ABSTRACT
Cryptolepis sanguinolenta (Lindl.) Schlt. is a popular medicinal plant species in Ghana that is used in the treatment of malaria. Despite the heavy demand for this species, harvesting is done solely from the wild, resulting in declining populations. As part of the ongoing research to develop domestication protocols for its cultivation, a field study was conducted to develop a cropping cycle and determine the effect of staking and plant age on plant growth and active component (cryptolepine) concentration in the roots. Staking had no significant effect on root dry weight but was important to the production of seed pods possibly resulting from better flower positioning. The highest cryptolepine concentration (on average 1.84 mg/100 mg of root material) coincided with the peak average root dry weight (52.8 g) at 289 days after planting (DAP), signifying the most ideal time to harvest roots. Interestingly, the cryptolepine content (1.82 mg/100 mg) in seedlings prior to the start of the experiment was comparable to the concentration found, 289 DAP (1.84 mg/100 mg). The first 105 DAP were characterized by low yields of root dry weight (13.5 g) followed by a period of rapid growth in which the root dry weight increased almost linearly until 289 DAP. Although, dry matter partitioned to the vines increased towards the end of the experimental period (60%), dry matter partitioned to the roots remained fairly constant (30%) throughout the experimental period.
Key words: Cultivation, Cryptolepis sanguinolenta, cryptolepine, domestication, malaria, wild harvesting.
Ghana, like other developing countries is home to a diverse population of medicinal plant species. It is estimated that ~80% of the population in developing countries depend on indigenous medicinal plant species to meet their primary healthcare needs (Cunningham,1993). The medicinal plant industry in Ghana today, serves as a major source of income to those in its manufacturing and raw material collection sectors (Ofori et al., 2012). Medicinal plants therefore do not only play an important role in meeting the health care needs of the populace but are also important in the economic growth of developing economies such as that of Ghana.
Several of these indigenous medicinal plant species have been proven to be effective in the treatment of malaria. According to the World Health Organization (WHO, 2012) World Malaria report, shows that 219 million cases of malaria were reported in 2010, leading to 660,000 malaria deaths. For communities where allopathic medicines are not readily available, herbal treatment is usually the only source of remedy.
Cryptolepis sanguinolenta (Lindl.) Schlt. is an important medicinal plant with a long history of use in the treatment of malaria in Ghana. It belongs to the family Periplocaceae but is sometimes classified under the Apocynaceae family. The plant has restricted distribution in the West and parts of the Central African sub-region, in countries such as Senegal, Nigeria, Cameroun, Central African Republic, Congo, DR. Congo, Uganda and Angola (Jansen and Schmelzer, 2010).
In Ghana, the plant is found mainly on the slopes of the Akwapim and Kwahu mountain ranges and can also be found in the Volta, Central and Western regions of Ghana (Willcox et al., 2004). It is widely used by traditional healers and has been incorporated into a number of herbal products being sold on the market in Ghana, such as Class Malacure, Herbaquine, Nibima, Phyto-Laria and Malaherb. An aqueous extract of the roots yields indoloquinoline alkaloids, mainly cryptolepine (Figure 1), an N-methyl derivative of quindoline (Dwuma-Badu, 1978; Tachie et al., 1991,1993). The several related minor alkaloids include 11-hydroxycryptolepine, cryptoheptine, isocryptolepine (Cryptosanguinolentine), neo cryptolepine, cryptoq uindoline, quindoline, quindolinone, biscryptolepine and cryptospirolepine have been isolated (Tackie et al., 1993; Sharaf et al., 1996).
These alkaloids have been shown to have anti-microbial (Sawer et al., 2005), anti-hyperglycemic (Luo et al., 1998; Bierer et al., 1998) and anti-malarial (Tempesta, 2010; Bugyei et al., 2010; Ansah et al., 2005) properties. A clinical study conducted on patients between the ages of 11 and 50 years by Bugyei et al. (2010), proved the effectiveness of a tea bag formulation made from the roots of C. sanguinolenta in the treatment of acute malaria. Earlier studies have shown that alkaloids isolated from C. sanguinolenta are effective against chloroquine-resistant strains of the malaria parasite (Wright et al., 1996; Cimanga et al., 1997; Kirby et al., 1995).
