ABSTRACT
Croton macrostachyus, one of the Croton species found in Ethiopia, is used traditionally for treatment of a number of health ailments including diabetes, malaria, stomachache, ascariasis, abdominal pain, gonorrhea, wounds, ringworm infestation and hemorrhoids. In the present study, phytochemical screening of ethanol extract from leaves of C. macrostachyus revealed the presence of terpenes, flavonoids, alkaloids and saponins. Silica gel column chromatographic separation of the ethanol extract afforded a pentacyclic triterpeniod reported for the first time from the genus. The structure of the compound was determined using combination of spectroscopic techniques via UV-Vis, IR, 1H NMR, 13C NMR and DEPT-135.
Key words: Croton macrostachyus, pentacyclic triterpenoids, anti-malaria, spectroscopic techniques, phytochemical screening.
Medicinal plants have been used to treat various health ailments for a long period of time in different countries. Natural products have been playing dominant role in drug discovery efforts for treatment of human and livestock diseases (Newman and Cragg, 2012). With the upsurge in the use of plants medicines, a through scientific investigation of these plants is imperative based on the need to validate their folklore use. Croton species are among the most common traditional medicinal plants used in Africa, Asia, and South America for treatment of diabetes (Moshi et al., 2000), digestive problems (Yirga et al., 2011), malaria (Mohammed et al., 2007; Alshawsh et al., 2009; Mesfin et al., 2009), insomnia and head-ache (Bum et al., 2012), hemorrhoids and ulcers (Antonio, 2007). The genus has been reported to have a number of biological activities for instance anti-hypertensive, anti-inflammatory, antimalarial and anti-viral (Mbiantcha et al., 2013; Habtamu et al., 2012; Prozesky et al., 2001). Triterpenoids, either pentacyclic or steroidal, volatile oils containing mono and sesquiterpenoids, shikimate-derived compounds and phenolic compounds are among secondary metabolites reported from the genus Croton. Reports on the pharmacological activity of mactostachyus species suggested that the genus is a potential source of enourmous bioactive compounds (Antonio, 2007). In Ethiopia, people use the stem and roots of the plant for diarrhea treatment and decoction of the leaf to treat malaria (Giday et al., 2007). Recent studies by Sendeku et al. (2015) and Taye et al. (2011) indicated that the methanol and ethanol extracts from leaves of C. macrostachyus showed antibacterial activity while the aqueous extracts were comparatively ineffective. Following the potential of the plant in traditional medicine and its efficient pharmacological activity, further analysis is required to determine the possible bioactive components.
In the ongoing study to analyze the chemical constituents of medicinal plants found in Ethiopian flora, we hereby report a comprehensive phytochemical screening, isolation and a complete characterization of one triterpenoid from the leaves of C. macrostachyus.
Instruments
Nuclear Magnetic Resonance (NMR) analysis was recorded on a Bruker Avance 300 MHz spectrometer with deutrated CDCl3 using tetramethylsilane (TMS) as internal standard. Structural elucidations were done on the basis of NMR spectra both 1D (1H NMR, 13C NMR and DEPT-135) and IR. All NMR data were obtained in CDCl3. Chemical shifts are reported in parts per million (ppm, δ). Spectral splitting patterns are designated as s: singlet, d: doublet, t: triplet, q: quartet, m: complex multiplet (chemically non-equivalent H’s), br s: broad signal. 13C NMR spectra were proton decoupled and recorded on a 100 MHz Bruker spectrometer using TMS as the internal standard. Column chromatography (CC) was performed over silica gel (Merck, 230-400 mesh). Analytical thin layer chromatography (TLC) was carried out using pre-coated 0.2 mm silica gel 60 F254 on aluminum foil and compounds on TLC were detected under UV lamp at 254 and 365 nm. UV-Vis spectrum was recorded on UNICAM UV-300 double beam spectrophotometer in the range of 200 to 1000 cm-1 using CHCl3 as internal standard. Infrared (IR) spectrum was recorded on Perkins-Elmer Bx Infrared spectrometer using KBr disc in the range 4000 to 400 cm-1. Melting point was recorded by Mettler Toledo apparatus, Type FP62, and it was uncorrected. All reagents were obtained from Sigma Aldrich and used as received unless noted otherwise.
Preliminary phytochemical screening test was done based on standard procedures according to Sofowora (1982), Trease and Evans (1989) and Harborne (1973).
