ABSTRACT
Maize weevil (Sitophilus zeamais Motschulsky) is a major maize (Zea mays L.) storage insect pest in the tropics which reduces the quantity and quality of maize hence facilitating establishment of aflatoxin and other mycotoxins. The objective of this study was to evaluate maize weevil resistance on selected inbred lines. Twenty eight inbred lines with 2 checks (MTPO701-reistant and Duma 41-susceptible) were used in this experiment. Thirty unsexed adult insects were introduced into 250 ml glass jars with grains of the lines at room temperature. Evaluation of weevil damage was done at 10, 60 and 120 days after maize weevil infestation. Each category of storage period was replicated 4 times and experiment was set at the same time. Data was collected on percent weevil damage, grain weight loss and number of live and dead weevils on each inbred line. ANOVA analysis showed significant differences (P ≤ 0.05) on weight loss. The selection of the resistant genotypes was based on percent weight loss after 60 days. Resistant lines selected included KEN2/TZL2.25# and LEPOOL-1/TZL2-2-1. These lines showed resistance to maize weevil damage and hence can be stored up to 4 months. At 120 days there was maximum damage and most lines could not be differentiated on the basis of resistance. KEN2/TZL2-2-5# showed consistency in resistance to maize weevils at all storage periods. High heritability at 60 days showed that selection for weevil resistance in these inbred lines is effective and feasible. Results in this study also revealed high, positive and significant correlation relationship between percent damage, weight loss and live weevils. The maize weevil resistant lines can be used to improve resistance of high yielding varieties in breeding programmes.
Key words: Inbred lines, maize, post-harvest, resistance, Sitophillus zeamais.
Maize (Zea mays L.) is the most important cereal crop in Kenya and is consumed in various regions (Kang’ethe, 2011; Suleiman and Kurt, 2015). While farmers may achieve high yields in the farm, they experience much grain losses during storage due to insect pests. Research has focused on increased field maize productivity while postharvest handling has not received adequate attention yet; insect pests at maize storage cause devastating yield and quality losses (Tefera et al., 2011; Kumar and Kalita, 2017). Due to surplus maize in the market after harvesting, coupled with low prices, farmers mainly store their maize to take advantage of higher prices of their produce when the demand is high (Suleiman and Kurt, 2015). In most tropical countries, harvested grains are mainly stored by farmers for considerable periods in various types of storage structures made of mud, bamboo strips and plastic sacks (Bilgami and Sinha, 1987; Ranjan et al., 1992; Kumar and Kalita, 2017). These unimproved traditional storage methods inevitably provide suitable conditions for the growth of insects and microorganisms responsible for the quality loss in stored grains. Most insect pests have been reported to be associated with stored maize and their by‑products causing loss of food for human and animal consumption (Demianyk and Sinha, 1987; Kumar and Kalita, 2017). The main storage insect pests causing yield losses in maize include maize weevil, large grain borer, red flour beetle, Indian meal moth and lesser grain borer. These insect storage pests may destroy 10 to 15% of grain and contaminate the grains with undesirable odors and flavors. Among the pests, maize weevil has been identified to cause major grain losses in stored maize and creates a higher risk of establishment of aflatoxin and other mycotoxins in the grains (Tefera et al., 2011; Kumar and Kalita, 2017). The female weevils bore holes into grain kernels, lay eggs in the holes, and then cover the holes with gelatinous plugs (Subramanyam and Hagstrum, 2012).
The larvae feed inside the kernel and the adult eventually chew their way out of the kernels (Subramanyam and Hagstrum, 2012). Postharvest maize weevil infestation commences in the field but most damage occurs during storage (Demissie et al., 2008; Goftishu and Belete, 2014). We therefore require control measures that are effective both in the field level and under storage. As a remedy to control of these pests, synthetic insecticides have been widely used on stored grains. However, there are global concerns due to environmental hazards, chemical residues on food, insecticides resistance development and associated costs (Cherry et al., 2005; Nicoloupolou-Stamati et al., 2016). Host plant resistance offers sustainable control measure to weevil infestation in the field level, under storage and minimizes the major concerns associated with use of insecticides. Studies have found resistance to weevil infestation to be heritable (Derera et al., 1999; Goftishu and Belete, 2014). Most studies on host plant resistance to maize weevil have focused on grain factors contributing to resistance and inheritance mechanism of resistance (Derera et al., 2001; Zunjare et al., 2014). Despite the increased understanding of the inheritance of weevil resistance and of the resistance mechanisms in the maize grains, there has been very little application of this knowledge in maize breeding programmes (Dhliwayo and Pixley, 2002; Zunjare et al., 2014). Maize inbred lines represent a resource for studies in genetics and plant breeding towards crop improvement (Mwololo et al., 2012; Zunjare et al., 2014). These lines are mainly used in development of hybrids. Progress has been made in developing maize cultivars resistant to post-harvest insect pests (Goftishu and Belete, 2014). Understanding the level of responses of different maize inbred lines especially against S. zeamais infestation is important to decide the course of resistance breeding strategy. The objective of this study was to screen and identify resistant inbred lines to weevil attack so as to develop resistant maize hybrids.
