Full Length Research Paper
ABSTRACT
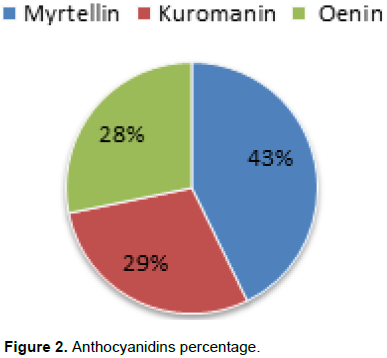
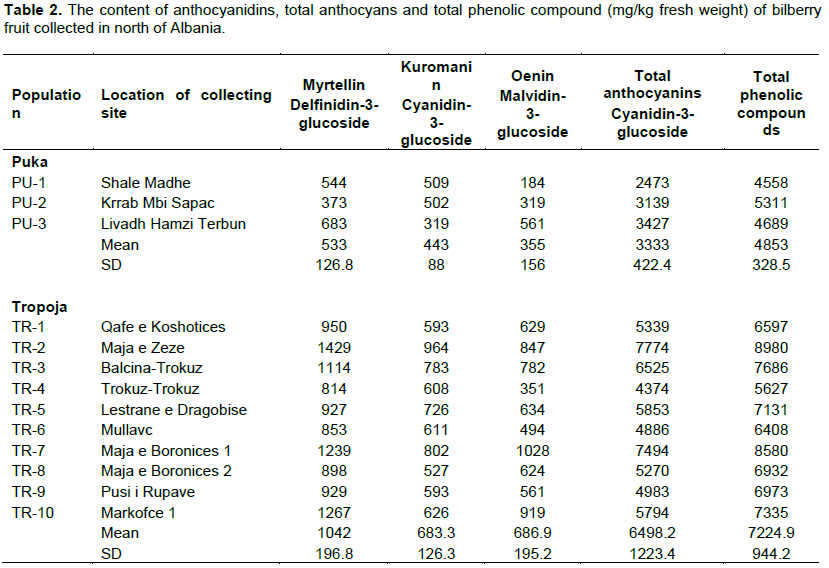
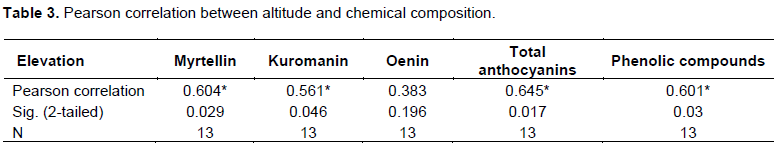
The objective of this study was to evaluate the content of anthocyanin and total phenols, in some wild bilberry populations, grown in natural conditions in northern Albania. This study aimed at identifying the wild bilberry populations, whose fruits are rich in anthocyanin and as such, can be selected and cultivated for production in the future. Thirteen bilberry fruit (Vaccinium myrtillus L.) samples were collected from wild bilberry populations in Puka and Tropoja region. Collected fruit samples were evaluated for chemical traits (myrtellin, kuromanin, oenin, total anthocyans, and total phenolic compounds) using high-performance liquid chromatography (HPLC) analyses. This study confirmed significant chemical differences between two regions (Puka-Tropoja) as well as within one region. The bilberry fruit analyses showed that the total anthocyans (calculated as cyanidin-3-glucozide) concentration ranges from a minimum of 2473 mg/kg FW (Shale e madhe Iballe/Puke) to a maximum 7774 mg/kg FW (Maja e zeze Doberdol/Tropoje). In addition, it was confirmed that the total phenolic compounds (min 4558 to max 8980 mg/kg FW) was significantly higher for bilberry fruits samples collected in Tropoja compared to the ones collected in Puka region. Statistical analyses showed that elevation gradient has a great effect on chemical composition of bilberry fruit samples. It also showed that among analysed anthocyanidins, myrtellin have the highest concentration (43%).
Key words: Chemical variability, wild bilberry (Vaccinium myrtillus L.) populations, northern part of Albania.
