ABSTRACT
Tobacco use is a leading cause of cancer morbidity and mortality. Out of several possible components of tobacco, the free radical present in tobacco induces oxidative stress which ultimately leads to the damage of DNA. In the present study, an attempt was made to find the natural potential tobacco free radical scavenger, by using different standard assays. Viola odorata has been known for diverse therapeutic applications since the ancient time, so in the present study, the tobacco free radical scavenging activity of V. odorata was explored. A significant increase in inhibitory concentration (IC50) values of V. odorata ethanolic extract was observed, when mixed with ethanolic extract of tobacco, which signify that most of the anti-oxidant activity of V. odorata has been utilized in the inhibition of tobacco free radicals, leading to increment of IC50 values in different assays. This positive finding was validated by four different types of assays. The various standard assays like DPPH, nitric oxide, Fe2+ chelating and hydroxyl radical scavenging were explored to support the study.
Key words: Viola odorata, Nicotiana tabacum, antioxidant property, mixture of extract, tobacco related cancer.
The two important factors responsible for the pathogenesis of human cancers are environmental factors and genetic disorders, out of which 93% of cancers are caused by the former factor and rest 7% are due to the latter (Seto et alet al., 2010). Tobacco related cancer (TRC) caused by smoking/chewing tobacco contributes to 30% of the environmental factors, leading to human cancer (Anand et alet al., 2008). Thus, it is very clear that consuming tobacco is one of the major public health issues. According to the World Health Organization, one out of two people smoking throughout their lives will end up having TRC (WHO, 2012). In addition to nicotine, tobacco mainly contains polyaromatic hydrocarbons (PAH) and N-nitrosamine carcinogens (Xue et alet al., 2014). The tobacco specific nitrosamines such as 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and N'-nitrosonornicotine (NNN), are carcinogenicto humans (IARC, 2007). The NNK and NNN cause cancer by deoxyribonucleic acid (DNA) adductions and mutations by receptor-mediated effect on tumor growth (Xue et alet al., 2014; Takahashi et alet al., 2010). Tobacco smokes contains free radicals which induce oxidative damage or stress (Valko et alet al., 2006). The cause of oxidative stress is the production of oxidant species like reactive oxygen species (ROS) and reactive nitrogen species (RNS). The bulk of ROS are generated by the mitochondrial respiratory chain through incomplete reduction of molecular oxygen to water during oxidative phosphorylation, in addition to during microsomal and peroxisomal oxidations (De Marco, 2003).
The tobacco smoke has large amounts of nitric oxide and other unstable oxidants like hydroquinones, semiquinones and quinones (Xue et alet al., 2014; Hecht, 2003). These compounds induce redox cycling and are responsible for oxidative damage (Hecht, 2011; Pryor et alet al., 20111998). The NNK leads to increased levels of 8-hydroxy-2'-deoxyguanosine adducts in lung tissues, when orally administrated or intraperitoneally injected into mice and rats. The 8-Hydroxy-2'-deoxyguanosine is an important pre-mutagenic lesion produced from ROS that is a marker of DNA oxidative damage (Rosa et alet al., 1998; Chung and Xu, 1992). The activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) is also responsible for increased level of ROS during tumor progression (Lee and Kim, 2013). So, it has been thought that if any healthy free radical scavenger is consumed along with tobacco, then the concentration of ROS and RNS can be decreased significantly, which subsequently reduce the risk of TRC.
Viola odorata (family Violaceae) is commonly known as sweet violet and has been used to treat anxiety, insomnia and hypertension (Mousavi et alet al., 2016). The pharmacological exploration have shown that this plant also has diuretic, laxative (Vishal et alet al., 2009), antihypertensive and antidyslipidemic (Siddiqi et alet al., 2012), antibacterial (Pränting et alet al., 2010), anticancer (Gerlach et alet al., 2010), hepatoprotective (Qadir et alet al., 2014), lung-protective (Koochek et alet al., 2003) and excellent antioxidant activities (Ebrahimzadeh et alet al., 2010). The plethora of therapeutic applications of V. odorata, fortified the authors to evaluate the free radical scavenging activity of the plant extract over the tobacco extract in continuation of their endeavor in the field of natural product chemistry (Khan et alet al., 2016). To the best of the author’s knowledge, this type of cumulative synergistic study is not reported in the literature so far.