Source of maize germplasm
Maize grains used in this study were from twenty eight inbred lines which had been planted at the Kiboko nursery in July, 2016. The genotypes used were provided by the Kenya Agricultural and Livestock Research Organization (KALRO), Katumani centre. The inbred lines originated from Kenya, Zimbabwe and France. The grains were selected on the basis of their high resistance to aflatoxin contamination.
Field trial design and management
The experimental materials were evaluated at KALRO Kiboko. Kiboko is located in Makueni County. The mean annual rainfall is 530 mm and is spread over two very short rainy seasons. It lies at an altitude of 975 meters above sea level and between latitude 02° 15’ S and longitude 37° 75’ E. Sand-clay type of soil occupies this location. Temperatures are uniformly high with mean maximum value of 35.1°C and the minimum of 14.3°C. The twenty eight entries were planted in the Kiboko experimental site. Field sizes for the inbred lines were 87.5 m by 18 m and 87.5 m by 30 m, respectively. Each plot measured 5 m by 0.75 m. Fertilizer was applied at a standard rate of 30 kg Calcium Ammonium Nitrate (CAN) and 30 kg Di ammonium Phosphate (DAP). Supplementary irrigation was administered when needed. The fields were kept free from weeds by hand weeding. Number of rows per plot was 2 and distance between stations, 0.25 m. Treatments were laid in a randomized complete block design (RCBD) with 4 replicates.
Grain preparation, insect culture and infestation
At harvest, sieving was done to remove any dirt, dust or broken grains. The mature maize weevil insects used for the evaluation were sourced from CIMMYT/KARI-Kiboko post-harvest testing laboratory. The insects had been reared on commercial hybrid maize H614 under controlled conditions of 28°C and 70% relative humidity (Tefera et al., 2011). Fifty grams of grains was put in 250 ml capacity no-choice glass jars at room temperature and then thirty unsexed adult insects were introduced into the glass jars (Tefera et al., 2011). Glass jars were then covered with a lid made of wire mesh (1 ml) to allow for adequate ventilation and prevent escape of the weevils (Tefera et al., 2011).
Categories of samples
At harvest, the maize was arranged in three categories. Each category describes the time when the samples were assessed for insect damage. One category represented materials under storage for 10 days; the second had materials under storage for 60 days while the third, materials were stored for 120 days. Each category consisted of 28 entries replicated 4 times. The experimental set up for these genotypes was done at the same time.
Experimental design
Treatments were laid out in a completely randomized design and kept on wooden shelves at room temperature in the laboratory. The experiment consisted of 28 germplasms replicated 4 times and put in 3 categories. A total of 336 samples were assessed in this experiment. MTP0701 (resistant check) and DUMA 4(susceptible check) to weevil infestation were incorporated in the study. Assessment of the trials was done at 10, 60 and 120 days of storage.
Data collection and assessment
Data was collected on weight of damaged and undamaged grains, live and dead weevils. On each assessment date (10, 60 and 120 days), the glass jars were opened, contents separated into grains, insects and dust using 4.7 and 1 mm sieves (Endecotts Ltd UK). Mortality was assessed. All maize weevils were separated and removed (by hand) from the maize at the end of these three storage periods. Separation of the damaged and undamaged kernels was done using grain tunneling and holes as the criteria (Tefera et al., 2011); these were counted and the percentage of damaged grain and grain weight loss computed. The percent damage was determined using the converted percent damage method (Baba -Tiertor, 1994):
where: GD = Damaged grain
WDG = Weight of damaged grain
WDUD = Weight of damaged and undamaged grains
Weight loss was determined by the count and weight method of Gwinner et al. (1996).
Weight loss (%) = (Wu x Nd) - (Wd x Nu) X 100 / Wu x (Nd + Nu)
Where, Wu = Weight of undamaged grain,
Nu = Number of undamaged grain,
Wd = W eight of damaged grain, and
Nd = Number of damaged grain.