INTRODUCTION
Bilberry plant is an important plant due to its edible fruit and medicinal use of both fruit and leaves. The bilberry contains anthocyanin which is considered to be an important natural antioxidant (Prior et al., 1998; Huang et al., 2012; Saral et al., 2015; Zoratti et al., 2016). Anthocyanins are the colorant/pigment of the fruit and flowers (Chandra et al., 2001; Fe et al., 2010; Huang et al., 2014). Bilberry fruits have been used for different purposes such as medicinal fruit due to positive effects on cardiovascular system and vascular diseases (Jungfer, 2013; Basu et al., 2010). The bilberry leaf decoction has been proven to have positive effect on diabetes type 2 (Ferreira et al., 2010; Upton, 2001). Bilberry (Vaccinium myrtillus L.) fruit is 60 to 70% richer in anthocyanins compared to blueberry (Vaccinium corumbosum) (Burdulis et al., 2009; Moze et al., 2011; Primetta et al., 2013; Gonçalves et al., 2015) which is richer in total phenols (Jaakola et al., 2002; Stajcic et al., 2012; Rimpapa et al., 2015 ). The anthocyanins are found mainly in skin and pulp of the bilberry fruit (Akerstrom, 2010; Oancea et al., 2013; Saral et al., 2015).
Albania is characterised by a great diversity in plants (especially wild plants) due to its hilly/mountainous landscape and Mediterranean climate. Although, the country is very rich in plant genetic resources (especially wild plants), chemical evaluations and sustainable use of medicinal and aromatic plant (MAPs), resources remain low (Asllani, 2004).
Though the bilberry fruit in Albania is consumed either fresh or as processed juice, it is emphasized that so far no studies have been conducted on the factors influencing the quality of the fruit, which according to the literature data, mostly depends on the content of anthocyanin and phenols in the fruit. The cultivation potential of this plant in Albania is very high, particularly in the areas adjacent to the natural growth habitat.
Bilberry is considered as a niche product with a real potential for economic value and market. Within MAPs, bilberry plant (for fruit) has greater economic importance where quality production is obtained from natural populations of bilberry in several region of Albania. Bilberry plant is naturally grown as wild populations in the northern part of Albania, mainly in the regions of Tropoja, Puka, Dibra and Kukes (Demiri, 1983). The study area covered the two most significant regions as regards the wild bilberry production and bilberry covered surface in the country.
Spontaneous and uncontrolled collection (damage to fruiting branches during collection) has significantly affected the distribution and the number of bilberry plants in its natural habitat. Under such circumstances, the cultivation of the bilberry and its controlled production, in order to guarantee the natural regeneration of its natural habitats has become an emergency.
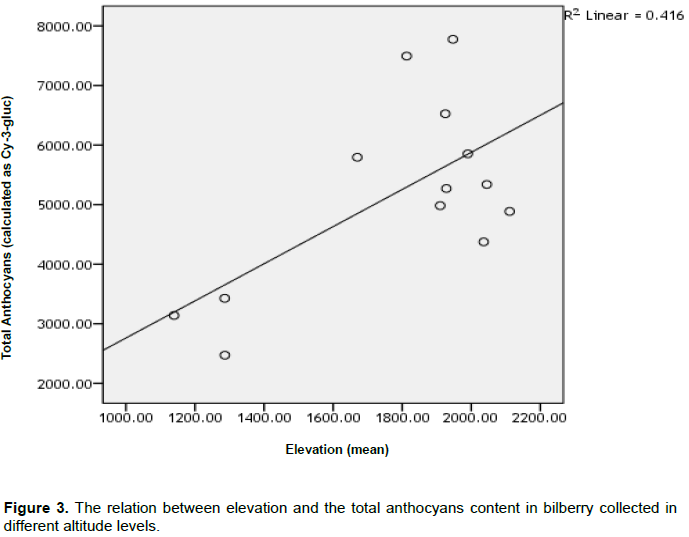
The main purpose of this study was to identify the main factors affecting the content of anthocyanin and phenols in the fruit as well as the impact of other climate factors such as elevation, exposure of the land, or plant associations, where bilberry grows etc.
In this context, this study is of interest due to the fact that it is able to identify some populations that are rich in anthocyanins and phenols, and can be used for cultivation in the future. On the other hand, the data of this study, which evaluate the impact of elevation, land exposure, etc, will serve precisely to the above-mentioned purpose.