Plant material
V. odorata was collected from an authorized dealer of Ayurvedic plants, Sadar bazar, New Delhi. The Nicotiana tabacum leaves were collected from Munger, Bihar, India. The sample was authenticated by Dr Sunita Garg, CSIR-National Institute of Science Communication and Information Resources, New Delhi, India and was deposited in the Raw Material Herbarium and Museum, Delhi (RHMD) under voucher number NISCAIR/RHMD/Consult/2017/ 3081-30. The collected plant material were air dried in shadow and grinded for extraction.
Preparation of extract
Air dried N. tabacum leaves were extracted using 99% ethanol by ultrasonication for 2 h. The combined extract were filtered and concentrated under reduced pressure at 35°C by using rotatory evaporator. The crude extracts were totally dried under high vacuum repeatedly until constant weight was obtained. The dried crude extract was kept over calcium chloride in desiccator for further study.
V. odorata (leaves and flower) was firstly extracted with petroleum ether at room temperature for 2 days to remove nonpolar impurities and waxes (fraction CPEEx). The crude extract was then dissolved in diethyl ether and left for 2 days at room temperature (extract CDEEEx). The solution was occasionally stirred with the help of glass road. After filtration, the crude extract was dissolved in 99% ethanol and left for 2 days at room temperature (extract CEEx). The combined petroleum ether extract (CPEEx), diethyl ether extract (CDEEEx) and ethanol extract (CEEx) were concentrated under reduced pressure at 35°C by using rotatory evaporator and desiccated separately for further study (Tang et al., 2010).
Determination of total flavonoid content
The total flavonoid content was determined by colorimetric aluminum chloride (AlCl3) method with slight modification (Ahn et alet al., 2007). The 0.5 mL of 2% AlCl3 in ethanol and 0.1 mL of 1 M potassium acetate were added to 0.5 mL of sample or standard. After 1 h at room temperature, the absorbance of the reaction mixture was measured at 415 nm with a double beam ultraviolet/visible spectrophotometer. The quercetin (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one) was used as standard.
Determination of total phenol content
Total phenolic compound contents were determined by the Folin-Ciocalteau reagent and gallic acid (trihydroxybenzoic acid) (Agbor et alet al., 2014). The method is based on the theory that Folin-Ciocalteau reagent shows blue color by reaction with phenolic compounds. The extract samples (0.5 mL) were mixed with Folin-Ciocalteu reagent (5 mL, 10% v/v, diluted with de-ionized water) and aqueous sodium carbonate (4 mL, 1 M). The mixture was allowed to stand for 30 min at room temperature and the phenols were determined by measuring absorbance at 765 nm. The standard curve was constructed by different concentration of gallic acid (mg/mL) in methanol : water solutions (50:50 v/v).
Antioxidant activity
Assay of free radical scavenging activity (DPPH)
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical was used for determination of free radical scavenging activity of the extracts based on the method given by Brand-William et alet al. (1995). This method depends on the decrease in absorbance at 517 nm, of colored solution of DPPH in methanol by reference or extract. Ascorbic acid in methanol (1 mg/mL) was used as reference. Different concentrations of each extract were added to methanolic solution of DPPH (100 µM) in equal volumes and left for 20 to 30 min at room temperature. The absorbance was recorded at 517 nm and DPPH radical scavenging activity was calculated as follows:
Inhibition (%) = ( Absblank – Abssample/Absblank) × 100
Where, Absblank is the absorbance of the control, containing all reagents except extract. The inhibitory concentration (IC50) values represent the concentration of sample, which is required to inhibit 50% of DPPH free radicals.
Assay of nitric oxide-scavenging activity
The theory of the assay depends on the estimation of nitrite ion by Griess reagent (Jagetia and Baliga, 2004). The nitrite ion is formed by the reaction of nitric oxide with oxygen, in which nitric oxide is generated by the sodium nitroprusside at biological pH. The Griess reagent usually contains 0.2% naphthylethylenediamine dihydrochloride (NEDD), and 2% sulphanilamide in 5% phosphoric acid. The competitive scavenging activitiyactivity of nitrite ion with oxygen leads to lesser production of nitrite ions. To carry out the experiment, sodium nitroprusside (10 mM), in phosphate buffer at pH 7, was mixed with different concentrations of each extracts dissolved in methanol and left for 2 to 2.5 h at room temperature under dark and anhydrous condition. The absorbance was recorded at 546 nm after adding 0.5 mL of Griess reagent in the experimental mixture. Quercetin was used as positive control or reference.
