ABSTRACT
Artemisia monosperma (Delile) callus was induced using seedling explant cultured on Murashige and Skoog solid medium (M&S) supplemented with 1 mg/L naphthalene acetic acid (NAA) and 1 mg/L kinetin (Kn). Cultures were maintained on 2 mg/L 2,4-dichlorophenoxy acetic acid (2, 4- D) and 1 mg/L Kn and treated with different elicitors. Total flavonoids and phenolics were determined by aluminum chloride-potassium acetate and Folin-Ciocalteu colorimetric methods, respectively. Yeast extract 10 mg/L (Y2) showed higher productivity and viability of callus cultures than Fusarium oxysporum and calcium chloride elicitors. Y2 shows an increase of 2.4 and 1.5 times non-elicited calli for flavonoids and phenolic compounds production, respectively. Pockets of embryogenic calli were transferred to M&S solid media supplemented with 0.5 mg/L NAA and 0.5 mg/L benzyl aminopurine (BAP), followed by hormonal free media where different stages of embryos were monitored, giving regenerated plantlets. HPLC analysis of methylene chloride and ethyl acetate fractions of parent plant (P), Y2 elicited embryogenic callus (EC.Y2) and Y2 elicited non-embryogenic callus (NEC.Y2) showed that, quercetin content in methylene chloride fraction of NEC.Y2 is 7.2 times the same fraction of P, while vanillic acid ethyl ester content in ethyl acetate fraction of EC.Y2 (EEC.Y2) is 8.6 times the same fraction of P. EEC.Y2 showed highest anti-oxidant activity with IC50 7.22±0.14 µg/mL compared with ethyl acetate fraction of P with IC50 20.01±0.82 µg/mL (IC50 of L-ascorbic acid = 1.24±0.07 µg/mL). Using MTT assay, EEC.Y2 exhibited potent cytotoxic activity against colon carcinoma cells and moderate activity against hepatocellular and lung carcinoma cells with IC50 18.9±1.4, 22.3±0.9 and 41.6±1.2 µg/mL, respectively; compared with doxrubcin as reference standard.
Key words: Artemisia monosperma, callus cultures, flavonoids, phenolic compounds, anti-oxidant, cytotoxicity.
Flavonoids are one of the largest groups of secondary metabolites and widely distributed in leaves, seeds, barks and flowers of plants with more than 4000 different structures. Most of flavonoids possess coronary heart
disease prevention, hepatoprotective, anti-inflammatory, anti-oxidative and anticancer activities, while some flavonoids exhibit potential antiviral activities (
Kumar and
Pandey, 2013). The evolving commercial importance of flavonoids and a need for renewable resources of valuable chemicals has led to attempts in developing alternative systems for their production e.g. callus culture (Jedinák et al., 2004). Phenolic compounds also have been reported to have multiple biological effects including antioxidant, anti-inflammatory, chemopreventive and anti-cancer activities (Servili et al., 2014).
The application of elicitors, which is currently the focus of researches, has been considered as one of the most effective methods to improve the synthesis of secondary metabolites in medicinal plants (Patel and Krishnamurthy, 2013). Yeast extract and Fusarium oxysporum (Azeez and Ibrahim, 2013) as biotic elicitors and calcium chloride (Rashid et al., 2011) as abiotic elicitor are suitable elicitors for induction of secondary metabolites production.
Artemisia monosperma (Delile), family Asteraceae, is reputed in traditional medicine for its antispasmodic, anthelmintic and anti-hypertensive activities (Wagner and Wolff, 1977). Volatile oils (Saleh, 1985), flavonoids (Elgamal et al., 1997), alkaloids (Zaki et al., 1984) and coumarins (Hammoda et al., 2008) were previously isolated and identified from A. monosperma parent plant. To the best of our knowledge, there is no report for any tissue culture studies on this plant. So, the aim of our study is to establish a stable callus culture to investigate the effect of biotic and abiotic elicitors on the flavonoids and phenolic contents production, as well as its anti-oxidant and cytotoxic activities compared with the parent plant.
Plant material
The aerial parts of A. monosperma, Delile (Athir) plant, family Asteraceae (Compositae) were collected from Bir El-Abd road, North Sinai, Egypt, in April 2013 during the flowering and fruiting stages. The plant was identified by Dr. Abdel-Halim Abdel-Mogaly, Herbarium of Horticultural Research Institute, Agricultural Research Centre, Ministry of Agriculture, Dokki, Giza, Egypt, where voucher specimen No. 1814-CAIM is kept there. Seeds were collected by rubbing out the capitulum of the shade dried parent plant.
In vitro seed germination
Seeds of A. monosperma were surface sterilized by immersion in 70% ethanol for 1 min followed by 2 min in 30% hydrogen peroxide and rinsed three times with sterile distilled water. The sterilized seeds were cultured on sterile Whatman No.1 filter paper in sterile Petri dishes, containing liquid M&S medium (Murashige and Skoog, 1962), supplemented with 30 g/L sucrose and adjusted to pH 5.8 before autoclaving. The seeds were incubated at 25±2°C under continuous light using fluorescent white lamps. Germination of seeds was evaluated using seed germination percentage according to the following equation:
Callus induction and maintenance
Non-embryogenic callus
Callus induction was carried out using M&S media supplemented with 1 mg/L NAA, 1 mg/L Kn and incubated in darkness at 25±2°C. Most seedlings produced sufficient calli for subculture within 5 to 8 weeks. Callus maintenance was carried out using M&S media supplemented with different hormonal combinations as 0.5 mg/L NAA and 0.5 mg/L Kn; 1 mg/L NAA and 1 mg/L Kn; 0.5 mg/L NAA and 0.5 mg/L BAP; 1 mg/L 2,4-D and 0.2 mg/L Kn. Yellowish, friable and healthy callus was maintained on two types of M&S solid media; the first supplemented with 2 mg/L 2,4-D and 1 mg/L Kn (M&S1) and the second supplemented with 1 mg/L 2,4-D & 0.5 mg/L Kn (M&S2). All media were supplied with 30 g/L sucrose and solidified with 8 g/L agar. Callus was sub-cultured into fresh medium every four weeks and incubated at 25±2°C with 12 h, photoperiod.