Plant material
The leaves of C. macrostachyus (Bissana in Amharic, local language) were collected from Dawuro area, in Southern Nation and Nationalities of People Region (SNNPR), located at a distance of 512 km from the capital city, Addis Ababa, Ethiopia in July 2009. The plant material was identified by Dr. Ensermu Kelbessa, Department of Biology, Faculty of Natural Sciences, Addis Ababa University (AAU) and a voucher specimen (CM-001) was deposited at National Herbarium of Ethiopia, Addis Ababa University. The leaves of C. macrostachyus samples were collected and spread to enhance drying at room temperature and then grounded into fine powder using mortar and kept in a transparent polyethylene bangs until extraction.
Preparation of crude ethanol extract
250 g powdered leaf samples of C. macrostachyus was soaked with petroleum ether for 3 days with occasional shaking and filtered to remove the fat contents. After filtration, the air dried marc was further soaked in 650 ml of ethanol for three days at room temperature. The ethanol extract was then filtered and concentrated using Rotary Evaporator at 40°C, air dried and weighted to yield 36.6 g (15.3%) dark green crude extract. The ethanol extract was suspended in water and partitioned with diethylether and ethyl acetate successively and combined to give a total yield of 4.7 g dark green solid. TLC analysis of the organic layer and aqueous extract showed six and eight spots, respectively, using chloroform/ethyl acetate (9:1) and n-hexane: chloroform (4:1) as eluent.
Isolation and purification of compound 1
3.5 g Crude organic layer part (3.5 g) was subjected to silica gel column chromatography (silica gel, 70 g) with increasing gradient of ethyl acetate in chloroform followed by methanol in chloroform as an eluent. A total of 31 fractions, each 50 mL, were collected and analyzed by TLC. Fractions from Fr 12 to 18 (5 to 20% ethyl acetate in chloroform) showed two spots on TLC. They were combined and further purified using silica gel column chromatography to give a single clear spot (compound 1) using chloroform/ethyl acetate (1:1) as eluent.
Isolation and purification of compound 2
Greenish ethanol extract (4.7 g) was dissolved in 15 ml chloroform, subjected to silica gel column chromatographic separation (80 g silica gel) and eluted with increasing gradient of chloroform in n-hexane and then ethyl acetate in chloroform successively. A total of 17 fractions (each 50 ml) were collected. The constituent profile of each fraction was monitored by TLC (40% ethyl acetate in chloroform) and visualized under UV-Vis light (λmax 254 and 366 nm). Based on their TLC profile, fractions 4 to 8 were combined and purified to give a single spot on TLC analysis (chloroform: ethyl acetate (9:1)). Fraction 9 to 14 were also combined, purified and taken to spectroscopic analysis due to their amount and level of purity. The last two fractions were dark green in color and show cloudy red spots on fluorescent UV-Vis light. Fractionation stopped after observing the dark green part at bottom. The fraction then labeled compound 2 was obtained as a pale yellow precipitate. (M.pt: 244.1°C±2, 44.8 mg).
Preliminary phytochemical screening
Detections of common secondary metabolites were performed for ethanol extract of leaves of C. macrostachyus using the preceding analytical procedures (Kebede et al., 2015).
Test for terpenes
250 mg of ethanol extract was mixed with 2 ml of CHCl3 and 30 ml of concentrated H2SO4 was added carefully to form a layer (Debjyoti, 1995). Reddish-brown coloration of the interface was inspected.
Test for flavonoids
250 mg of ethanol extract was dissolved in small amount of dilute NaOH and concentrated HCl (3 ml) was added (Farnsworth, 1996). A yellow solution that turns to colorless was inspected.
Test for tannins
Small quantity of the ethanol extract was mixed with water and heated on water bath. The mixture was filtered and small amount of solid FeCl3 was added to the filtrate (Sofowora, 1982). Dark-green solution was inspected.
Test for alkaloids
250 mg of the crude extract was mixed with 2 ml of concentrated hydrochloric acid. The mixture was then filtered and mixed with small amount of amyl alcohol at room temperature (Ganjewala et al., 2009). The mixture was kept for observation of the color resulted from the alcoholic layer.
Test for saponins
250 mg of the ethanol extract was shaken with 5 ml of distilled water for 30 min and then heated to boil. Appearance of creaming mix of small bubbles (frothing) was inspected (Farnsworth, 1996).
Test for anthraquinones
500 mg of the ethanol extract was boiled with concentrated hydrochloric acid for few minutes in water bath and filtered. The filtrate was allowed to cool and equal volume of CHCl3 was added to it. Few drops of ammonia were added to the mixture and heated in water bath. Formation of rose-pink color was inspected (Sofowora, 1982).