Assay of metal chelating activity
Ferric ions (Fe2+)-chelating activity of extracts were determined by following the method of Dinis et al. (1994) and Gülçin et alet al. (2004) with minor modification. The Fe2+ capacity was recorded spectrophotometrically at 562 nm. Briefly, the plant extract at different concentrations in methanol was added into 0.1 mL of FeCl2 (1 mM) followed by addition of 0.1 mL of ferrozine (3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-p,p′-disulfonic acid monosodium salt hydrate) solution (5 mM). The experiment solution was incubated at room temperature for about 10 to 15 min. Finally, the absorbance value of the mixture was quantified spectrophotometrically at 562 nm using same formula used for DPPH assay. Where Absblank was the absorbance of the control, and Abssample was the absorbance of the extract or positive control.
Assay of hydroxyl radical scavenging activity
The assay for hydroxyl radical scavenging activity was developed according to Kim et alet al. with minor modifications (Kim and Minamikawa, 1997; Topal et alet al., 2016). The extract at different concentrations in methanol was mixed with 0.4 mL of FeSO4 (10 mM), 0.4 mL ethylenediaminetetraacetic acid (EDTA, 10 mM), 0.4 mL of 2-deoxyribose, 0.2 mL of phosphate buffer and 250 µL of hydrogen peroxide. The experimental mixture was incubated at room temperature for about 4 to 5 h. In the next step 1 mL of trichloroacetic acid (2.5%) and 1 mL thiobarbituric acid (1%) were added to the experimental mixture and heated for 5 minutes at 80°C. After cooling, its absorbance was measured at 520 nm. The hydroxyl radical scavenging activity (%) was calculated using the same formula which was used for DPPH activity. The IC50 was calculated by plotting the inhibition percentage against extract concentrations.
Determination of antioxidant properties of mixture of extracts
The combined activity of both extracts was determined by mixing the different w/w combination. The scavenging activity of the combined extract was determined by the described assays. For this, the mixture of extract was incubated at room temperature with occasionally shaking for about 3 to 3.5 h and then heated at 50 to 60°C for 10 min. This experimental results obtained by interval of times showed that 3 to 3.5 h are sufficient time for this kind of study. The different possible combinations of w/w ratio of mixture were examined with 50 to 10% of tobacco extract with V. odorata extract. The most important results are shown in the current manuscript.
Statistical analysis
The data of various analyses were expressed as mean ± standard deviation. All tests were carried out in triplicate to improve the accuracy. The data reported in the present manuscript were analysed using one way analysis of variance (ANOVA) followed by Dunnet’s test. In the experiments, P values of <0.05 were taken to be significant.
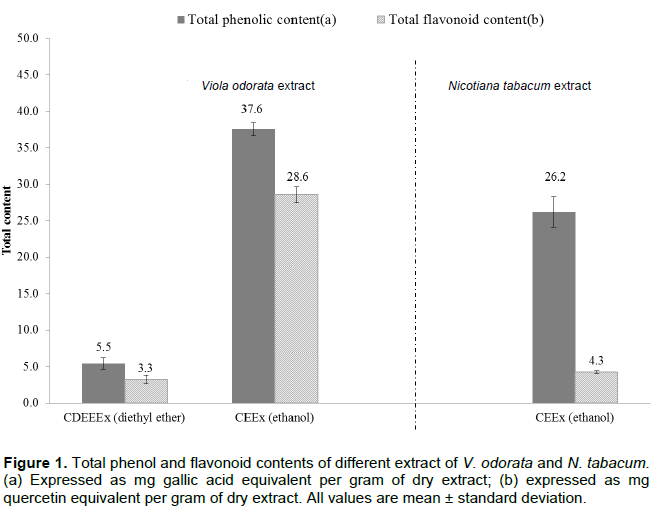
Total phenolic and total flavonoid contents
The total phenolic and total flavonoid contents in the different extracts expressed as gallic acid equivalent and quercetin equivalent are shown in FigureTable 1. The total phenolic and total flavonoid contents were greater in the ethanolic extract [CEEx] than in the diethyl ether extract [CDEEEx]. The petroleum ether extract [CPEEx] showed no positive results in the phytochemical screening. The results were calculated from the standard gallic and quercetin calibration curves (R2 = 0.99).