Growth parameters
Growth curves were carried out for calli growing on M&S1 and M&S2 media according to Godoy-Hernández and Vázquez-Flota (2006). Growth dynamics in callus cultures were calculated as follow:
Growth index (GI) = (Ge - G start)/G start (Verpoorte et al., 1998).
Where Ge = Weight of biomass at the end of generation (final weight); G start = Weight of biomass at zero time (initial weight).
Specific growth rate (μ):
μ = (ln x - ln xo)/t (Godoy-Hernández and Vázquez-Flota, 2006).
Where xo is the initial biomass and x is the biomass at time t.
Doubling time (dt) which is the time required for the biomass of a population of cells to double where dt = ln (2)/ μ (Godoy-Hernández and Vázquez-Flota, 2006).
Somatic embryogenesis
After 3 months of culture on M&S media supplemented with 1 mg/L NAA and 1 mg/L Kn, pockets of yellowish green embryogenic calli with nodular structures were transferred into M&S solid media supplemented with 0.5 mg/L NAA and 0.5 mg/L BAP for 3 to 4 months where different stages of embryos were monitored by using microscope. The well proliferated cultures were further kept on hormonal free medium solidified with 6 g/L agar for five months. The developed undifferentiated plantlets were cultured on M&S media supplemented with different combinations of thidiazuron (TDZ), 2,4-D, kn and indole acetic acid (IAA).
Quantitative determination of total flavonoids and total phenolic contents of parent plant and callus cultures
Total flavonoids
The powdered parent plant (1 g) and 40 g of the fresh weight of callus (equivalent to 2 g dry weight) were extracted according to Al-Gendy et al. (2015). Total flavonoids of both parent plant and callus extracts were determined according to aluminium chloride- potassium acetate method (Woisky and Salatino, 1998). The absorbance of the reaction mixture was measured at λmax 415 nm by using 6715 UV/VIS (SENWAY) spectrophotometer. Flavonoids were expressed as rutin and quercetin equivalent. The calibration curves of rutin and quercetin were done by using standard solutions of 5, 10, 20, 40 and 80 μg/mL in 80% ethanol (v/v) and 12.5, 25, 50, 66.6 and 100 μg/mL in 80% ethanol (v/v), respectively and treated similarly (Hung and Morita, 2008).
Total phenolic contents
Total phenolics of parent plant and callus cultures were determined using Folin-Ciocalteau colorimetric method (Sellappan et al., 2002). The absorbance was measured at λmax 765 nm. Concentration of phenolic contents was expressed as gallic acid equivalent. The calibration curve of gallic acid was constructed by using standard solutions of 12.5, 25, 33.3, 50, 66.6 and 100 μg/mL in 80% ethanol (v/v) (Al-Gendy et al., 2013).
Elicitation of callus cultures
Dried commercial yeast extract at different conc. 5, 10 and 15 mg/L (Ahmed and Baig, 2014) and calcium chloride obtained from El-Nasr Pharmaceutical Chemicals Company (0.2, 0.4 and 0.6 mg/L) were added as dried powder separately to M&S1 media before autoclaving (Baldi and Dixit, 2008). Fusarium oxysporum hyphae, supplied from Faculty of Agriculture, Zagazig University was washed with distilled water, filtered, dried at 40°C till constant weight and finely powdered before addition to M&S1 media at 1, 2 and 3 mg/L (Patel and Krishnamurthy, 2013). M&S1 media was adjusted to pH 5.8 after addition of these elicitors and then autoclaved. Control media was prepared by substituting the elicitor with distilled water. The embryogenic callus was similarly elicited with 10 mg/L yeast extract.
Extraction and fractionation
The dried powdered (25 g) parent plant (P) was cold macerated with 70% ethyl alcohol till exhaustion, concentrated at 50°C to yield 3.4 g of a sticky dark green residue. Additionally, 230 g of fresh weight non-embryogenic callus elicited with 10 mg/L yeast extract (NEC.Y2) and 330 g of fresh weight embryogenic callus elicited with 10 mg/L yeast extract (EC.Y2) were extracted according to Al-Gendy et al. (2015) as mentioned above to yield 5.3 g and 6.7 g of a sticky dark brown residue of NEC.Y2 and EC.Y2, respectively. The alcoholic callus extracts were dissolved in excess amount of methyl alcohol, filtered and concentrated at 50°C to yield 2.1 and 3.6 g of sticky dark brown residue of NEC.Y2 and EC.Y2, respectively. The dried alcoholic extracts of P, NEC.Y2 and EC.Y2 were defatted with hexane and fractionated by methylene chloride and ethyl acetate. Each fraction was dried over anhydrous sodium sulfate and concentrated under vacuum to afford methylene chloride fractions (0.6, 0.25 and 0.11 g) and ethyl acetate fractions (0.7, 0.2 and 0.19 g) for P, NEC.Y2 and EC.Y2, respectively. All fractions were stored at -20°C until use. Methylene chloride and ethyl acetate fractions (20 mg, each) were dissolved in 5 mL of 95% ethyl alcohol and quantitatively estimated for their flavonoids and phenolic contents as mentioned above.
