (Promega, WI, USA) was used following the manufacturer’s instructions. Birefly, 50 µl of GSH-Glo® Reagent were incubated for 30 min in 24-well opaque microplates containing the treated and non-treated cells, before adding 100 µl of Luciferin Detection agent. Following 15 min incubation, Glutathione Reductase reduces oxidized glutathione (GSSG) to reduced glutathione (GSH) in the presence of NADPH. Subsequently, the chromogen reacts with the thiol group of GSH to produce a colored compound that absorbs at 405 nm. The plates were read in a GloMax®-Multi+ Microplate Multimode Reade (Molecular Devices; Sunnyvale, CA, USA). The GSH content was calculated from a GSH standard curve. The luminiscence data were reported as RLU. GSH and GSSG in SW480 cells after treatment were then calculated and the ratio of GSH/GSSG was calculated by the following formula:
Ratio GSH/GSSG treated = [μM GSH–(μM GSSG × 2)]/μM GSSG.
Statistical analysis was performed according to student’s t-test by one way analysis of variance. Significant difference was taken as p<0.05. Each reported value was the mean ± SD from 3 independent experiments.
Determination of intracellular ROS
The 2′-7′-Dichlorodihydrofluorescein diacetate (DCFH-DA) is one of the most widely used to measured directly the redox-state of a cell. The DCFH-DA is a cell permeable, nonfluorescent precursor of DCF that can be used as an intracellular probe for oxidative stress. The intracellular esterases cleave DCFH-DA at the two ester bonds, producing a relatively polar and cell membrane-impermeable product, the H2DCF. This nonfluorescent molecule accumulates intracellularly and subsequent oxidation yields the highly fluorescent product DCF. Cells were seeded in culture dishes (2×104 cells per 2.5 cm internal diameter) and treated with ABJ at 3, 6, 8 and 10% v/v final concentrations per well for 48 h, 37°C and 5% CO2, cells were resuspended in pre-warmed PBS containing 8 µM DCFH-DA and incubated for 30 min at RT in darkness. For positive control, H2O2 (1.05%, v/v) was added to cell culture 15 min before read. Data were presented as Relative Fluorescence Units (RFU) signal, measured at 520 nm excitation/485 nm in a G GloMax®-Multi+ Microplate Multimode Reade (Molecular Devices; Sunnyvale, CA, USA).
Statistical analysis
The data were presented as mean ± standard error (SE) from three independent experiments. Comparisons between groups were done by one- and two-way ANOVA. Comparison between treated and not treated with Andean berry juice was done by two-tailed paired t-test. Results were considered significant when p<0.05. These analyses were done with the GraphPad Prism version 7.00 for Windows (GraphPad Software, San Diego California, USA).
Effect of ABJ on SW480 cell growth
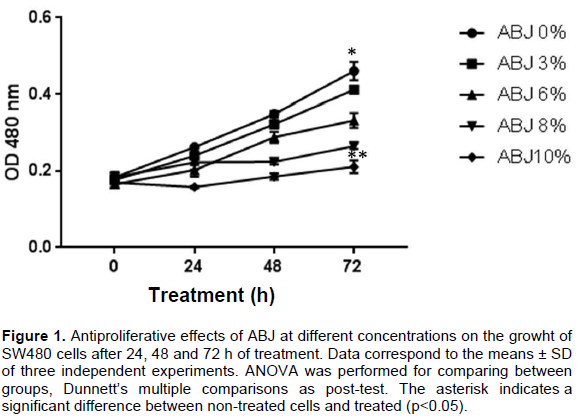
The growth inhibitory effects of ABJ on SW80 cells were tested using the colorimetric method of sulforhodamine B (SRB) as described by Gossé et al. (2005). Figure 1 shows that cells treated at 3, 6, 8 and 10% ABJ for 24, 48 and 72 h, the SW480 cell proliferation was inhibited in a concentration-dependent manner. After 48 h, a significant inhibition of SW480 cell growth (p <0.05) at 6, 8 and 10% ABJ compared to non-treated cells was observed. This effect was observed until 72 h of cells exposed at 8% (p<0.004) and 10% (p<0.0034) of ABJ compared to non-treated cells under the same conditions. The IC50 at 24, 48 and 72 h was 19, 8 and 3% of ABJ, respectively.