Free radical scavenging activities
The DPPH free radical scavenging activity of ethanolic extracts of V. odorata, N. tabacum and mixture of both are reported in Table 21. It was found that the radical scavenging activities of the extract were in linear relation with concentration, that is, increased with the concentration. The IC50 value of CEEx (V. odorata) and CEEx (N. tabacum) were 112.36 ± 0.8 μg/mL and 312.68 ± 0.6 μg/mL, respectively, thus signifying that CEEx (V. odorata) has a very good antioxidant property. The IC50 of ascorbic acid (vitamin C) was found to be 4.98 ± 0.3 µg/mL for DPPH assay. The IC50 of nitric oxide radical scavenging activity (NORSA) of CEEx (V.odorata) was found to be 1.26 µg/mL, which may not be considered good as compared to the IC50 of Quercetin (19.86 ± 0.6 µg/mL). The CEEx extracts of V. odorata and N. tabacum also showed good metal chelating activity, which was 168.43 ± 0.2 μg/mL and 168.43 ± 0.2 µg/mL, respectively. The hydroxy radical scavenging activity of CEEx extracts of V. odorata and N. tabacum were found to be 228.65 ± 0.9 μg/mL and 356.36 ± 0.8 µg/mL, respectively.
The main focus of the current manuscript is the scavenging properties of combined extract of V. odorata and N. tabacum. The combined DPPH assay revealed the scavenging activity of mixture of extract with IC50 235.16 ± 0.2 μg/mL. The other assays, that is, NORSA, metal chelating activity and hydroxyl radical scavenging gave the IC50 values of 1.41 ± 0.3, 223.62 ± 0.2 and 315.12 ± 0.3 µg/mL, respectively. For this combined extract analysis, the best found combination, that is, 80% w/w of V. odorata and N. tabacum, was used.
The DPPH assay is a widely accepted and used model for exploration of radical scavenging ability of various extracts and samples. The DPPH is a stable nitrogen-centered free radical, in which the color changes from violet to yellow upon reduction. The potential compound or substance capable of inhibiting this free radical is treated as antioxidants and consequently a radical scavenger. It has been accepted that higher total phenol and flavonoids contents lead to good DPPH-scavenging activity (Xu and Chnang, 2007). From Table Figure 1, it is shown that the ethanolic extract of V. odorata has good antioxidant activity as expressed in terms of DPPH scavenging activity. The activity of combined ethanolic extract significantly decreased (CEEx (V. odorata)) which can be rationalized on the basis of competitive inhibition of tobacco extract free radicals. The free radicals responsible for TRC significantly was inhibited by the V. odorata, which is shown by increase in IC50 values (235.16 ± 0.2 from 112.36 ± 0.8 µg/mL). As NORSA is found to be in the order of mg/mL, nevertheless significant increase in IC50 was observed in the same case from 1.26 ± 0.9 to 1.41 ± 0.3 mg/mL. These results also favor the hypothesis that V. odorata has the capability to inhibit the ROS present in tobacco which leads to TRC.
The Fe2+ catalyzed the oxidative processes, leading to the formation of hydroxyl radicals and hydro peroxide decomposition reactions. Minimizing the concentration of Fe2+ is directly correlated with the decrease in oxidative damage, because it causes the production of oxyradicals (Ak and Gülçin, 2008). The ferrozine formed red colored complex and in the presence of other chelating agent, the red color fades. The absorbance of Fe2+- ferrozine complex varied linearly, which means that the activity was increased with increase in concentration from 12.5 to 1000 µg/mL. The IC50 for Fe2+ chelating ability of CEEx (V. odorata) was 168.43 ± 0.2 (µg/mL) and it significantly increased to 223.62 ± 0.2 (µg/mL) in the presence 20% of tobacco extract w/w (Table 21). This also gave the support that V. odorata has a significant tobacco free radical scavenging activity. The principal for hydroxyl radical scavenging activity, is the conversion of 2-deoxuribose to malondialdehyde, which react with thiobarbituric acid giving rise to a pink pigment. The increase in IC50 value form 228.65 ± 0.9 to 315.12 ± 0.3 µg/mL signify the importance of V. odorata as a free radical scavenger of tobacco.