HPLC analysis
Methylene chloride and ethyl acetate fractions of P, NEC.Y2 andEC.Y2 were investigated according to the method described byMattila et al. (2000) and analyzed for their flavonoids and phenolic constituents by HPLC (Hewlett Packard, series 1050) using C18 hypersil BDS column with particle size 5 µm. The separation was carried out with methanol and acetonitrile as a mobile phase, flow with 1 mL/min. The identification depended on diode-array and electro-array detectors and based upon comparison of retention time with the available authentics (DvoÅ™áková et al., 2008).
Antioxidant assay by DPPH method
The antioxidant activities of total extract, methylene chloride and ethyl acetate fractions of P, NEC.Y2 and EC.Y2 in addition to total extract of NEC were evaluated according to DPPH free radical scavenging activity method (Ratty et al., 1988; Sahu et al., 2013). Briefly, 2 mL of 0.1 mM solution of DPPH in methanol was added to 2 mL of the serial dilutions of the samples at concentration of 10, 20, 40, 60, 80 and 100 µg/mL. L-ascorbic acid was used as standard at 0.25, 0.5, 0.75, 1, 2, 4, 6 and 8 µg/mL. After incubation for 30 min at room temperature, the absorbance was measured at λmax 517 nm and the activity is expressed as percentage DPPH-radical scavenging that is calculated according to the following equation:

Where AC is the absorbance of the control solution, AS is the absorbance of sample in DPPH solution. The percentage of DPPH radical-scavenging was plotted against the different extract concentrations to determine the concentration (µg/mL) of extract required to scavenge DPPH by 50% (IC50).
Evaluation of cytotoxic activity
Cytotoxic activity of ethyl acetate fraction of EC.Y2 against colon (HCT-116), hepatocellular (HepG-2) and lung (A-549) carcinoma cell lines was detected using MTT assay (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide) where doxrubcin was used as standard. Serial dilutions of samples and standard were used at concentration of 0, 1.56, 3.125, 6.25, 12.5, 25 and 50 µg/mL (Mosmann, 1983; Elaasser et al., 2011).
Statistical analysis
All the results in the current study were calculated using (Microsoft Excel 2010) and recorded in triplicate. Each value represents the mean ± S.D of three samples and all bars in the figures represent S.D. The IC50 was determined as the drug conc. which resulted in 50% reduction in cell viability or inhibition of the biological activity
Plant cell culture systems represent a potential renewable source of valuable medicinal compounds, flavors, fragrances and colorants, which can not be produced by microbial cells or chemical synthesis (Mulabagal and Tsay, 2004).
Induction and maintenance of callus cultures
The germination percentage of sterilized seeds was 30.9%. Induction of A. monosperma non-embryogenic calli sufficient for subculture, was successful on M&S media supplemented with 1 mg/L NAA and 1 mg/L Kn. After 5 weeks, the cultures were maintained on M&S1 and M&S2 and the best growing calli were followed for 31 days. Fresh and dry weights were determined every 3 days (Figure 1). Both M&S1 and M&S2 produced yellowish, friable and healthy callus but M&S1 media showing higher growth parameters (GI and μ) and lower dt as shown in Table 1.
Somatic embryogenesis and plantlets regeneration
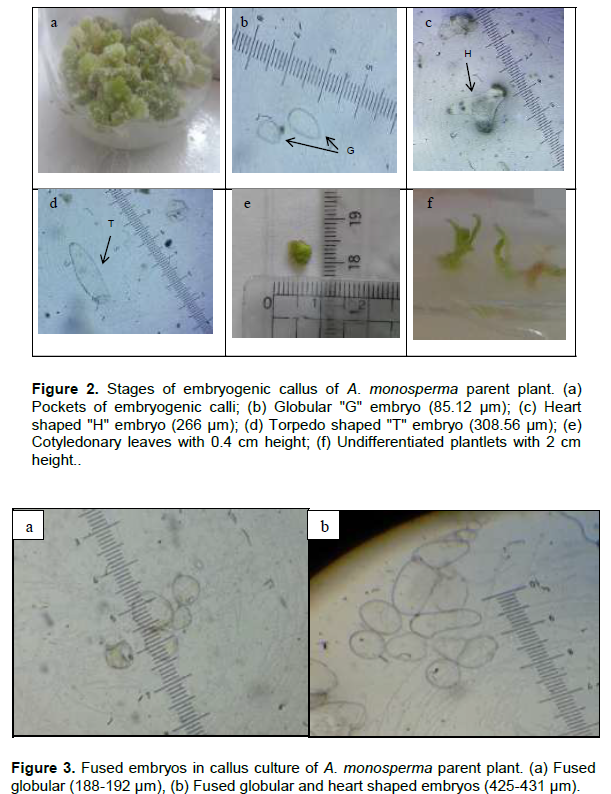
Pockets of yellowish green embryogenic calli with nodular structures appeared on the surface of the non-embryogenic callus after 3 months of culture on M&S medium supplemented with 1 mg/L NAA and 1 mg/L Kn (Figure 2a). Embryogenic callus was maintained on M&S solid media supplemented with 0.5 mg/L NAA and 0.5 mg/L BAP. Somatic embryos as globular (G: 60-120 µm), heart-shaped (H: 100-295 µm) and torpedo-shaped (T: 270-440 µm) were monitored (Figure 2b, c and d). Mature embryos successfully germinated into cotyledonary embryo, which further developed into cotyledonary leaves of 0.4 cm height (Figure 2e). When proliferated calli were moved to hormonal free medium, they kept the embryogenic potential and showed further embryo development giving undifferentiated plantlets of nearly 2 cm height (Figure 2f). When the embroids were transferred to M&S solid media supplemented with 0.5 mg/L TDZ, 1 mg/L 2,4-D and 0.1 mg/L Kn in a trial for regeneration of the whole plant, browning of callus was observed. Also, other trials were done by culturing on M&S solid media supplemented with 2 mg/L IAA alone or in combination with 0.5 mg/L Kn, where no change in behavior was observed but abnormal embryos as fused globular and torpedo shaped were detected (Figure 3). No further differentiation of the whole plant occurred even after culturing on M&S solid media supplemented with different hormonal types and concentrations may be due to the appearance of abnormal embroids. In a previous report, somatic embryos also failed to mature in Stevia rebaudiana (Pande and Gupta, 2013) which is in agreement with our results.