Effect of ABJ on SW480 cell viability, cytotoxic and activation of caspase 3
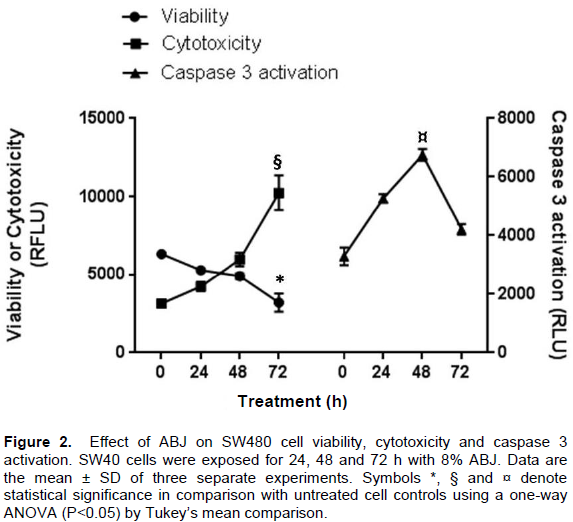
Figure 2 shows the effect ABJ on SW480 cell viability,cytotoxic effect and caspase 3 activation after treatment at 8% ABJ (IC50 for 48h). The SW480 cell viability was reduced in a time-dependent manner whereas the cytotoxic effect evidenced by the increase on the RFU values corresponding to dead or dying cells were increased in time (Figure 2). Under the same conditions, the activation of caspase-3 was paralled to cytotoxic effect until 48 h. After 72 h of 8% ABJ treatment, the caspase-3 activity fell-down which suggests that most cells are dead by apoptosis and only a small number of these are dying by the apoptosis triggered by ABJ during all the treatment.
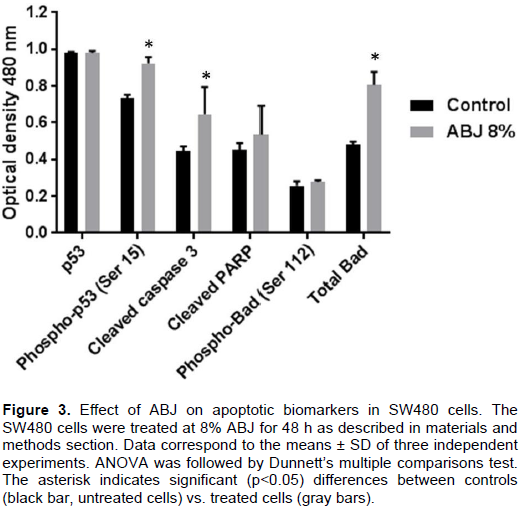
Effect of ABJ on apoptotic biomarkers in SW480 cells
Figure 3 shows that ABJ’IC50 induces phosphorylation of p53 at Ser15 with significant statistical differences (p= 0.04) compared with untreated cells, as well as ABJ´IC50 induces cleavage of PARP and caspase-3. In the other hand, it has already been established that phosphorylation of Bad at Ser 112 inactivates its proapoptotic function. Our findings suggest that ABJ´IC50 does not induce Bad phosphorylation; this may lead the increase of apoptotic-promoting activity of Bad and thereby contribute to the death of this human colon cancer cell line.