Preliminary phytochemical screening of the crude ethanol extract of the leaf revealed the presence of various metabolites such as terpenoids, flavonoids, saponins and alkaloids whereas tannins and anthraquinones were not detected (Table 1).
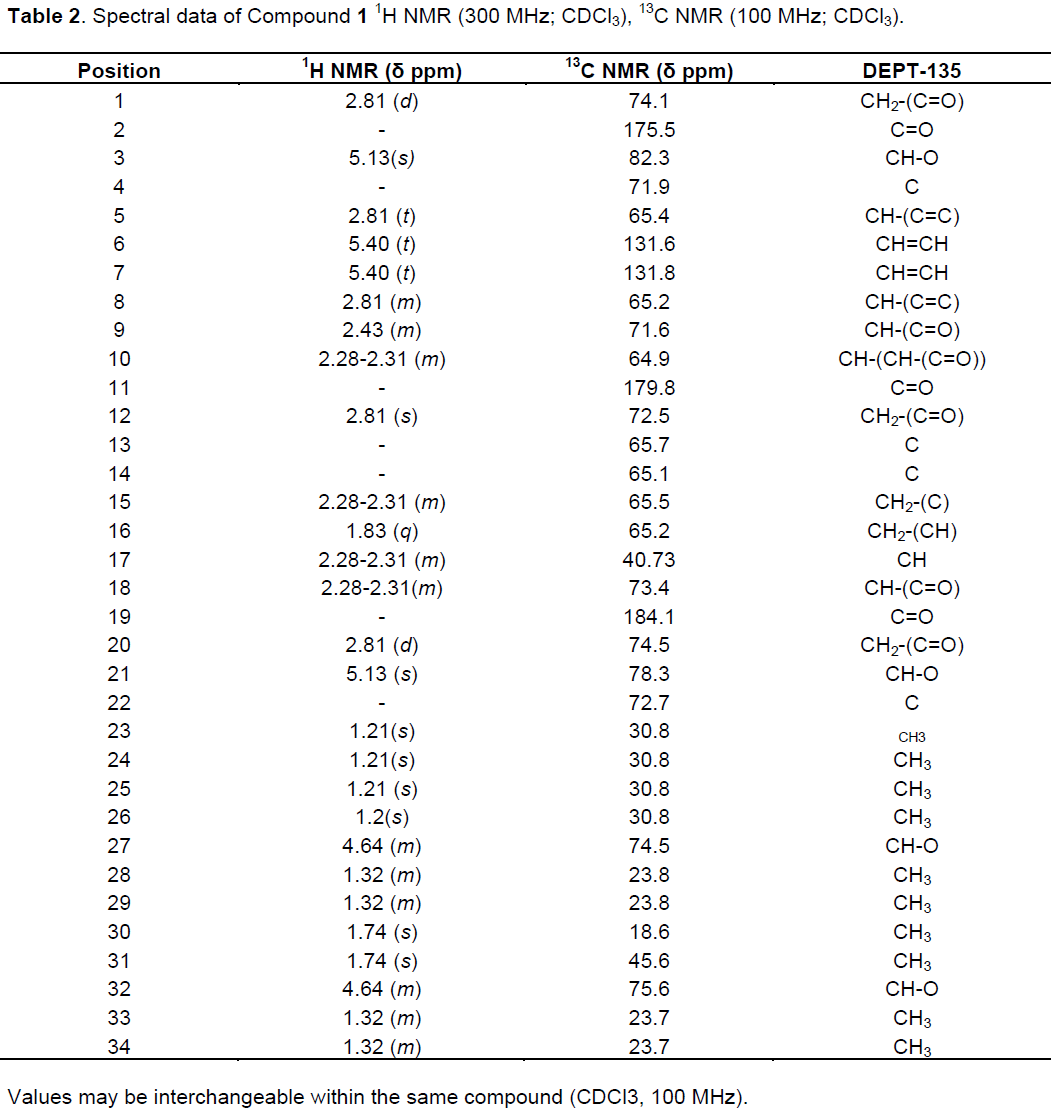
Silica gel column chromatographic separation of the crude ethanol extract afforded one pentacyclic triterpenoid and complete characterization of the compound is presented subsequently. All the NMR chemical shift values, δ, are presented in ppm. The IR spectrum of compound 1 indicated absorption peaks at 2926.56 and 2856.24 cm-1 (sp3 C-H methyl stretching and sp2 C-H methylene, respectively), 1738.71 cm-1 (carbonyl group of ketone moiety), 1468.91 cm-1 (olefinic carbon), 1377.08 and 1365.51 cm-1 (C-O of ether functionality). The UV-Vis spectrum revealed absorption peak λmax at 380 nm attributed to n-π* transition.