Quantitative determination of total flavonoids and total phenolics of parent plant and callus cultures
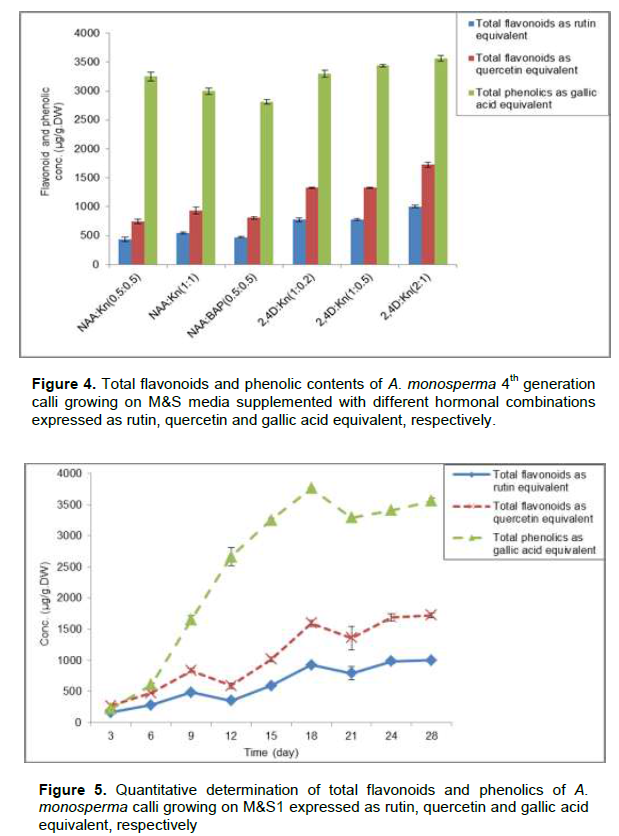
The quantitative determination of total flavonoids and phenolic contents of A. monosperma 28th day old of 4th generation calli growing on M&S media supplemented with different hormonal combinations was recorded as shown in Figure 4. M&S1 showed calli with the highest content of flavonoids (1002.8±20.1 and 1723±40.6 µg/g.DW) expressed as rutin and quercetin equivalent,respectively which is nearly 2.3 times its content on M&S media supplemented with 0.5 mg/L NAA and 0.5 mg/L Kn. Also M&S1 showed calli with the highest phenolic contents (3561.1±50.1 µg/g.DW) expressed as gallic acid equivalent which is 1.26 times its content on M&S media supplemented with 0.5 mg/L NAA & 0.5 mg/L BAP. Callus maintained on M&S1 media was followed every 3 days for its flavonoids and phenolic contents expressed as rutin, quercetin and gallic acid equivalent, showed that, the 28th day old calli have highest content of flavonoids while 18en day old calli have highest content of phenolics (Figure 5).
Elicitation of callus culture
M&S1 was treated with different elicitors as yeast extract, Fusarium oxysporum and calcium chloride because this media showed the best growth parameters of callus cultures in addition to its highest flavonoids and phenolic
contents. These elicitors were previously reported to increase the concentrations of some metabolites.
Yeast (Y)
Y2 (10 mg/L) resulted in maximum increase in flavonoids content (2452±85.7 and 4226±92.4 µg/g.DW) expressed as rutin and quercetin equivalent, respectively after 24 days of subculture representing 2.4 times control (Y0). Flavonoids content increased gradually from zero time till the stationary phase after 24 days (Figure 6a and b). Also Y2 showed highest phenolic contents (5401±95.3 µg/g.DW) expressed as gallic acid equivalent at 28th day which is nearly 1.51 times Y0 (Figure. 6c). Y1 (5 mg/L) and Y3 (15 mg/L) increased flavonoids and phenolic contents, which is nearly 1.46 and 1.42 times Y0, respectively (Figure 6a, b and c). Different flavonoids and phenolic compounds were enhanced through the addition of yeast extract in callus and suspension cultures of Iphiona mucronata (Al-Gendy et al., 2015), flavonolignans in hairy root cultures of Silybum marianum (Hassanloo et al., 2008) and artemisinin in callus culture of Artemisia. annua (Baldi and Dixit, 2008).
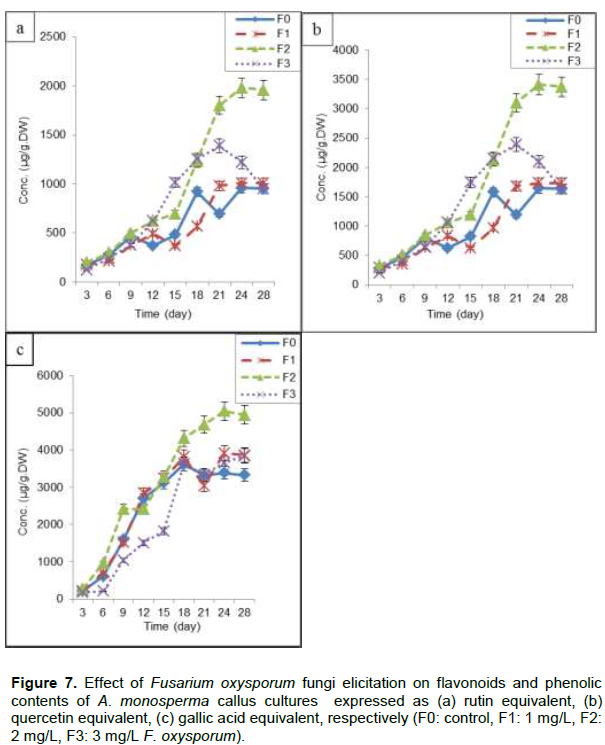
F. oxysporum fungi (F)
F2 (2 mg/L) resulted in maximum flavonoids content (1980±80.5 and 3412±87.5 µg/g.DW) expressed as rutin and quercetin equivalent, respectively after 24 days of subculture (Figure 7a and b), which is nearly 1.98 times control (F0) and also 1.94, 1.98 times F1 (1 mg/L) and F3 (3 mg/L), respectively. Also F2 gives maximum content of phenolics at 24th day of subculture (5048±91.4 µg/g.DW) expressed as gallic acid equivalent, representing 1.65 times F0, 1.37 and 1.31 times F1 and F3, respectively (Figure 7c). Application of fungal preparations as elicitors has become one of the most important and successful measures to enhance secondary metabolites production in plant cell cultures as phenolic acids and diterpenes from Rosemary (Rosmarinus officinalis L.) leaf and callus cultures (Rashid et al., 2011), hyoscyamine and scopolamine in root cultures of Hyoscyamus niger and H. muticus (Namdeo, 2007) and four major isomers of boswellic acid in callus culture of Boswellia serrata (Ghorpade et al., 2011).