Intracellular ROS level and GSH depletion assay
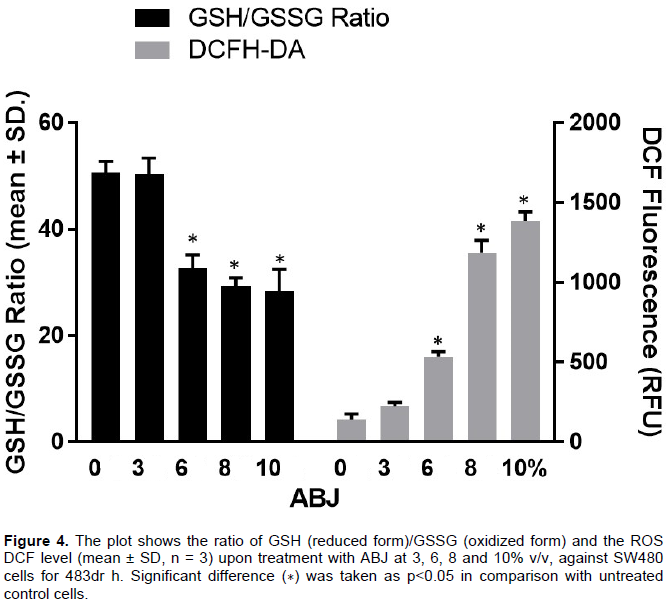
Ratio of reduced glutathione (GSH) and oxidized glutathione (GSSG) was measured in SW480 cells after 48 h of incubation after treatment with ABJ. Increased levels of oxidized form of glutathione (formed as results of ROS scavenging) are indicative of oxidative stress. As can be seen from Figure 4, ABJ produced significant glutathione depletion as evident by the increased formation of GSSG particularly at the concentration of 8% v/v compared to vehicle control.
Figure 4 shows the effect of ABJ on modulation of the intracellular oxidative state of SW480 cells after 48 h of incubation, the intracellular ROS production was also measured by DCF method. The ABJ (from 3 to 10% v/v) induced a significant dependent-increase of intracellular ROS at 6% v/v in SW480 cells. Maximum increase ROS level was detected at concentration of >8% v/v of ABJ.
In this study, the antiproliferative and proapoptotic effects of an Andean berry juice on colon adenocarcinoma cell line SW480 was evaluated. These juices contain most variety of the berry phytochemicals which allows to do an evaluation of their anticarcinogenic capacities (Franco et al., 2016). Although we have previously reported the free radical scavenging capacity of Vaccinium meridionale Sw freeze-dried aqueous extract by chemical methods and the antiproliferative effects against colon cancer cell lines (Maldonado et al., 2014). In our study, by the first time, we show that this juice is a potential anticancer agent able to eliminate a colon cancer cell (SW480) through apoptosis involving the stress oxidative as mechanisms involved in these events.
It was found that ABJ had a IC50 value of 8% of fresh juice v/v, an effect also observed in similar studies evaluating the anticancer effects of 13 edible berries on 5 cancer cell lines (AGS stomach adenocarcinoma, MFC-7 and MDA-MB-231 mammary gland, PC-3 prostatic adenocarcinoma and Caco2 colorectal adenocarcinoma cell line) using juices made with a domestic extractor (Boivin et al., 2007). In that study, the authors found that most berries juices reduced the proliferation of cancer cells lines, but the extent of inhibition was different between the various berries juices, for example intestinal cancer cell line Caco-2 was inhibited by Cranberry, Raspberry and Blackcurren, but was much less susceptible by gooseberry and sea buckthorn than other cell lines (Boivin et al., 2007). They found a IC50 value of 25% v/v of Cranberry juice for colon cancer Caco-2 cells, higher than the IC50 of ABJ on SW480 cells. This discrepancy could be explained by the fact that Vaccinium meridionale Swartz contain high levels of antioxidants, approximately 1-fold, that other species of berries as Cranberrie (Mosquera et al., 2015).
In the other hand, Bermúdez Soto et al. (2007) reported that incubation of Caco 2 cells with a pre-digested chokeberry juice at a nontoxic dose (final pH 7.5 and osmolarity 325 miliosmoles L-1 in the culture medium) increased the cytotoxicity (20%) and reduced cell proliferation (30 to 40%). They also found with the use of microarrays and RT-PCR. An increase in the expression of tumor suppressor genes such as CEACAM1 and BMP2 (2.6 and 2.4 fold, respectively). In turn, they reported downregulation of genes related to tumor invasion and metastasis FGFR2 and S100A4 (-4.6 and -2.5 fold, respectively) in response to juice treatment (Bermúdez et al., 2007).
All these data support our results of the antiproliferative and proapoptotic effects observed here with an Andean berry juice on colon adenocarcinoma cells.