The 1H NMR spectrum showed peaks at δ 5.40 (t, 2H, H-7) and 5.13 (s, 2H, H-3) attributed to olefinic protons and a methine hydrogen next to a ketone and an ether group. An isopropyl group attached to heteroatom oxygen and a methylene attached to carbonyl group were clearly evident at δ 4.64 (m, 1H, H-27) and 2.43 (H-9), respectively.
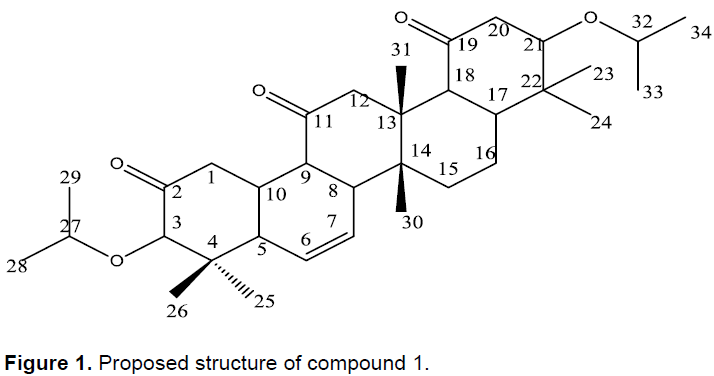
Furthermore, methyl groups were observed at δ (ppm) 1.83 (H-16), 1.74 (H-30, 31), 1.32 (H-33, 34) and 1.21 (H-25, 26). The 13C-NMR spectrum showed a total of 34 well-resolved carbon signals attributed to three ketone carbonyls [δ 184.1 (C-19), 179.80 (C-11) and 175.5 (C-2)], four quaternary carbons, twelve methines, five methylenes and ten methyls, also supported by DEPT-135. Of these, four of the methines were observed to be linked to hetroatom, possibly oxygen [δ 82.3 (C-3), 78.3 (C-21), 75.6 (C-32) and 74.5 (C-27)] and two olefinic carbons δ131.8 (C-6) and 131.6 (C-7)], also confirmed from DEPT-135 spectrum. Seven peaks observed in 13C NMR spectrum [δ 184.1 (C-19), 179.8 (C-11), 175.5 (C-2), 65.7 (C-22), 65.5 (C-4), 65.2 (C-14) and 65.1 (C-13)] were not detected in DEPT-135 spectrum suggesting that these carbons belong to quaternary carbons. The dawn field chemical shift of methyl group (C-31) compared to methyl (C-30) is attributed to the anisotropic effect of the ketone group (C-19), in agreement with the position of the carbonyl group at C-19 (Table 2). Based on the aforementioned spectroscopic data and comparison with literature the compound was found to be a pentacyclic triterpeniod (Figure 1) reported for the first time from the genus.
Compound 2 was found as pale yellow crystalline powder with Rf value of 0.58 (40% ethyl acetate in chloroform as eluent). The 1H NMR spectrum of the compound showed OH protons aromatic phenyl protons. The 13C NMR spectrum of the compound 2 showed a total of around sixty carbon atoms peaks of which one carbonyl carbon multiple aromatic and aliphatic carbons The idea was supported by the IR spectrum showing broad region for OH with methylene and characteristic aromatic C=C peaks with C=O peaks. However, we are not able to elucidate the structure from the spectroscopic data and further advanced spectroscopic studies are needed to elucidate the structure of compound 2.
Preliminary phytochemical screening test on the ethanol extract of leaves of C. macrostachyus revealed the presence of alkaloids, terpenes, flavonoids and saponins. Anthraquinones and tannins were not detected in the present work. The pentacyclic triterpenoid identified in this study was reported for the first time from C. macrostachyus. The traditional use of the plant may be attributed to its high content of polar bioactive constituents. Apart from the phytochemical screening of the ethanol leaf extract, isolation and NMR characterization of one triterpene was successfully achieved. This work will give background record to use C. macrostachyus as a potential drug source. Further work on isolation of the detected bioactive molecules in concert with their biological activities including antiviral and antibacterial effects is going on.
The authors have not declared any conflict of interest.
The authors are grateful to Dr. Ensermu Kelbessa and staff members of the National Herbarium of Ethiopia, Addis Ababa University, for identification of the plant material. They are grateful to Dr. Alay Hagos for conducting IR spectrophotometer at Netherlands. Prof. Ermias Dagne, Department of Chemistry, Addis Ababa University is duly acknowledged for allowing us to use 300 MHz NMR spectrometer. This research is partly supported by the School of Graduate Study, Hawassa University.