Calcium chloride (C)
Different concentrations of C were used but C2 (0.4mg/L) showed maximum concentration of flavonoids at 28th day of subculture (1465±70.5 and 2545±77.1 µg/g.DW) expressed as rutin and quercetin equivalent, respectively (Figure 8a and b) which is nearly 1.48 times control (C0). Only C2 increased phenolic contents (4802±80.9 µg/g.DW) after 24 days representing nearly 1.47 times C0 but C1 and C3 was not successful as it decreased phenolic contents to give 90 and 60% of C0, respectively (Figure 8c). The effect of calcium chloride as abiotic elicitor was extensively studied as enhancement of phenolic acids production from leaf and callus cultures of R. officinalis (Rashid et al., 2011) and artemisinin production in hairy roots culture of A.annua (Patraetal., 2013).
Through the use of different elicitors added at zero time of subculture and measuring flavonoids and phenolic contents expressed as rutin, quercetin and gallic acid equivalent, respectively (Figure 9a, b and c); it is clear that Y2 gave maximum flavonoids and phenolic contents at 28th day of subculture compared with F2 and C2 elicitors.
Comparative study of total extract, methylene chloride and ethyl acetate fractions obtained from P and different callus cultures for their flavonoids and phenolic contents revealed that, the ethyl acetate fraction of Y2 elicited embryogenic calli (EEC.Y2) represents 1.09, 1.1 and 3.2 times the ethyl acetate fraction of P (EP) for the total flavonoids and phenolic contents expressed as rutin, quercetin and gallic acid equivalent, respectively (Figure 10).
HPLC analysis of flavonoids and phenolic compounds
Quantitative analysis of A. monosperma parent plant confirmed the high flavonoids and phenolic contents and the obtained results encourage us to identify these contents in the ethyl acetate fractions of Y2 elicited embryogenic callus (EEC.Y2), Y2 elicited non-embryogenic callus (ENEC.Y2) and the parent plant (EP) in addition to methylene chloride fractions of Y2 elicited embryogenic callus (MEC.Y2), Y2 elicited non-embryogenic callus (MNEC.Y2) and the parent plant (MP) using HPLC technique against the available authentics of flavonoids and phenolic compounds. Eleven flavonoidal compounds (Table 2) and twenty three phenolic compounds (Table 3) were identified by comparing the retention time of their peaks with that of the available authentic flavonoids and phenolics injected under the same conditions of the experiment (Karimi et al., 2012). It is worthy to note that some peaks can't be identified due to the low concentration and/or the limited number of available authentics.
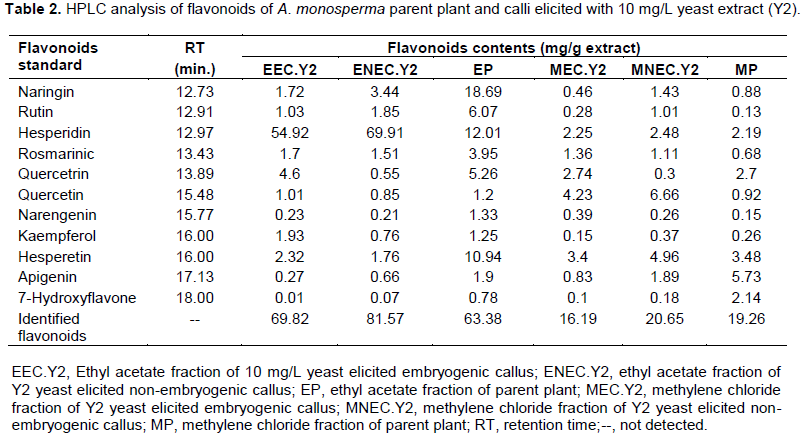
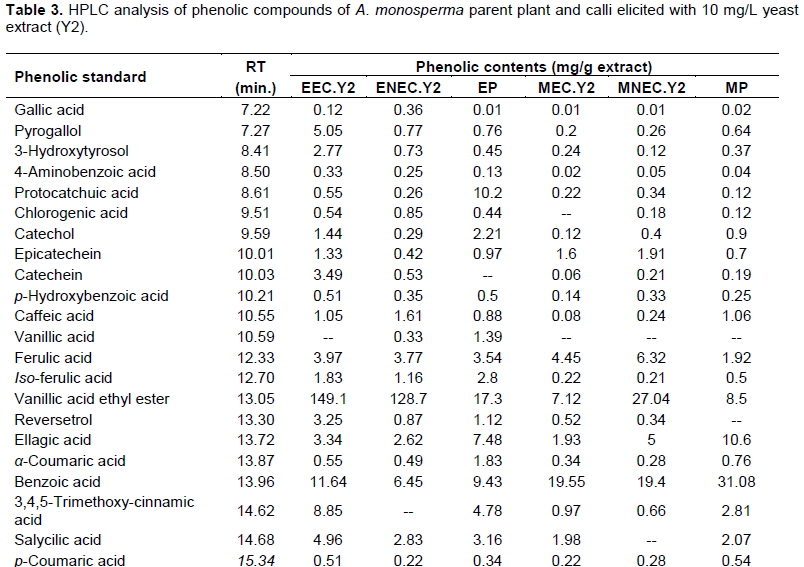