Apoptosis is a complex process that proceeds through at least two major pathways (intrinsic and extrinsic) which are regulated at multiple levels. The uncontrolled cell division and suppressed apoptosis are main characteristic features of cancer cells (Kang et al., 2006; Vidya et al., 2010).
Caspases are a group of proteins belonging to the group of cysteine ​​proteases. Caspases are essential mediators of the apoptosis. One of them is the caspase 3 that hydrolyzes DEVDG (Asp-Glu-Val-Asp-Gly) peptide and can activate caspase 7 favouring a DNA damage that would inevitably lead to cell suicide. Caspase 3 is an apoptosis-executing protein and can be activated by either two major pathways (Lemaire et al., 1998).
One of the possible mechanisms that could explain the death of carcinogenic cells in the presence of ABJ could be the apoptotic processes, so we decided to determine if the SW480 cells exposed to the ABJ treatment induce the activation of caspase 3. It was found out that a time-dependent increase manner in caspase 3 activity in response to the IC50 ABJ treatment which indicates that carcinogenic cell death is the result of an apoptotic process, because Caspase 3 is an executioner caspase and an important apoptotic biomarker. This proapoptocic effect on colon adenocarcinoma cells like Caco-2, SW480 and HT-29 with increase in caspase 3 activity has been reported for other berries juices and extracts such as Cranberry, blueberry, raspberry and acai berry (Safrin et al., 2016; Tamara et al., 2014). In our study, the ABJ juice does not only induced an increase in caspase 3 activity of SW480 cells but in the activation of others proapoptotic biomarkers such as the PARP cleavage fragment. The primary function of PARP-1 is to detect and repair DNA damage, however, cells with profound DNA damage amplify PARP-1 activity, in such a way that ATP deposits are depleted, resulting in a passive necrotic cell death, Caspases can block this process, thanks to the cleavage of PARP-1 which is considered as a distinctive sign of apoptosis (Eguchi et al., 1997).
On the contrary, Boivin et al. (2007) found that the death of colon adenocarcinoma cells Caco-2 exposed to different berries juices had no correlation with the increase in caspase 3 activity. These discrepant results can be explained by some differences present in both studies such as: (I) the use of different juices berries; Boivin et al. (2007) used a juice from Cranberry, Raspberry and Blackcurren made with a domestic extractor, while in this study, we used a juice from Freeze-dried powder of Andean berry, (II) the used of different adenocarcinoma cells lines (CaCo2 and SW480 cells, respectively), and III) different periods of incubation; they incubate for a period of 24 h and was done in a period of 48 h.
In order to determine the possible apoptotic mechanisms involved in the apoptotic effects observed in SW480 cells treated with 8% ABJ, the endogenous levels of p53 protein, phospho-p53 (Ser15), total Bad, phospho-Bad (Ser112), and cleaved poly (ADP-ribose) polymerase-1 (PARP) were measured. These molecules represent key signaling proteins in pathways controlling survival and apoptosis.
Severe oxidative stress induces apoptosis followed by DNA damage, p53 is phosphorylated and translocated to the nucleus where it triggers multiple mechanisms including modulation of the Bcl-2 family proteins, amplification of death signals and activation of Caspases (Thompson et al., 2004). In our study, it was observed that ABJ enhanced the activation of tumor suppressor protein p53 in SW480 cells; it was also found that ABJ upregulated the expression of tumor Bcl2 family proapoptotic protein Bad. This protein is regulated through its phosphorylation. Dephosphorylated Bad promotes apoptosis by binding to Bcl-2 family, while phosphorylation of Bad at Ser75 in a MAPK-dependent manner facilitating its inactivation. Promoting cells survival, in our study, it was found that the phosphorylated form of Bad was inhibited in the cells exposed to ABJ compared to control cells as well as the cell treated with ABJ, induced the proteolytic cleavage of caspase 3. Activation and execution of the apoptotic pathway in SW80 cells was also perceptible by increased level of cleaved PARP (Figure 5). However, we cannot determine which one of the two major apoptosis pathways is involved, since we did not make caspase 8 (apoptosis extrinsic pathway) or caspase 9 (apoptosis intrinsic pathway) determinations.