In conclusion, it has been found that V. odorata has a good antioxidant property, as proved by the ethanolic extract of the same. The various standard assays like DPPH, nitric oxide, Fe2+ chelating and hydroxyl radical scavenging was explored to sum up the good antioxidant property of V. odorata. In addition to this, V. odorata ethanolic extract was found to be a good scavenger of tobacco free radicals, responsible for tobacco related cancer as significant increment of IC50 value was observed in 20% w/w of tobacco and V. odorata extract. The detailed study to find out a more proper tobacco free radical scavenger is under investigation in our laboratory. This finding results in a possible solution to mix the highly antioxidant indexed therapeutically important plant to tobacco so that the emission of cancer causing free radicals can be inhibited and this may reduce the chance of occurrence of tobacco related cancer.
The authors declare that there is no conflict of interest.
REFERENCES
|
Agbor GA, Vinson JA, Donnelly PE (2014). Folin-Ciocalteau reagent for polyphenolic assay. Int. J. Food Sci. Nutr. Diet 3(8):147-156.
Crossref
|
|
|
|
Ahn MR, Kumazawa S, Usui Y, Nakamura J, Matsuka M, Zhu F, Nakayama T (2007). Antioxidant activity and constituents of propolis collected in various areas of China. Food Chem. 101(4):1383-1392.
Crossref
|
|
|
|
|
Ak T, Gülçin Ä° (2008). Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 174(1):27-37.
Crossref
|
|
|
|
|
Anand P, Kunnumakara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS, Sung B, Aggarwal BB (2008). Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 25(9):2097-2116.
Crossref
|
|
|
|
|
Brand-Williams W, Cuvelier ME, Berset C (1995). Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 28(1):25-30.
|
|
|
|
|
Chung FL, Xu Y (1992). Increased 8-oxodeoxyguanosine levels in lung DNA of A/J mice and F344 rats treated with the tobacco-specific nitrosamine 4-(methyhiitrosamine)-l-(3-pyridyl)-1-butanone. Carcinogenesis 13(7):1269-1272.
Crossref
|
|
|
|
|
De Marco F (2013). Oxidative stress and HPV carcinogenesis. Viruses. 5(2):708-731.
Crossref
|
|
|
|
|
Dinis TC, Madeira VM, Almeida LM (1994). Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 315(1):161-169.
Crossref
|
|
|
|
|
Ebrahimzadeh MA, Nabavi SM, Nabavi SF, Bahramian F, Bekhradnia AR (2010). Antioxidant and free radical scavenging activity of H. officinalis L. var. angustifolius, V. odorata, B. hyrcana and C. speciosum. Pak. J. Pharm. Sci. 23(1):29-34.
|
|
|
|
|
Gerlach SL, Rathinakumar R, Chakravarty G, Göransson U, Wimley WC, Darwin SP, Mondal D (2010). Anticancer and chemosensitizing abilities of cycloviolacin O2 from Viola odorata and psyle cyclotides from Psychotria leptothyrsa. Biopolymers 94(5):617-625.
Crossref
|
|
|
|
|
Gülçin Ì, Åžat Ä°G, Beydemir Åž, ElmastaÅŸ M, Küfrevioǧlu ÖÄ° (2004). Comparison of antioxidant activity of clove (Eugenia caryophylata Thunb) buds and lavender (Lavandula stoechas L.). Food Chem. 87(3):393-400.
Crossref
|
|
|
|
|
International Agency for Research on Cancer Working Group on the Evaluation of the Carcinogenic Risks to Humans (IARC) (2007). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans VOLUME 89. Smokeless tobacco and some tobacco-specific N-Nitrosamines. World Health Organization International agency for research on cancer. Available at:
View
|
|
|
|
|
Jagetia GC, Baliga MS (2004). The evaluation of nitric oxide scavenging activity of certain Indian medicinal plants in vitro: a preliminary study. J. Med. Food 7(3):343-348.
Crossref
|
|
|
|
|
Khan MF, Azad CS, Kumar A, Saini M, Narula AK, Jain S (2016). Novel Imbricatolic acid derivatives as protein tyrosine phosphatase-1B inhibitors: Design, synthesis, biological evaluation and molecular docking. Bioorg. Med. Chem. Lett. 26(8):1988-1992.
Crossref
|
|
|
|
|
Kim JW, Minamikawa T (1997). Hydroxyl radical-scavenging effects of spices and scavengers from brown mustard (Brassica nigra). Biosci. Biotechnol. Biochem. 61(1):118-23.