HPLC analysis of A. monosperma parent plant and the elicited calli fractions showed that some flavonoids were found in callus in higher concentration than parent plant as hesperidin content in ENEC.Y2 is 5.82 times EP and kaempferol in EEC.Y2 is 1.5 times EP. Additionally, the highest conc. of quercetin in MNEC.Y2 and MEC.Y2 is nearly 7.2 times more than MP. Some phenolics were found in callus in higher concentration than plant as vanillic acid ethyl ester and pyrogallol in EEC.Y2, which are 8.6 and 6.5 times EP, respectively. Also, MNEC.Y2 has the highest concentration of both ferulic acid and cinnamic acid, which are 3.2 and 3.5 times MP, respectively. On the other hand, some flavonoids and phenolic compounds were found in the parent plant in equal or higher concentration than callus as quercetrin in both EP and EEC.Y2 is 8.3 times ENEC.Y2. Additionally, iso-ferulic acid of EP is 1.5 and 2.4 times EEC.Y2 and ENEC.Y2, respectively. All the identified compounds were not reported to be isolated from A. monosperma parent plant except quercetin (El-Toumy et al., 2011) and p-coumaric acid only (Abdel-Mogib et al., 1990). Different flavonoids were determined by HPLC analysis of A. vulgaris and A. annua where quercetin and its derivatives was dominant (Nikolova et al.,2004).
Biological Activitities
Anti-oxidant activity
DPPH radical scavenging activity showed that EEC.Y2 has higher anti-oxidant activity (IC
50 7.22±0.14 µg/mL) than EP (IC
50 20.01±0.82 µg/mL) compared with L-ascorbic acid (IC
50 1.24±0.07 µg/mL) as showed in Figure 11. It was previously reported that some of the detected flavonoids and phenolic compounds showed high anti-oxidant activities as hesperidin, hesperetin (Parhiz et al., 2015) and
p-coumaric acid (
Kiliç and YeÅŸiloÄŸlu, 2013). Skowyra et al. (2014) reported the high antioxidant activity of
A.
annua species.
Cytotoxic activity
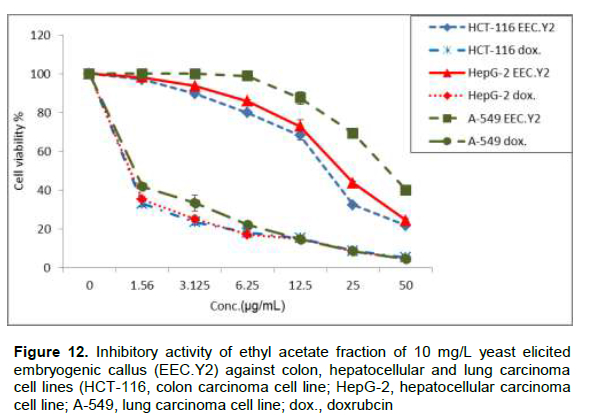
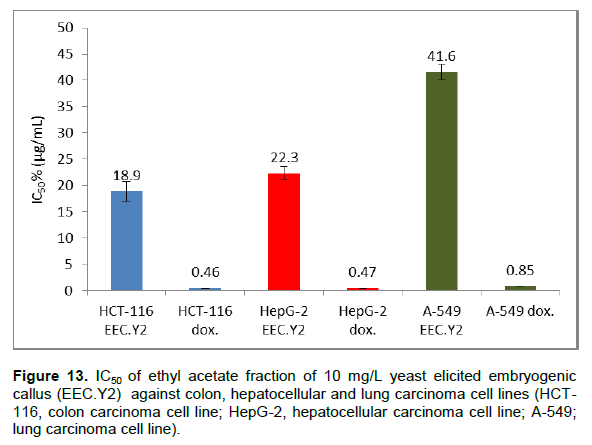
Due to the high flavonoids and phenolic contents and also antioxidant activity of EEC.Y2, it was evaluated for its cytotoxicity against colon (HCT-116), hepatocellular (HepG-2) and lung (A-549) carcinoma cell lines in vitro using doxrubcin as standard (Figure 12). The criteria used to categorize the activity of EEC.Y2 against human cancer cell lines based on the US National Cancer Institute (NCI) which proposed that crude extracts with potential cytotoxic activity are those presenting IC50 of ≤ 30 μg/mL (Baravaliel et al., 2012; Moo-puc et al., 2013). The criteria was modified from these of Geran et al. (1972) as follows: IC50 ≤ 20 µg/mL is highly active, IC50 = 21 - 200 µg/mL is moderately active, IC50 = 201 - 500 µg/mL is weakly active and IC50 > 501 µg/mL is inactive (Srisawat et al., 2013). So, EEC.Y2 (Figure 13) is highly active against HCT-116 (IC50= 18.9±1.4 µg/mL) and moderately active against HepG-2 cell lines (IC50= 22.3±0.9 µg/mL) and A-549 cell lines (IC50= 41.6±1.2 µg/mL). The cytotoxic activityofthisfractionmaybe attributed to its content of some flavonoids which previously reported to have high cytotoxic activity (Febriansah et al., 2014). It was previously reported that, polyacetylene capillin, a constituent of A. monosperma, inhibit proliferation of colon, larynx, lung and pancreatic carcinomacelllines(WhelanandRyan,2004).