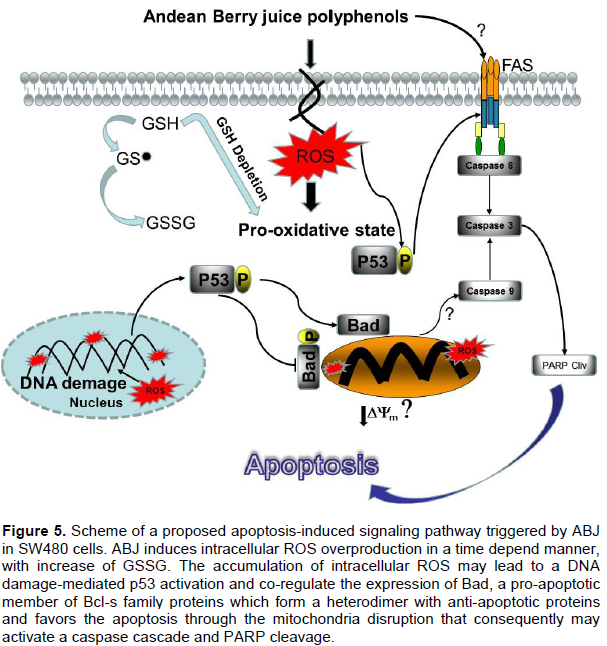
In a recent study, Carole Minker et al. (2015) identified which proapoptotic pathway is induced in human colon cancer cell lines (SW480 and SW620), exposed to proanthocyanidins (Pcys) extracted from 11 berries. They found that Lowbush blueberry extract triggers the strongest activity of all berries tested by the opposite blueberry. Pcys are less effective for DNA fragmentation. Finally, they concluded that Lowbush blueberry Pcy-induced apoptosis is mediated by TNF-R1, DR3, or DR6, these death receptors inducing caspases 8 and 9 activation (Minker et al., 2015). Further studies are in course in our group to gain more insight into the apoptotic mechanisms triggered by ABJ on colon cancer cell lines and in a preclinical model of colon carcinogenesis.
The ROS production was measured in the SW480 cells exposed to ABJ using DCHFDA, as shown in Figure 4, the ABJ induce ROS production in a concentration dependent manner, even ten-fold higher compared with untreated cells. This increase in ROS levels occurred when a decrease in GSH/GSSG ratio was also observed in ABJ-treated SW480 cells, indicating that SW480 cells are under a condition of oxidative stress during the apoptotic process, in spite of the antioxidant capacity previously reported and measured using free-cell living methods such as ORAC (Maldonado et al., 2014).
The concentration of ABJ used here on SW480 cells induced oxidative stress which may be involved in the apoptotic process because an increase of intracellular ROS may lead to a DNA damage able to activate the p53, in addition, the increase of ROS production may also activates the release of cytochrome c from mitochondria and favours the activation of caspse 9 previous to activation of caspase 3 (Pelicano et al., 2004; Lamy et al., 2008) demonstrated that apoptosis of SW480 cells treated with a flavonoid identified as lupulone at 40 mg/ml for 48 h was associated to the increase of ROS intracellular concentration involving release of cytochrome c, caspase 9 and caspase 3 activation. These effects were avoided when SW480 cells were exposing to vitamin C in the presence of lupulone (Lamy et al., 2008). Thus, these observations suggest that ABJ might activate in SW480 cells the intrinsic apoptotic pathway; however, this hypothesis must be confirmed in further studies.
Recently, Khan et al. (2014) proposed that the fact that carcinogenic cells have higher levels of copper compared with non-malignant cells and may be more susceptible to the transfer of electrons with antioxidants to generate ROS, so damage to DNA by copper may be an important route by which carcinogenic cells can die in the presence of polyphenols, while normal cells can survive (Azmi et al., 2006).
In conclusion, the present study shows a novel insight into the mechanism of action of ABJ induced apoptosis on human colon adenocarcinoma cells. A link was reported between apoptosis cell death and the increase of intracellular ROS, our date supported the potential of ABJ as a chemotherapeutic agent, an anti-proliferative activity for human colon adenocarcinoma.