Genotypes were categorized as resistant (1 to 5%), moderately resistant (5.1 to 8%), moderately susceptible (8.1 to 10%), susceptible (10.1 to 13) and highly susceptible (>13.1%) after 60 days based on percentage weight loss, which was found to be a key trait of discriminating genotypes in relation to resistance (Tefera et al., 2011; Mwololo et al., 2012).
Data analysis
The numbers of percentage weevil damage, grain weight loss, live and dead weevils were subjected to Genstat 14th edition software and means separated using Fishers least significance difference at 5% probability level. Heritability was measured based on grain damage. Broad sense heritability was estimated based on Johnson et al. (1955) where by the error mean sum of squares (EMS) was considered as error variance (σ2e). Genotypic variance (σ2g) was derived by subtracting error mean sum of squares (EMS) from the genotypic mean sum of squares (GMS) and divided by the number of replications as given by the formula:
σ2g = GMS-EMS/r
where GMS=Genotype mean sum of squares, EMS= error mean sum of squares and r = number of replications. Phenotypic variance (σ2P) was derived by adding genotypic variance with error variance as given by the formula:
σ2p = σ2e + σ2g
Broad sense was then calculated as;
H2b = σ2g/ σ2p
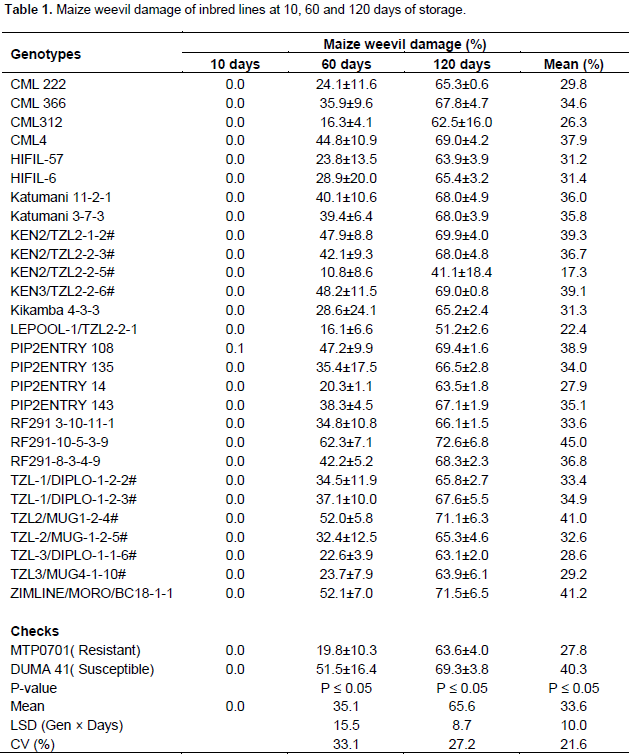
Maize weevil damage of inbred lines
There was no significant difference among inbred lines in response to weevil damage after 10 days of storage and damage on lines was less than 1%. The inbred lines differed significantly to weevil damage after 60 days of maize storage. The highest weevil damage was recorded in ZIMLINE/MORO/BC18-1-1, TZL2/MUG1-2-4 and RF291-10-5-3-9 as they were more damaged than the susceptible check. On the other hand, lines KEN2/TZL2-2-5#, LEPOOL-1/TZL2-2-1 and CML 312 were less damaged than the resistant check MTP0701 (Table 1). The inbred lines showed significant differences to maize weevil damage after 120 days of storage. KEN2/TZL2.2.5#, LEPOOL-1/TZL2-2-1, TZL-3/DIPLO-1-1-6#, CML 312 and PIP2ENTRY 14 were less damaged than the resistant check at this stage (Table 1). The combined effect of weevil damage at the 3 stages revealed that RF291-10-5-3-9 was highly damaged by the weevils (Table 1). On the contrary, line KEN2/TZL2-2-5 was the least damaged at all the 3 stages. KEN2/TZL2-2-5, CML312 and LEPOOL-1/TZ2 2-2-1 were resistant lines at 10, 60 and 120 days of storage on the basis of percent damage as they recorded less damage in relation to the resistant check MTP0701.