Crossref
|
|
|
|
|
Koochek MH, Pipelzadeh MH, Mardani H (2003). The effectiveness of Viola odorata in the prevention and treatment of formalin-induced lung damage in the rat. J. Herbs Spices Med. Plants 10(2):95-103.
Crossref
|
|
|
|
|
Lee JW, Kim JH (2013). Activation of the leukotriene B4 receptor 2-reactive oxygen species (BLT2-ROS) cascade following detachment confers anoikis resistance in prostate cancer cells. J. Biol. Chem. 288(42):30054-30063.
Crossref
|
|
|
|
|
Mousavi SH, Naghizade B, Pourgonabadi S, Ghorbani A (2016). Protective effect of Viola tricolor and Viola odorata extracts on serum/glucose deprivation-induced neurotoxicity: role of reactive oxygen species. Avicenna J. Phytomed. 6(4):434-441.
|
|
|
|
|
Pränting M, Lööv C, Burman R, Göransson U, Andersson DI (2010). The cyclotide cycloviolacin O2 from Viola odorata has potent bactericidal activity against Gram-negative bacteria. J. Antimicrob. Chemother. 65(9):1964-1971.
Crossref
|
|
|
|
|
Pryor WA, Stone K, Zang LY, Bermúdez E (1998). Fractionation of aqueous cigarette tar extracts: fractions that contain the tar radical cause DNA damage. Chem. Res. Toxicol. 11(5):441-448.
Crossref
|
|
|
|
|
Qadir MI, Ali M, Saleem M, Hanif M (2014). Hepatoprotective activity of aqueous methanolic extract of Viola odorata against paracetamol-induced liver injury in mice. Bangladesh J. Pharmacol. 9(2):198-202.
Crossref
|
|
|
|
|
Rosa JG, Prokopczyk B, Desai DH, Amin SG, El-Bayoumy K (1998). Elevated 8-hydroxy-2'-deoxyguanosine levels in lung DNA of A/J mice and F344 rats treated with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and inhibition by dietary 1, 4-phenylenebis (methylene) selenocyanate. Carcinogenesis 19(10):1783-1988.
Crossref
|
|
|
|
|
Seto M, Honma K, Nakagawa M (2010). Diversity of genome profiles in malignant lymphoma. Cancer Sci. 101(3):573-578.
Crossref
|
|
|
|
|
Siddiqi HS, Mehmood MH, Rehman NU, Gilani AH (2012). Studies on the antihypertensive and antidyslipidemic activities of Viola odorata leaves extract. Lipids Health Dis. 11(1):6.
Crossref
|
|
|
|
|
Takahashi H, Ogata H, Nishigaki R, Broide DH, Karin M (2010). Tobacco smoke promotes lung tumorigenesis by triggering IKKβ-and JNK1-dependent inflammation. Cancer cell. 17(1):89-97.
Crossref
|
|
|
|
|
Tang J, Wang CK, Pan X, Yan H, Zeng G, Xu W, He W, Daly NL, Craik DJ, Tan N (2010). Isolation and characterization of cytotoxic cyclotides from Viola tricolor. Peptides 31(8):1434-1440.
Crossref
|
|
|
|
|
Topal F, Nar M, Gocer H, Kalin P, Kocyigit UM, Gülçin Ä°, Alwasel SH (2016). Antioxidant activity of taxifolin: an activity–structure relationship. J. Enzyme Inhib. Med. Chem. 31(4):674-683.
|
|
|
|
|
Valko M, Rhodes CJ, Moncol J, Izakovic MM, Mazur M (2006). Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 160(1):1-40.
Crossref
|
|
|
|
|
Vishal A, Parveen K, Pooja S, Kannappan N, Kumar S (2009). Diuretic, laxative and toxicity Studies of Viola odorata aerial parts. Pharmacol. online 1:739-48.
|
|
|
|
|
WHO (World Health Organization) (2012). WHO global report on mortality attributable to tobacco. In WHO global report on mortality attributable to tobacco. Available at: http://www.who.int/tobacco/publications/surveillance/rep_mortality_attributable/en/
|
|
|
|
|
Xu BJ, Chang SK (2007). A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 72(2):S159-166.
Crossref
|
|
|
|
|
Xue J, Yang S, Seng S (2014). Mechanisms of cancer induction by tobacco-specific NNK and NNN. Cancers 6(2):1138-1156.
Crossref
|
|