The authors have not declared any conflict of interests.
REFERENCES
|
Azmi AS, Bhat SH, Hanif S, Hadi SM (2006). Plant polyphenols mobilize endogeneous copper in human peripheral lymphocytes leading to oxidative DNA breakage: A putative mechanism for anticancer properties. FEBS Lett. 580:533-538.
Crossref
|
|
|
|
Bermúdez-Soto MJ, Larrosa M, García-Cantalejo J, Espín JC, Tomás-Barberan FA, García-Conesa MT (2007). Transcriptional changes in human Caco-2 colon cancer cells following exposure to a recurrent non-toxic dose of polyphenol-rich chokeberry juice. Genes Nutr. 2(1):111-113.
Crossref
|
|
|
|
|
Boivin D, Blanchette M, Barrette S, Moghrabi A, Béliveau R (2007). Inhibition of cancer cell proliferation and suppression of TNF-induced activation of NFkappaB by edible berry juice. Anticancer Res. 27(2):937-48.
|
|
|
|
|
Boyle P, Levin B (2008). World Cancer Report. International Agency for Research on Cancer (IARC). (eds). Lyon
|
|
|
|
|
Capocasa F, Scalzo J, Mezzetti B, Battino M (2008). Combining quality and antioxidant attributes in the strawberry: The role of genotype. Food Chem. 111:872-878.
Crossref
|
|
|
|
|
Çelik H, Özgen M, Serçe S, Kaya C (2008). Phytochemical accumulation and antioxidant capacity at four maturity stages of cranberry fruit. Sci. Horticult. 117:345-348.
Crossref
|
|
|
|
|
Doll R, Peto R (1981). The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J. Natl. Cancer Inst. 66:1191-1308.
|
|
|
|
|
Eguchi Y, Shimizu S, Tsujimoto Y (1997). Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 57:1835-1840.
|
|
|
|
|
Ferguson P, Kurowska E, Freeman DJ, Chambers AF, Koropatnick DJ (2004). A flavonoid fraction from cranberry extract inhibits proliferation of human tumor cell lines. J. Nutr. 134:1529-1535.
|
|
|
|
|
Franco Tobón YN, Rojano BA, Alzate Arbeláez AF, Morales Saavedra DM, Maldonado Celis ME (2016). Efecto del tiempo de almacenamiento sobre las características fisicoquímicas, antioxidantes y antiproliferativas de néctar de agraz (Vaccinum meridionale Swartz). Arch. Lat. Nutr. 60(4):17-28.
|
|
|
|
|
Forman D, Bray F, Brewster DH, Gombe Mbalawa C, Kohler B, Pi-eros M, SteliarovaFoucher E, Swaminathan R, Ferlay J (2014). Introduction. In Forman D, Bray F, Brewster DH, Gombe Mbalawa C, Kohler B, Pi-eros M, Steliarova-Foucher E, Swaminathan R, Ferlay J (eds). Cancer Incidence in Five Continents, Vol. VIII,. IARC Scientific Publications, France. pp. 1-10.
Crossref
|
|
|
|
|
Gaviria C, Hernández, JD, Lobo M, Medina, CI, Rojano BA (2012). Cambios en la Actividad Antioxidante en Frutos de Morti-o (Vaccinium meridionale Sw.) durante su Desarrollo y Maduración. Rev. Fac. Nac. Agron. 65(1):87-6495.
|
|
|
|
|
Gaviria C, Ochoa C, Sáncez N, Medina C, Lobo M, Galeano P, Mosquera A, Tamayo A, Lopera Y, Rojano B (2009). Actividad antioxidante e inhibición de la peroxidación lipídica de extractos frutos de morti-o (Vaccinium meridionale SW). Bol. Latinoam. Caribe Plantas Med. Arom. 8(6):519-528.
|
|
|
|
|
Gossé F, Guyot S, Roussi S, Lobstein A, Fischer B, Seiler N, Raul F (2005). Chemopreventive properties of apple procyanidins on human colon cancer-derived metastatic SW620 cells and in a rat model of colon carcinogenesis. Carcinogenesis 26(7):1291-1295.