The widespread use and wild harvesting of C. sanguinolenta in non-sustainable ways (as the roots are the desired products), necessitates its sustainable management and conservation through cultivation. The threat to their supply is further heightened by forest clearings for farming activities. There is the need to come up with rapid and easy-to-adopt cultivation and propagation techniques for C. sanguinolenta to ensure a reliable and steady source of plant material thus conserving the wild population in their natural habitat.
Although the production of secondary metabolites such as alkaloids are controlled genetically, environmental conditions play an important role in the growth, formation and quality of these secondary metabolites (Li, 2000). Environmental conditions such as light intensity can be influenced by staking, an old cultural practice used to ensure that the leaves of plants get better exposure to sunlight and ventilation thus increasing the plant’s photosynthetic capacity (Norman, 1992). Staking has been reported to result in a two and half fold increase in tuber yield in Dioscorea plants compared to unstaked plants (Kurian and Sankar, 2007). Singh et al. (1996) observed a significant increase in tuber yield and diosgenin content (a major steroid drug precursor) with the staking of Dioscorea tubers.
The objective of this study was to determine the cropping cycle and evaluate the effect of staking on plant growth parameters and cryptolepine concentration (active ingredient) over a one year period. This research forms part of a series of experiments to develop domestication protocols for the cultivation of C. sanguinolenta.
Site conditions
A field experiment was carried out at the Plant Genetic Resources Research Institute’s experimental plots at Bunso in the Eastern region of Ghana from April, 2012 to April, 2013. The mean rainfall in Bunso during the given period was the highest in the month of June, 2012 (236.5 mm) and lowest in February, 2013 (1.1 mm). Table 1 shows maximum and minimum air temperatures, mean monthly rainfall and maximum and minimum relative humidity in Bunso during the experimental period. The soil of the experimental site was sandy loam in texture with pH 5.8, organic matter 3.1%, N: 0.10%, P: 32.4 mg/kg, K: 0.2 cmol/kg, Na: 0.4 cmol/kg, Ca: 11.6 cmol/kg, Mg: 3.6 cmol/kg, and CEC 25.6 cmol/kg, in soil depth of 0 to 15 cm.
Plant and cultivation/site preparation
Seeds of C. sanguinolenta were sown on nursery beds in February, 2012 and transplanted onto the field in April after the first rainfall (74 days after seeds were sown in the nursery). A 60 m × 12 m land was cleared and divided into three plots of equal size. Each plot was further divided into two and randomly assigned the treatment staked or unstaked. Treatments were replicated three times. Sixty-four mounds were created on each plot. In all, 192 C. sanguinolenta seedlings were planted. The spacing between mounds of height 24 to 36 cm was 80 × 80 cm.
Fifteen (15) seedlings were randomly selected at the start of the experiment for cryptolepine concentration analysis. Transplanted seedlings were given 14 days to establish in the field after which thirty plants were harvested (5 plants/replicate/treatment) at each sampling date 105, 197, 289 and 379 days after planting (DAP).
Staking was done using Gliricidia sepium. Cultural practices such as weeding was done once a month. Watering was done during the first two weeks after transplanting to ensure seedlings were properly established in the field.
Experimental design and data collection
The experiment was laid out in a completely randomized design (CRD). Each experimental plot of size 30 × 4 m contained four rows with eight plants within each row representing thirty two plants/treatment/experimental unit. The total field size was 60 × 12 m with a total of 192 plants. Plants on the outer periphery of the experimental field served as a boarder row and were not used as record plants. Observations were recorded on the following reproductive parameters: number of days from transplanting to flowering, pod set and pod ripening. Data were also taken on the following vegetative parameters: total number of vines per plant, average vine girth (measured in centimeters using a pair of veneer calipers), plant fresh weight (harvested plants were separated into leaves, stems and roots and weighed), plant dry matter was determined by oven drying the detached leaves and stems at 70°C for 48 h to constant weight. Roots were air-dried over one month to a constant weight and ground. Dry matter partitioning ratio of the accumulated total dry matter in the various plant tissues was calculated using the formula: dry matter ratio (DMR) = (DMt)/TDMp
× 1; where DMt and TDMp are dry matter in tissue and total dry matter in plant, respectively for a specific harvest date.
Chemical analysis
The powdered samples were analyzed to determine the active ingredient (cryptolepine) concentration. Of the fifteen plants used in the fresh and dry weight determination, three plants per treatment per plant age were set aside for the determination of cryptolepine content.