Elicitation of A. monosperma callus, using yeast and F. oxysporum fungi as biotic elicitors and calcium chloride as abiotic elicitor increased the flavonoids and phenolic compounds production, compared to the parent plant. The results favored the use of yeast extract compared with other elicitors. Embryogenic callus cultures afforded small non differentiated plantlets without further proliferation. The ethyl acetate fraction of yeast elicited embryogenic callus shows the highest antioxidant activity. Moreover, it has also high in vitro cytotoxic activity against colon carcinoma cells and moderate activity against hepatocellular and lung carcinoma cells.
The authors have not declared any conflict of interests.
REFERENCES
|
Abdel-Mogib M, Dawidar AM, Metwally MA, Abou-Elzahab M (1990). p-coumaric acid derivatives from Artemisia monosperma. Phytochemistry 29(8):2728-2729.
Crossref
|
|
|
|
Ahmed SA, Baig MMV (2014). Biotic elicitor enhanced production of psoralen in suspension cultures of Psoralea corylifolia L. Saudi J. Biol. Sci. 21(5):499-504.
Crossref
|
|
|
|
|
Al-Gendy AA, Bakr RO, El-Gindi OD (2015). Production of flavonoids and phenolic compounds by elicitation of Iphiona mucronata (Forssk.) Asch. & Schweinf (Asteraceae) callus and suspension cultures. Int. J. Pharmacogn. Phytochem. 30(1):1293-1300.
|
|
|
|
|
Al-Gendy AA, Bakr RO, El-gindi OD (2013). Somatic embryogenesis and plant regeneration from callus and suspension cultures of Iphiona mucronata (Forssk.). Eur. Sci. J. 9(27):37-49.
|
|
|
|
|
Azeez HA, Ibrahim KM (2013). Effect of biotic elicitors on secondary metabolite production in cell suspensions of Hypericum triquetrifolium turra. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Horticult. 70(1):26-33.
|
|
|
|
|
Baldi A, Dixit VK (2008). Enhanced artemisinin production by cell cultures of Artemisia annua. Curr. Trends. Biotechnol. Pharm. 2(2):341-348.
|
|
|
|
|
Baravalia Y, Vaghasiya Y, Chanda S (2012). Brine shrimp cytotoxicity, anti-inflammatory and analgesic properties of Woodfordia fruticosa kurz flowers. Iran. J. Pharm. Res. 11(3):851-861.
|
|
|
|
|
DvoÅ™áková M, Guido LF, Dostálek P, Skulilová Z, Moreira MM, Barros AA (2008). Antioxidant properties of free, soluble ester and insoluble-bound phenolic compounds in different barley varieties and corresponding malts. J. Inst. Brew. 114(1):27-33.
Crossref
|
|
|
|
|
Elaasser MM, Abdel-Aziz MM, El-Kassas RA (2011). Antioxidant, antimicrobial, antiviral and antitumor activities of pyranone derivative obtained from Aspergillus candidus. J. Microbiol. Biotech. Res. 1(4):5-17.
|
|
|
|
|
Elgamal MHA, Ouf SA, Hanna AG, Yassin FY (1997). Phytochemical and mycological investigation of Artemisia monosperma. Folia Microbiol. 42(3):203-210.
Crossref
|
|
|
|
|
El-Toumy SA, Omara EA, Brouard I, Bermejo J (2011). Evaluation of hepatoprotective effect of Artemisia monosperma against carbon tetrachloride-induced hepatic damage rat. Aust. J. Basic. Appl. Sci. 5(6):157-164.
|
|
|
|
|
Febriansah R, Putri DDP, Sarmoko, Nurulita NA, Meiyanto E, Nugroho AE (2014). Hesperidin as a preventive resistance agent in MCF-7 breast cancer cells line resistance to doxorubicin. Asian Pac. J. Trop. Biomed. 4(3):228-233.
Crossref
|
|
|
|
|
Geran RI, Greenberg NH, Macdonald MM, Schumacher AM, Abbott BJ (1972). Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemoth. Rep. 3(2):1-103.
|
|
|
|
|
Ghorpade RP, Chopra A, Nikam TD (2011). Influence of biotic and abiotic elicitors on four major isomers of boswellic acid in callus culture of Boswellia serrata Roxb. Plants Omics J. 4(4):169-176.
|
|
|
|
|
Godoy-Hernández G, Vázquez-Flota FA (2006). Growth measurements: estimation of cell division and cell expansion. Methods Mol. Biol. 318:51-58.
|
|
|
|
|
Hammoda HM, Ela MA, El-Lakany AM, El-Hanbali O, Zaki CS, Ghazy NM (2008). New constituents of Artemisia monosperma Del. Pharmazie 63(8):611-614.
|
|
|
|
|
Hasanloo T, Rahnama H, Sepehrifar R, Shams MR (2008). The influence of yeast extract on the production of flavonolignans in hairy root cultures of Silybum marianum L. Gaertn. In 4th Kuala Lumpur
Crossref
|
|
|
|
|
International Conference on Biomedical Engineering 2008. Springer
|
|
|
|
|
Hung PV, Morita N (2008). Distribution of phenolic compounds in the graded flours milled from whole buckwheat grains and their antioxidant capacities. Food Chem. 109(2):325-331.
Crossref
|
|
|
|
|
Karimi E, Oskoueian E, Hendra R, Oskoueian A, Jaafar HZE (2012). Phenolic compounds characterization and biological activities of Citrus aurantium bloom. Molecules 17(2):1203-1218.
Crossref
|
|
|
|
|
Kiliç I, YeÅŸiloÄŸlu Y (2013). Spectroscopic studies on the antioxidant activity of p-coumaric acid. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 115:719-724.