Crossref
|
|
|
|
|
Kang YH, Lee KA, Ryu CJ, Lee HG, Lim JS, Park SN, Paik SG, Yoon DY (2006). Mitomycin C induces apoptosis via Fas/FasL dependent pathway and suppression of IL-18 in cervical carcinoma cells. Cancer Lett. 237(1):33-44.
Crossref
|
|
|
|
|
Khan HY, Zubair H, Faisal M, Ullah MF, Farhan M, Sarkar FH, Ahmad A, Hadi SM (2014) Plant polyphenol induced cell death in human cancer cells involves mobilization of intracellular copper ions and reactive oxygen species generation: a mechanism for cancer chemopreventive action. Mol. Nutr. Food Res. 58(3):437-46.
Crossref
|
|
|
|
|
Kraft TFB, Schmidt BM, Yousef GG, Knight CTG, Cuendet M, Kang Y-H, Pezzuto JM, Seigler DS, Lila MA (2005). Chemopreventive Potential of Wild Lowbush Blueberry Fruits in Multiple Stages of Carcinogenesis. J. Food Sci. 70:S159-S166.
Crossref
|
|
|
|
|
Lamy V, Roussi S, Chaabi M, Gossé F, Lobstein A, Raul F (2008). Lupulone, a hop bitter acid, activates different death pathways involving apoptotic TRAIL-receptors, in human colon tumor cells and in their derived metastatic cells. Apoptosis 13(10):1232-42.
Crossref
|
|
|
|
|
Lemaire C, Andreau K, Souvannavong V, Adam A (1998). Inhibition of caspase activity induces a switch from apoptosis to necrosis. FEBS Lett. 425:266-270.
Crossref
|
|
|
|
|
Maldonado-Celis ME, Arango-Varela SS, Rojano BA (2014). Free radical scavenging capacity and cytotoxic and antiproliferative effects of Vaccinium meridionale Sw. agains colon cancer cell lines. Rev. Cubana Plant Med. 19(2):172-184.
|
|
|
|
|
Minker C, Duban L, Karas D, Järvinen P, Lobstein A, Muller CD (2015). Impact of Procyanidins from Different Berries on Caspase 8 Activation in Colon Cancer. Oxid. Med. Cell. Long. 2015:154164.
Crossref
|
|
|
|
|
Pelicano H, Carney D, Huang P (2004). ROS stress in cancer cells and therapeutic implications. Drug Resist. Updat 7:97-110.
Crossref
|
|
|
|
|
Safrin S, Giampieri F, Gasparrini M, Forbes-Hernandez TY, Varela-López A, Quiles JL, Mezzetti B, Battino M (2016). Chemopreventive and Therapeutic Effects of Edible Berries: A Focus on Colon Cancer Prevention and Treatment. Molecules 21(2):169.
Crossref
|
|
|
|
|
Safrin S, Giampieri F, Gasparrini M, Forbes-Hernandez TY, Varela-López A, Quiles JL, Mezzetti B, Battino M (2016). Chemopreventive and Therapeutic Effects of Edible Berries: A Focus on Colon Cancer Prevention and Treatment. Molecules 21(2):169.
Crossref
|
|
|
|
|
Tamara Y. Forbes-Hernández, Giampieri F, Gasparrini M, Mazzoni L, Quiles JL, Alvarez JM, Battino M (2014). The effects of bioactive compounds from plant foods on mitochondrial function: A focus on apoptotic mechanisms. Food Chem. Toxicol. 68:154-82.
Crossref
|
|
|
|
|
Thompson T, Tovar C, Yang H, Carvajal D, Vu BT, Xu Q, Wahl GM, Heimbrook DC, Vassilev LT (2004). Phosphorylation of p53 on key serines is dispensable for transcriptional activation and apoptosis. J. Biol. Chem. 279:53015-53022.
Crossref
|
|
|
|
|
Vidya PR, Senthil MR, Maitreyi S, Ramalingam K, Karunagaran D, Nagini S (2010). The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-kappaB inhibition. Eur. J. Pharmacol. 649(1-3):84-91.
Crossref
|
|