High performance liquid chromatography (HPLC) assay for cryptolepine
A 100 ppm or 0.1 mg/ml solution of a reference standard of cryptoplepine was prepared by accurately weighing 0.5 mg of the reference standard and dissolving it in 5 ml of absolute ethanol. Standard solutions of concentrations 1, 5, 10 and 50 ppm were prepared from the stock standard solution. These solutions were run on the HPLC. The area under the curve (AUC) for each standard solution was calculated from the chromatogram obtained from the assay. Six replicates were obtained for each concentration. The average AUC for each concentration was then calculated.
A graph of the average AUC was plotted against concentration (C) in ppm to obtain a calibration curve. The slope (m), the y-intercept (b) and the correlation coefficient (r2 = 0.9999) were calculated using Microsoft Excel 2010. The equation of a straight line was then deduced, AUC = mC + b. The crude extracts were obtained by maceration of 100 mg of each plant material in ethanol (50 ml × 24 h × 3) followed by concentration to dryness using the rotary evaporator. Each residue was then reconstituted in 20 ml of ethanol and kept in a reagent bottle in the refrigerator at 4°C. one millilitre of each crude extract (test solution) was placed in vials and loaded unto the auto sampler of the HPLC machine after attaining room temperature. Twenty microlitres of each test solution was injected and six runs were done for each. Chromatograms were obtained and the AUC for each peak determined. The average of the AUCs was calculated and using the equation of the straight line obtained from the calibration curve, the concentration of cryptolepine in each crude extract was calculated.
The HPLC analysis was carried out at the Ghana Standards Authority, Accra, Ghana with a Varian 920-LC model liquid chromatograph equipped with a Varian Prostar 410 auto sampler, Varian Prostar 220/230/240 pumps, and 335 models Diode Array Detector (DAD), and the Galaxie software for data acquisition and processing. The reverse phase HPLC method was employed. The analyses were carried out in an isocratic elution mode using methanol: water (90:9) modified with dichloroacetic acid (DCA) to a pH of 2.4 as a mobile phase and a Pursuit C18 (5 µm particle size) column (250 × 4.6 mm id; Varian; Cat. no. 1215-9307). The DAD detection wavelength was monitored at 366 nm (Figure 1).
Statistical analysis
Data were analyzed by GENSTAT using version 9. Analysis of variance (ANOVA) and differences between means were determined for significance at p≤0.05 using least square difference.
General observations
In a preliminary experiment, it was found that seeds from mature pods germinated best (68%), followed by seeds from semi-mature pods (28%) and no germination from green unripe pods (0%). Germination occurred on average 18 days after sowing.
Cyme type flowers were observed in July, 105 DAP at which time approximately 70% of the plants were in flower (Figure 2). Flowering was more pronounced in plants that were not staked, compared to those that were staked. Fruits in the form of paired linear spreading follicles were prominent in October 197 DAP (Figure 3). At the peak of fruiting, staked plants produced more pods than unstaked plants; approximately 80 to 100 pods per staked plant were counted.
It was expected that the unstaked plants would produce more pods since they produced more flowers. However, the opposite was observed which may be as a result of the flowers in the staked plants being better positioned or exposed for pollination to take place. It is recommended that for production of C. sanguinolenta, staked plants are set aside for the production of seeds. Mature pods contained on average 30 seeds per pod. Seeds were approximately 11 mm long with a tuft of silky hairs at the end of each seed (Figure 4). Pods progressed from dark green during the early stages of development to pale green then dark brown and finally pale brown just before the pods split longitudinally along their side.
To obtain maximum germination, seeds from mature pods should be sown within two weeks of harvesting, as seeds do not store well and lose their ability to germinate after this period.
By January, 289 DAP the plants had lost about 90% of their leaves in response to the dry harmattan weather (characterized by high day time temperatures, low relative humidity and extremely low rainfall patterns) (Table 1). Leaf loss was more pronounced in the staked plants than those that were not staked. Majority of the pods were mature by January 289 DAP and had split to reveal seeds characterized by a tuft of hair. The plants regained about 70% of their leaves by April (379 DAP) with the presence of leaves being more pronounced in the unstaked than staked plants. No flowers or pods were observed in April 379 DAP.