REFERENCES
|
Alshawsh MA, Mothana RA, Al-Shamahy HA, Alsllami SF, Lindequist U (2009). Assessment of anti-malarial activity against Plasmodium falciparum and phytochemical screening of some Yemeni medicinal plants. Evid. Based Complement. Altern. Med. 6(4):453-6.
Crossref
|
|
|
|
Antonio S, Maria L, Faria S, Giuseppina N (2007). Traditional uses, Chemistry and pharmacology of croton species (Euphorbiaceae). J. Braz. Chem. Soc. 18:11-33.
Crossref
|
|
|
|
Bum EN, Ngah E, Ngo Mune RM, Ze Minkoulou DM, Talla E, Moto FCO, Ngoupaye GT, Taiwe GS, Rakotonirina A, Rakotonirina SV (2012). Decoctions of Bridelia micrantha and Croton macrostachyus may have anticonvulsant and sedative effects. Epilepsy Behav. 24:319-323.
Crossref
|
|
|
|
Debjyoti D (1995). Biochemistry 8th Ed. Academic publishers, Kolkata. P 39.
|
|
|
|
Farnsworth NR (1996). Phytochemical screening of the biologically active compounds from higher plants. J. Pharm. Sci. 55:227-276.
|
|
|
|
Ganjewala D, Dipita B (2009). Effect of leaf positions on total phenolics, flavonoids and proantho-cyanidins content and Antioxidant Activities in Lantana camara. J. Sci. Res. 2:363-369.
|
|
|
|
Giday M, Teklehaymanot T, Animut A, Mekonnen Y (2007). Medicinal plants of the Shinasha, Agew-awi and Amhara peoples in northwest Ethiopia. J. Ethnopharmacol. 110:516-525.
Crossref
|
|
|
|
Habtamu G, Legesse A, Yinebeb T, Asrat H (2012). Isolation of Crotepoxide From Berries of Croton macrostachyus and Evaluation of Its Anti-Leishmanial Activity. J. Pharmacogn. Phytochem. 1(4):15-24.
|
|
|
|
Kebede T, Kibret F, Fikre M, Milkyas E (2015). Phytochemical Screening and Characterization of Olean-18-ene Type Triterpeniod from the Roots of Lantana camara. Sci. Technol. Arts Res. J. 4(1):91-94.
Crossref
|
|
|
|
Mbiantcha M, Nguelefack TB, Ndontsa BL, Tane P, Kamanyi A (2013). Preliminary Assesement of Toxicity of Croton macroxtachyus stem bark extracts. Int. J. Pharm. Chem. Biol. Sci. 3(1):113-132.
|
|
|
|
Mesfin A, Giday M, Anmut A, Teklehaymanot T (2012). Ethnobotanical study of antimalarial plants in Shinile District, Somalia Region, Ethiopia, and in vivo evaluation of selected ones against Plasmodium berghei. J. Ethnopharmacol. 139:221-227.
Crossref
|
|
|
|
Moshi MJ, Uiso FC, Mahunnah RLA, Mbwambo ZH, Kapingu MC (2000). A survey of plants used by traditional healers in the management of noninsulin dependent diabetes mellitus. East Centr. Afr. J. Pharm. Sci. 3:30â€39.
|
|
|
|
Newman DJ, Cragg GM (2012). Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 75(3):311-335.
Crossref
|
|
|
|
Prozesky EA, Meyer JJ, Louw AI (2001). In vitro antiplasmodial activity and cytotoxicity of ethnobotanically selected South African plants. J. Ethnopharmacol. 76:239-244.
Crossref
|
|
|
|
Sendeku W, Alefew B, Mengiste D, Seifu K, Girma S, Wondimu E, Bekuma G, Verma D, Berhane N (2015). Antibacterial Activity of Croton macrostachyus Against Some Selected Pathogenic Bacteria. Biotechnol. Int. 8(1):11-20.
|
|
|
|
Sofowora A (1982). Historical review of traditional medicine. In, medicinal plants and traditional medicine in Africa. John Wily and Sons Ltd., Chichester, USA. pp. 9-12.
|
|
|
|
Taye B, Giday M, Animut A, Seid J (2011). Antibacterial activities of selected medicinal plants in traditional treatment of human wounds in Ethiopia. Asian Pac. J. Trop. Biomed. 1(5):370-375.
Crossref
|
|
|
|
Yirga G, Teferi M, Kasaye M (2011). Survey of medicinal plants used to treat human ailments in Hawzen district, Northern Ehiop. Inter. J. Biodivers. Conserv. 3:709-714.
|