Maize weevil grain weight loss of inbred lines
The inbred lines did not differ significantly after 10 days of weevil infestation. Their weight loss varied from 0 to 0.3% (Table 2). There was significant difference in grain weight loss after 60 days of storage. The lowest was recorded in inbred lines KEN2/TZL2-2-5# and LEPOOL-1/TZL2-2-1 while the highest was in ZIMLINE/MORO/BC18-1-1 (Table 2). Two lines were resistant, 4 were moderately resistant, 5 were moderately susceptible, 11 were moderately resistant and 7 were highly susceptible (Table 2). The inbred lines showed significant differences in grain weight loss after 120 days of storage. Even though much grain weight was lost at this stage, KEN2/TZL2-2-5# lost the least weight at 16%. Combined analysis showed KEN2/TZL2-2-5# had the least grain weight loss (7.0%) while KEN2/TZL2-1-2# and ZIMLINE/MORO/BC18-1-1 had the most weight loss (18.2%).

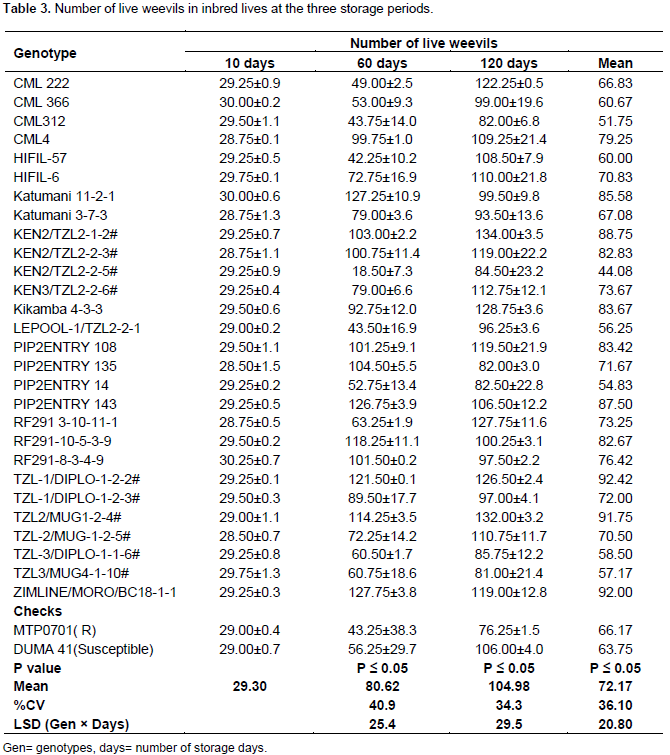
Number of live weevils in inbred lines
After 10 days of storage, the inbred lines did not show any significant difference in number of live weevils. Ninety eight percent of introduced weevils were still alive after 10 days of maize storage in the jars. Inbred lines showed significant differences in number of live weevils at 60 days of storage. Ninety seven percent of inbred lines recorded increased number of live weevils. KEN3/TZL2-2-5 had fewer weevils than the introduced number (18 live weevils) at 60 days of storage while the rest of the lines had more weevils than the initial number (Table 3). The highest number of live weevils was observed in lines; Katumani 11-2-1, PIP2ENTRY 143 and ZIMLINE/MORO/BC18. The lines had more than 120 live weevils (Table 3). Inbred lines did not differ in number of live weevils after 120 days of storage. The least number of live weevils was found in MTP0701. The highest number of weevils was found in TZL2/MUG1-2-4# and KEN2/TZL2-1-2# (Table 3). Combined ANOVA results showed that there were significant differences in inbred lines, storage periods and the interaction of the two factors in response to number of live weevils. The number of live weevils increased steadily with the number of storage periods. However, in PIP2ENTRY135, RF291-8-3-4-9, RF291-10-5-3-9, Katumani11-2-1, PIP2ENTRY 143 and ZIMLINE/MORO/BC18 the live weevils increased up to the 60th day and decreased at the 120th day.