The plant’s root architecture changed from a prominent taproot system to that of a thickened multi-root system with no root hairs, by the end of the experiment (Figure 5). No pests or diseases problems were encountered during the experimental period. It is proposed based on the cropping cycle shown in Figure 6, that roots which are the economic yield of C. sanguinolenta could be harvested thrice in a cropping cycle (November, December and January) by staggering the sowing of seeds over a 3 month period (February, March and April). Staggering the sowing of seeds will allow for sustained harvesting over the growing period, thus help to reduce the frequency of harvesting from the wild.
In this study, we attempted to develop cultivation protocols for the domestication of C. sanguinolenta, currently harvested solely from the wild, and as well determine when cryptolepine (the main active ingredient) concentration is at its peak in the roots of the plant.
The cultural practice of staking, which tends to be labour intensive, had no significant influence on the growth parameters studied. However, plant age showed a significant effect on the plant growth parameters studied (vine girth, leaf, root and vine dry weights) (Table 2). Vine girth did not change significantly over the 289 DAP period, however, a significant decrease in girth (1.6 cm) was observed at 379 DAP. This decrease in vine girth 379 DAP may be as a result of the decrease in photosynthetic activity considering a reduction in the total number of leaves during the Harmattan season 289 DAP (in January) lasting until February (Table 1). The decrease in leaf dry weight (11.3 g) 289 DAP, coincided with the dry Harmattan season when the vines lost ~ 90% of their leaves.
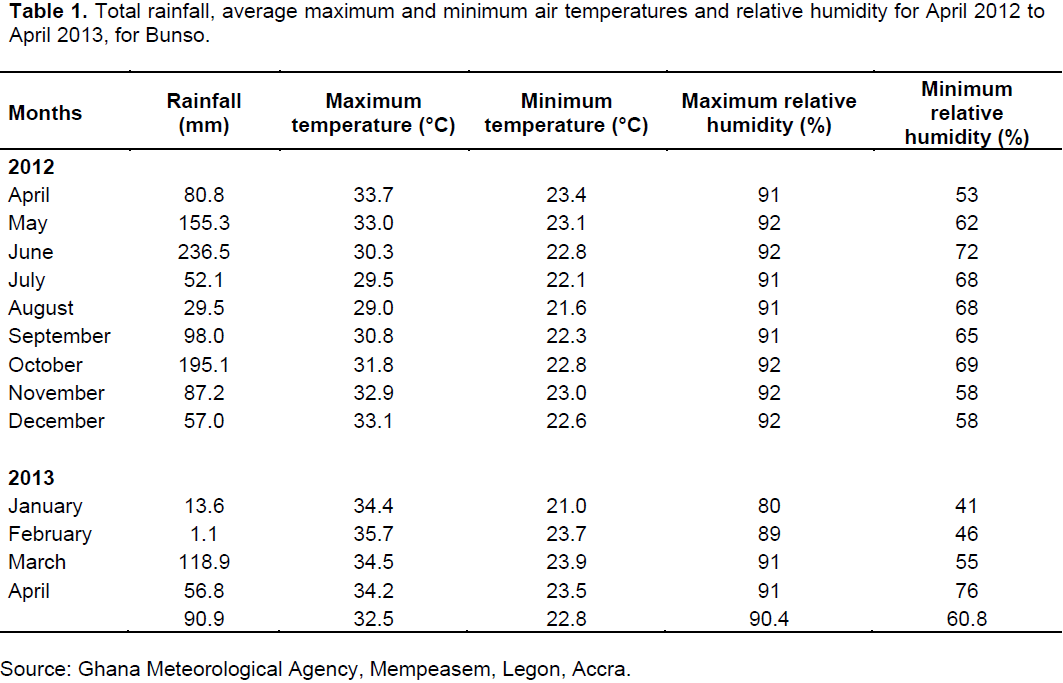
The root dry weight per plant was found to be the highest (52.8 g) 289 DAP (Table 2), suggesting the most ideal time to harvest the roots for maximum economic gain. Total dry weight accumulation over the 289 DAP period increased linearly, with very little variation between the 289 and 379 DAP period. Dry matter partitioning in plant parts is according to the need as defined by the growth and developmental stages of the plant and influenced by its growing environment (Singh et al., 2008). The total dry matter increased linearly during the growing period until maturity at 289 DAP (Table 2). The ratio of accumulated dry matter partitioned to leaves, roots and vines during successive growth periods defined as DAP are shown in Figure 7. At 105 DAP, which coincided with the period of peak flowering (the reproductive phase), the plant allocated fairly equal amounts of dry matter to the leaves (40%), roots (30%) and vines (30%). During subsequent harvests, dry matter partitioned to the leaves, vines and roots decreased, increased and remained constant respectively. At 197 DAP, which coincided with the period of peak pod formation, equal amounts of dry matter (40%) were partitioned to the vines and roots while that of the leaves decreased (20%). The dry matter partitioning to the leaves decreased further during the harmattan period in favour of partitioning to the vines at 289 DAP. The dry matter partitioning trends observed throw light on the plant’s distribution system and may serve to inform ways of increasing dry matter partitioning efficiency to the roots through the use of fertilizers that promote root development.