Crossref
|
|
|
|
|
Kumar S, Pandey AK (2013). Chemistry and biological activities of flavonoids: an overview. Scientific World J. 2013:162750. doi: 10.1155/2013/162750.
Crossref
|
|
|
|
|
Mattila P, Astola J, Kumpulainen J (2000). Determination of flavonoids in plant material by HPLC with diode-array and electro-array detections. J. Agric. Food Chem. 48(12):5834-5841.
Crossref
|
|
|
|
|
Moo-Puc R, Chale-Dzul J, Caamal-Fuentes E (2013). Bonellia albiflora: A mayan medicinal plant that induces apoptosis in cancer cells. Evid. Based Complement. Altern. Med. 2013:1-8.
Crossref
|
|
|
|
|
Mosmann T (1983). Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65 (1-2):55-63.
Crossref
|
|
|
|
|
Mulabagal V, Tsay HS (2004). Plant cell cultures - An alternative and efficient source for the production of biologically important secondary metabolites. Int. J. Appl. Sci. Eng. 2(1):29-48.
|
|
|
|
|
Murashige T, Skoog F (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 15(3):473-497.
Crossref
|
|
|
|
|
Namdeo AG (2007). Plant cell elicitation for production of secondary metabolites: A review. Phcog. Rev. 1(1):69-79.
|
|
|
|
|
Nikolova M, Gevrenova R, Ivancheva S (2004). High-performance liquid chromatographic separation of surface flavonoid aglycones in Artemisia annua L. and Artemisia vulgaris L. J. Serb. Chem. Soc. 69(7):571-574.
Crossref
|
|
|
|
|
Pande SS, Gupta P (2013). Plant tissue culture of Stevia rebaudiana (Bertoni): A review. J. Pharmacogn. Phytother. 5(1):26-33.
|
|
|
|
|
Parhiz H, Roohbakhsh A, Soltani F, Rezaee R, Iranshahi M (2015). Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: an updated review of their molecular mechanisms and experimental models. Phytother. Res. 29(3):323-331.
Crossref
|
|
|
|
|
Patel H, Krishnamurthy R (2013). Elicitors in plant tissue culture. J. Pharmacogn. Phytochem. 2(2):60-65.
|
|
|
|
|
Patra N, Srivastava AK, Sharma S (2013). Study of various factors for enhancement of artemisinin in Artemisia annua hairy roots. Int. J. Chem. Eng. Appl. 4(3):157-160.
Crossref
|
|
|
|
|
Rashid KI, Ibrahim KM, Hamza SJ (2011). Effect of some biotic and abiotic elicitors on phenolic acids and diterpenes production from Rosemary (Rosmarinus officinalis L.) leaf and callus analyzed by high performance liquid chromatography (Hplc). J. Al-Nahrain Univ. 14(3):104-109.
|
|
|
|
|
Ratty AK, Sunamoto J, Das NP (1988). Interaction of flavonoids with 1,1-diphenyl-2-picrylhydrazyl free radical, liposomal membranes and soybean lipoxygenase-1. Biochem. Pharmacol. 37(6):989-995.
Crossref
|
|
|
|
|
Sahu RK, Kar M, Routray R (2013). DPPH free radical scavenging activity of some leafy vegetables used by tribals of odisha, India. J. Med. Plants Stud. 1(4):21-27.
|
|
|
|
|
Saleh MA (1985). Volatile components of Artemisia monosperma and Artemisia judaica growing in the Egyptian deserts. Biochem. Syst. Ecol. 13(3):265-269.
Crossref
|
|
|
|
|
Sellappan S, Akoh CC, Krewer G (2002). Phenolic compounds and antioxidant capacity of Georgia-grown blueberries and blackberries. J. Agric. Food Chem. 50(8):2432-2438.
Crossref
|
|
|
|
|
Servili M, Sordini B, Esposto S, Urbani S, Veneziani G, Di Maio I, Selvaggini R, Taticchi A (2014). Biological activities of phenolic compounds of extra virgin olive oil. Antioxidants 3(1):1-23.
Crossref
|
|
|
|
|
Skowyra M, Gallego MG, Segovia F, Almajano MP (2014). Antioxidant properties of Artemisia annua extracts in model food emulsions. Antioxidants 3(1):116-128.
Crossref
|
|
|
|
|
Srisawat T, Chumkaew P, Heed-Chim W, Sukpondma Y, Kanokwiroon K (2013). Phytochemical screening and cytotoxicity of crude extracts of Vatica diospyroides symington type LS. Trop. J. Pharm. Res. 12(1):71-76.
Crossref
|
|
|
|
|
Verpoorte R, Van der Heijden R, ten Hoopen HJG, Memelink J (1998). Metabolic engineering for the improvement of plant secondary metabolite production. Plant Tissue Cult. Biotechnol. 4:3-20.
|
|
|
|
|
Wagner HK, Wolff PM (1977). New Natural Products and Plant Drugs with Pharmacological, Biological or Therapeutical Activity. Proceedings of The First International Congress on Medicinal Plant Research, Section A (Eds), held at the University of Munich, Germany, Springer-Verlag Berlin.
Crossref
|
|
|
|
|
Whelan LC, Ryan MF (2004). Effects of the polyacetylene capillin on human tumour cell lines. Anticancer Res. 24(4):2281-2286.
|
|
|
|
|
Woisky RG, Salatino A (1998). Analysis of propolis: some parameters and procedures for chemical quality control. J. Apic. Res. 37(2): 99-105.
Crossref
|
|
|
|
|
Zaki D, Abdel Aziz M, El-Gengeihy S, Morsi N (1984). Antimicrobial potentiation of some Egyptian desert plants. Herba Hung. 23:73-84.
|
|