MATERIALS AND METHODS

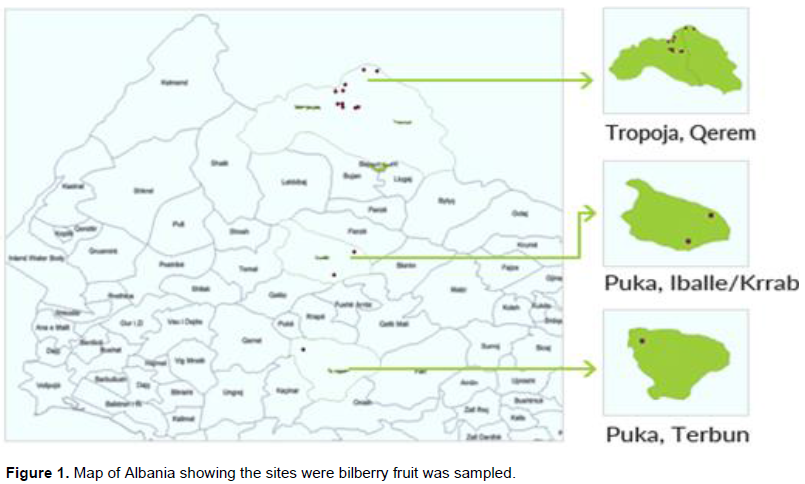
RESULTS AND DISCUSSION
CONCLUSIONS
CONFLICT OF INTERESTS
REFERENCES
|
Akerstrom A (2010). Factors affecting anthocianidin concentration in fruit of Vaccinium myrtillus L. Doc. Thes. 1-58. |
|
|
Asllani U (2004). The essentials oils of Medicinal and Aromatic Plants in Albania. |
|
|
Basu A, Rhone M, Lyons TJ (2010). Berries: emerging impact on cardiovascular health. Nutr. Rev. 68(3):168-177. |
|
|
Burdulis D, Sarkinas A, Jasutien I, Stackevicien E, Nikolajevas L, Janulisi V (2009). Comperative study of anthocyanin composition, antimicrobial and antioxodant activity in bilberry (Vaccinium myrtillus L.) and blueberry (Vaccinium corymbosum L.) fruits. Acta Pol. Pharm. 66(4):399-408. |
|
|
Cesa S, Carradori S, Bellagamba G, Locatelli M, Casadei MA, Masci A, Paolicelli P (2017). Evaluation of processing effects on anthocyanin content and colour modifications of blueberry (Vaccinium spp.) extracts: Comparison between HPLC-DAD and CIELAB analyses. Food Chem. 232:114-123. |
|
|
Chandra A, Rana J, Li Y (2001). Separation, identification, quantification and method validation of anthocyanins in botanical supplement raw materials by HPLC and HPLC-MS. J. Agric. Food Chem. 49(8):3515-3521. |
|
|
Dahlø ES (2011). Variation in chemical composition and genetic differentiation among bilberry (Vaccinium myrtillus L.) populations on a latitudinal gradient (Master's thesis, Institute for biology). |
|
|
Demiri M (1983). Excursion flora of Albania. Shtëpia Botuese e Librit Shkollor, Tiranë Albanian. |
|
|
Eder R, Wendelin S, Barna J (1994). Classification of red wine cultivars by means of anthocyanin analysis. Mitt. Klosterneuburg 44:201-212. |
|
|
Ferreira F, Peixoto F.P, Nunes E, Sena C, Seiça R, Santos M.S (2010). Vaccinium myrtillus improves liver mitochondrial oxidative phosphorylation of diabetic Goto-Kakizaki rats. J. Med. Plants Res. 4(8):692-696. |
|
|
Gonçalves C, Guine R, Teixeira D, Gonçalves F (2015). Evaluation of bioactive phenols in blueberries from bifferent cultivars. Int. J. Biol. Food Vet. Agric. Eng. 9(4):281-284. |
|
|
Habanova M, Haban M, Kobidova R, Schwarzova M, Gazo J (2013). Analysis of biologically active substances in bilberry (Vaccinium myrtillus L.) in selected natural localities of Slovak republic. J. Central Eur. Agric. 14(3):357-366. |
|
|
He F, Mu L, Yan GL, Liang NN, Pan QH, Wang J, Reeves M, Duan CQ (2010). Biosynthesis of anthocyanins and their regulation in collared grapes. Molecules 15(12):9057-9091. |
|
|
Huang YW, Liu YM, Wang J, Wang XN, Li CY (2014). Anti-Inflammatory effect of the blueberry anthocyanins malvidin-3-glucoside and malvidin-3-galactoside in endothelial cells. Molecules 19:12827-12841. |
|
|
Huang YW, Zhang HC, Liu W, Li CY (2012). Survey of antioxidant capacity and phenolic composition of blueberry, blackberry and strawberry in Nanjing. J. Zhej. Uni. Sci. 13(2):94-102. |
|
|
Huber E, Wendelin S, Kobler A, Berghofer E, Eder R (2005). Determination of phenolic composition and antioxidant capacity or ripened red grape variety. Mitt. Klos. 55:3-21. Available at: |
|
|
Jaakola L, Hohtola A, Määttä K, Törrönen S, Kärenlampi S (2002). Flavonoid biosynthesis in bilberry (Vaccinium myrtillus L.). Int. Horticult. Congress Environ. Stress Horticult. Crops 618:415-419. |
|
|
Jovancevic M, Balijagic J, Menkovic N, Savikin K, Zdunic G, Jankovic T, Dekic-Ivancevic M (2011). Analysis of phenolic compounds in wild population of bilberry (Vaccinium myrtillus L.) from Montenegro. J. Med. Plants Res. 5(6):910-914. |