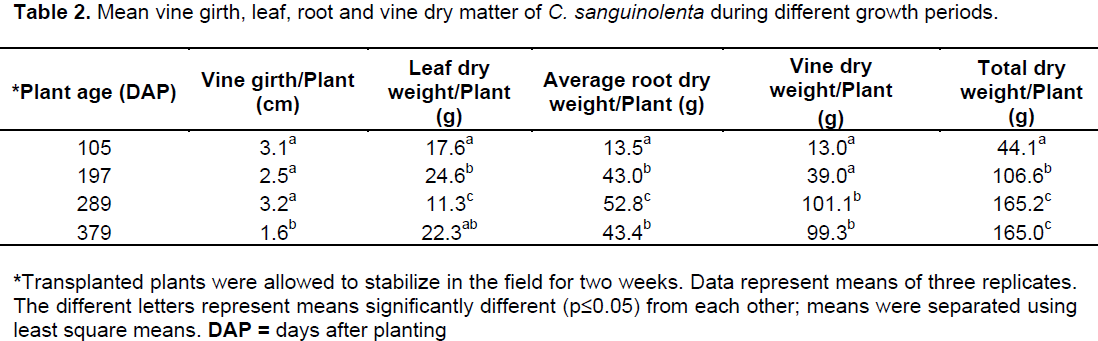
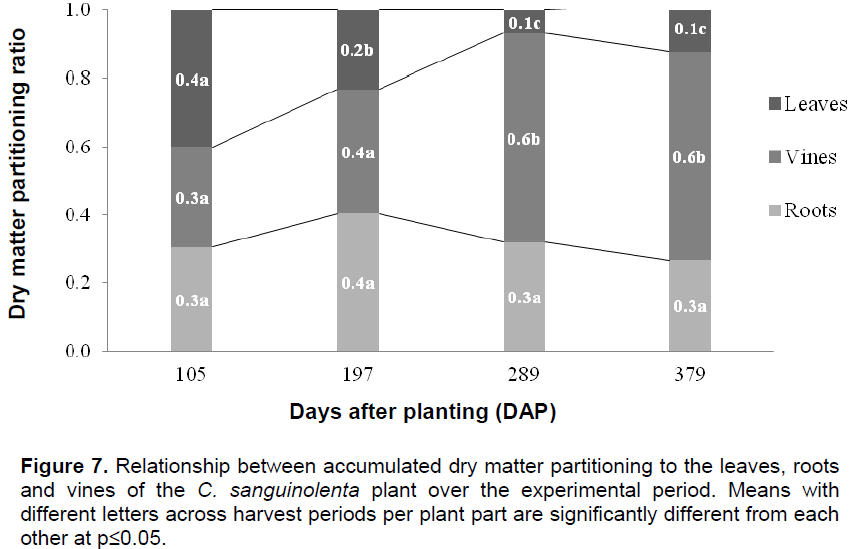
A significant interaction was found between the treatments (staking versus no staking) and plant age which influenced the concentration of cryptolepine in the roots of C. sanguinolenta. Interestingly, cryptolepine concentration was found to be among the highest in seedling plants at the start of the experiment (1.82 mg/100 mg) which then dropped sharply by105 DAP in both staked (0.54 mg/100 mg) and unstaked (0.46 mg/100 mg) plants which coincided with the period of flowering. The high cryptolepine concentration observed in the seedling plants at the start of the experiment is in line with the general view that the high concentration of alkaloids in seedling plants serves as a deterrent to herbivores thereby increasing its chances of survival (Harborne, 1993). A sharp decrease in the cryptolepine concentration was observed during the plant’s growing period and was found to be at its lowest 105 DAP which coincided with the period of peak flowering in the plant. This trend may explain the age old hypothesis that because of the structural similarity between alkaloids and growth hormones, alkaloids may have a hormonal influence on plant growth (Waller and Nowacki, 1978). This might explain the reason for the decline in cryptolepine concentration during the active growth period of C. sanguinolenta. An increase in concentration of the active ingredient followed the peak phase of flowering in the plant. Cryptolepine was at its highest in both the staked (1.75 mg/100 mg) and unstaked plants (1.92 mg/100 mg) 289 DAP (Figure 8). By the end of the experimental period, the cryptolepine concentration was significantly higher in unstaked (1.75 mg/100 mg) versus staked plants (1.33 mg/100 mg) (Figure 8).
In this experiment, C. sanguinolenta plants were grown under rain fed conditions in an open field simulating as much as possible their growth conditions in the wild. The highest cryptolepine concentration coincided with the peak of root dry weight at 289 DAP, signifying the most optimum time to harvest roots. It is possible to increase the number of times the roots are harvested from once to thrice a year by staggering the sowing of seeds over a three month period (February, March and April). Prolonging the harvest period has the advantage of minimizing dependency on collections from the wild. No pest or disease problems were encountered during the study period. It was observed that staking of the plants promoted pod formation and as such staking of a few plants is recommended for the production of seeds for replanting. For crop production for medicinal purposes C. sanguinolenta plants should be left unstaked.
The authors have not declared any conflict of interest.
The authors wish to thank the Volkswagen Foundation of Germany for providing the research funds and Mr Paul Osei-Fosu of Ghana Standard Authority for his immense help with the HPLC analysis.
REFERENCES
|
Ansah C, Khan A, Gooderham NJ (2005). In vitro genotoxicity of West African antimalarial herbal Cryptolepis sanguinolenta and its major alkaloid cryptolepine. Toxicology 208(1):141-147.
Crossref
|
|
|
|
Bierer DE, Fort DM, Mendez CD, Luo J, Imbach PA, Dubenko LG, Jolad SD, Gerber RE, Litvac J, Lu Q, Zhang P, Reed MJ, Waldeck N, Bruening RC, Noamesi BK, Hector RF, Carlson TJ, King SR (1998). Ethnobotanical-directed discovery of the antihyperglycaemic properties of cryptolepine: its isolation from Cryptolepis sanguinolenta, synthesis, and invitro and in vivo activities. J. Med. Chem. 41(6):894-901.
Crossref
|
|
|
|
|
Bugyei KA, Boye GL, Addy ME (2010). Clinical Efficacy of a Tea-Bag Formulation of Cryptolepis Sanguinolenta Root in the Treatment of Acute Uncomplicated Falciparum malaria. Ghana Med. J. 44(1):3-9.
|
|
|
|
|
Cimanga K, De Bruyne T, Pieters L, Vlietinck AJ, Turger CA (1997). In vitro and in vivo antiplasmodial activity of cryptolepine and related alkaloids from Cryptolepis sanguinolenta. J. Nat. Prod. 60:688-691.
Crossref
|
|
|
|
|
Cryptolepine Structure- CSID:74137,
View
|
|
|
|
|
Cunningham AB (1993). African medicinal plants. Setting priorities at the interface between conservation and primary healthcare. Plant and People Initiative. UNESCO, France. Available at:
View
|
|
|
|
|
Dwuma-Badu D, Ayim JS, Fiagbe NI, Knapp JE, Schiff PI, Slatkin DJ (1978). Constituents of west African medicinal plants: XX Quindoline from Cryptolepis sanguinolenta. J. Pharm. Sci. 67(3):433-4.
Crossref
|
|
|
|
|
Harborne JB (1993). Introduction to ecological biochemistry. 4th edition. Academic Press, London.
|
|
|
|
|
Jansen PCM, Schmelzer GH (2010). Cryptolepis sanguinolenta (Lindl.) Schltr. In: Schmelzer GH and Gurib-Fakim A (Eds.), Prota 11(2): Medicinal plants/Plantes médicinales 2. [CD-Rom]. PROTA, Wageningen, Netherlands.
|
|
|
|
|
Kirby GC, Paine A, Warhurst DC, Noamesi BK, Phillipson JD (1995). In vitro and in vivo antimalarial activity of cryptolepine, a plant-derived indoloquinoline. Phytother. Res. 9:359-363.