|
|
Jungfer E (2013). Authenticity determination of selected Vaccinium species using HPLC-MS. PhD. Dissertation. unpublished. |
|
|
Moze S, Polak T, Gasperlin L, Koron D, Vanzo A, Poklar Ulrih N, Abram V (2011). Phenolics in Slovenian bilberries (Vaccinium myrtillus L.) and blueberries (Vaccinium corymbosum L.). J. Agric. Food Chem. 59(13):6998-7004. |
|
|
Oancea S, Moiseenco F, Traldi P (2013). Total phenolic and anthocyanin profile of Romanian wild and cultivated blueberries by direct infusion ESI-IT-MS/MS. Rom. Biotechnol. Lett. 18(3):8350-8360. |
|
|
Primetta AK, Jaakola L, Ayaz FA, Inceer H, Riihinen KR (2013). Anthocyanin fingerprinting for authenticity studies of bilberry (Vaccinium myrtillus L.). Food Control 30(2):662-667. |
|
|
Prior R, Cao G, Martin A, Sofic E, McEwen J, O'Brien C, Lischner N, Ehlenfeldt M, Kalt W, Krewer G, Mainland M (1998). Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity and variety of Vaccinium species. J. Agric. Food Chem. 46(7):2686-2693. |
|
|
Rimpapa Z, Toromanovic J, Tahirovic I, Sapcanin A, Sofic E (2007). Total content of phenols and anthocyanins in edible fruit from Bosnia. Bosn. J. Basic Med. Sci. 7(2):117-20. |
|
|
Rohloff J, Uleberg E, Nes A, Krogstad T, Nestby R, Martinussen I (2015). Nutritional composition of bilberries (Vaccinium myrtillus L.) from forest fields in Norway – Effects of geographic origin, climate, fertilization and soil properties. J. Appl. Bot. Food Qual. 88(1):274-287. |
|
|
Saral O, Olmez Z, Sahin H (2015). Comparison of antioxidant properties of wild blueberries (Vaccinium arctostaphylos L. and Vaccinium myrtillus L.) with cultivated blueberry varieties (Vaccinium corymbosum L.) in Artvin region of Turkey. Turk. J. Agric. Food Sci. Technol. 3(1):40-44. |
|
|
Skenderi G (2003). Herbal Vade Mecum: 800 Herbs, Spices, Essential Oils, Lipids, etc., Constituents, Properties, Uses, and Caution. CreateSpace. pp. 46-51. |
|
|
Stajcic SM, Tepić AN, Äilas SM, Šumić ZM, ÄŒanadanović-Brunet JM, Ćetković GS, Vulić JJ, Tumbas VT (2012). Chemical composition and antioxidant activity of berry fruits. Acta Period. Technol. (43):93-105. |
|
|
Uleberg E, Rohloff J, Jaakola L, Trost K, Junttila O, Haggman H, Martinussenand I (2012). Effects of temperature and photoperiod on yield and chemical composition of northern and southern clones of bilberry (Vaccinium myrtillus L.). J. Agric. Food Chem. 60:10406-10414. |
|
|
Upton R (2001). Bilberry Fruit Vaccinium myrtillus L. Standards of Analysis, Quality Control, and Therapeutics. Am. Herbal Pharmacopoeia Ther. Compend. 14:1-25. |
|
|
Westhoff V, Eddy Van Der Maarel (2014). The Braun-Blanquet approach. Buc. University. |
|
|
Zoratti L, Jaakola L, Häggman H, Giongo L (2015b). Modification of sunlight radiation through colored photo-selective nets affects anthocyanin profile in Vaccinium spp. berries. PloS one 10(8):e0135935. |
|
|
Zoratti L, Klemettila H, Jaakola L (2016). Bilberry (Vaccinium myrtillus L.) ecotypes. In: Nutritional Composition of Fruit Cultivars (Simmonds M, Preedy VR eds). Academic Press. pp. 83-99. |
|
|
Zoratti L, Sarala M, Carvalho E, Karppinen K, Martens S, Giongo L, Häggman H, Jaakola L (2014). Monochromatic light increases anthocyanin content during fruit development in bilberry. BMC Plant Biol. 14(1):377. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0