Crossref
|
|
|
|
|
Kurian A, Sankar A (2007). Medicinal Plants, New India Publishing 356.
|
|
|
|
|
Li TSC (2000). Medicinal Plants: Culture, Utilization and Phytopharmacology. CRC publishers. P 536.
|
|
|
|
|
Luo J, Fort DM, Carlson TJ, Noamesi BK, Nii-Amon-Kotei D, King SR, Tsai J, Quan J, Hobensack C, Lapresca P, Waldeck N, Mendez CD, Jolad SD, Bierer DE, Reaven GM (1998). Cryptolepis sanguinolenta: an ethnobotanical approach to drug discovery and the isolation of a potentially useful new antihyperglycaemic agent. Diabetic Med. 15:367-374.
Crossref
|
|
|
|
|
Norman JC (1992). Tropical vegetable crops. Arthur Stockwell Ltd, Great Britain.
|
|
|
|
|
Ofori DA, Darko BO, Gyimah A, Adam KA, Jimoh SO, Jamnadass R (2012). Ethobotany, Propagation and Conservation of Medicinal Plants in Ghana. Ghana J. For. 28(1):29-38.
|
|
|
|
|
Sawer IK, Berry MI, Ford JL (2005). The killing effect of cryptolepine on Staphylococcus aureus. Lett. Apl. Microbiol. 40:24-29.
Crossref
|
|
|
|
|
Sharaf MHM, Schiff Jr PL, Tackie AN, Phoebe Jr CH, Martin GE (1996). Two new indoloquinoline alkaloids from Cryptolepis sanguinolenta: cryptosanguinolentine and cryptotackieine. J. Heterocycl. Chem. 33:239-243.
Crossref
|
|
|
|
|
Singh RS, Bhattacharya TK, Ghosh AC (1996). Variability in Yield of Tuber and Diosgenin in Plants Developed by Seed and Single-Node Leaf-Cutting and Vine Staking of Medicinal Yam (Dioscorea-Floribunda). Indian J. Agric. Sci. 66(8):443-445.
|
|
|
|
|
Singh RK, Singh JP, Lal SS (2008). Dry Matter Partitioning Relative to Development in High Yielding Indian Potato Cultivars Under Short Day Tropical Conditions. Potato J. 35(3-4):161-166.
|
|
|
|
|
Tachie AN, Sharaf MHN, Schiff PL, Boye GL, Crouch RC, Martin GE (1991). Assignment of proton and carbon NMR spectra of the indoquinoline alkaloid cryptolepine. J. Heterocycl. Chem. 28:1429-1435.
Crossref
|
|
|
|
|
Tackie AN, Boye GL, Sharaf MHM, Schiff PL Jr, Crouch RC, Spitzer TD, Johnson RL, Dunn J, Minick D, Martin GE (1993). Cryptospirolepine, a Unique Spiro-nonacyclic Alkaloid Isolated from Cryptolepis sanguinolenta. J. Nat. Prods. 56(5):653-670.
Crossref
|
|
|
|
|
Tempesta MS (2010). The Clinical Efficacy of Cryptolepis Sanguinolenta in the treatment of Malaria. Ghana Med. J. 44(1):1-2.
|
|
|
|
|
Waller GR, Nowacki E (1978). Alkaloid Biology and Metabolism in Plants. Plenum Press, New York. Available at: Phytochemistry 18(4):707-708.
|
|
|
|
|
Willcox M, Bodeker G, Rasoanaivo P, Addae-Kyereme J (2004). Traditional Medicinal Plants and Malaria. CRC Press. P 464.
|
|
|
|
|
World Health Orgaanization (WHO) (2012). World Malaria Report 2012 Fact Sheet. Available at: www.who.int/malaria
|
|
|
|
|
Wright CW, Phillipson JD, Awe SO, Kirby GC, Warhurst DC, Quertin-Leclerq J, Angenot L (1996). Antimalarial activity of cryptolepine and some other anhydronium bases. Phytother. Res. 10:361-363.
Crossref
|
|