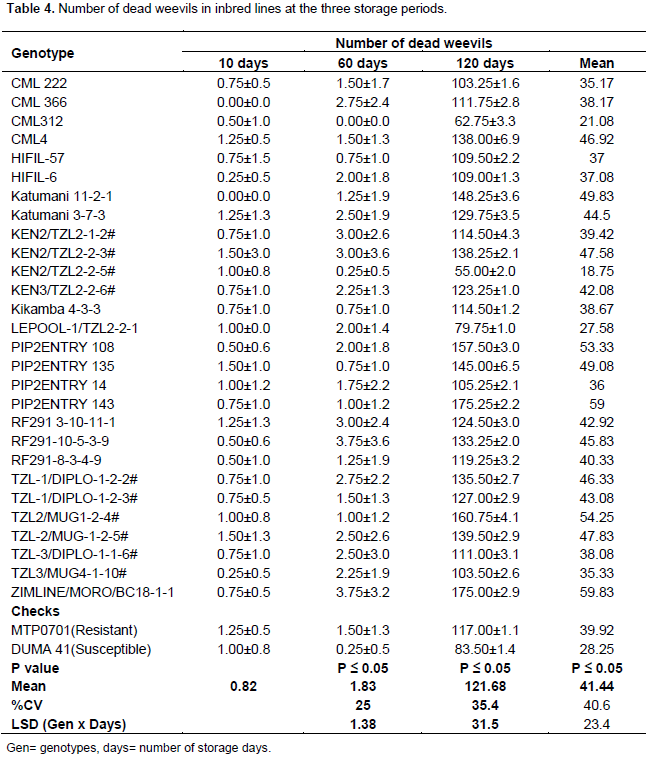
Number of dead weevils in inbred lines
There were no significant differences in number of dead weevils among inbred lines after 10 days of storage. Ninety three percent of the inbred lines had dead weevils after 10 days of storage. However the numbers of dead weevils at this storage period were few and ranged from 0 to 2 (Table 4). The inbred lines differed notably in the number of dead weevils after 60 days of storage. The numbers of dead weevils at this stage were still few and ranged from 0 to 4 (Table 4). Most dead weevils were found in RF291-10-5-3-9 and ZIMLINE/MORO/BC18 lines. There was a significant difference in number of dead weevils after 120 days of storage among inbred lines. This was the most recorded storage period with highest number of dead weevils (Table 4). Eighty nine percent of inbred lines had more than 100 dead weevils (Table 4). The highest number of dead weevils was found in ZIMLINE/MORO/BC18. There were significant differences among inbred lines, storage periods and the interaction of inbred lines and storage periods in number of dead weevils. The mean numbers of dead weevils were fewer at 10 and 60 days than at 120 days (Table 4).
Heritability of maize weevil resistance
Heritability in the broad sense of weevil resistance was estimated as described by Johnson et al. (1955). It was necessary to estimate variances first so as to calculate heritability in the broad sense. Percent damage was used to calculate heritability. It was found that heritability varied with the storage period. Heritability was 34% at 10 days, 56% at 60 days and 43% at 120 days. The highest heritability was recorded after 60 days of weevil infestation (Table 5).
Correlations
The result (Table 6) shows a linear association between variables measured after maize weevil infestation on inbred lines. All the variables showed significant (at P ≤ 0.05) and positive association. Weevil damage was found to correlate strongly with weight loss (r = 0.9), live (r = 0.8) and dead weevils (r = 0.7). However, in all variables, weight loss (%) was strongly correlated to weevil damage (%). The number of live weevils and dead weevils gave a correlation coefficient of 0.5 (Table 6).

Maize weevil damage of inbred lines
Significant differences in genotypes response to weevil damage is attributed to genotypic effects because the inbred lines were exposed to identical capacity of weevil infestation and environment. These differences in the resistance of the maize varieties indicate the inherent ability of the studied lines to resist S. zeamais attack. The resistance could either be due to antibiosis as a result of biochemical compounds which are toxic to insects or physical factors such as grain hardness (Garcia-Lara et al., 2004; Siwale et al., 2009; War et al., 2017). Resistance can also be attributed to pericarp surface texture, nutritional factors such as amylose, lipid and protein content (Dobie, 1974; Tipping et al., 1988; Tefera et al., 2013) or non-nutritional factors, especially phenolic compounds (Serratos et al., 1987; Tefera et al., 2013). Gerema et al. (2017) reported that high level of grain damage depends on the number of emerging insects and grains permitting high level of adult emergence. Weevil damage increased progressively from 10 to 60 to 120 days in all inbred lines. These results are similar with those of Tefera et al. (2011) and Togola et al. (2013) who reported the same trend.
According to Tefera et al. (2016), despite the shape, size and hardness of the grain, its chemical and nutritional composition are important primarily in resisting insect attack and damage, the length of exposure of the grain to the pest may affect the level of infestation of maize varieties by S. zeamais thus compromising extent of maize damage. The grain is then left exposed to micro-organisms leading to the production of mycotoxins thus lowering the quality and also rendering it undesirable for consumption (Mejia, 2007). Maximum weevil damage on lines was recorded at 120 days with 98% of lines having damages of more than 60%. This showed that resistance alone was not enough to suppress S. zeamais population build up but it can reduce losses due to weevil infestation since no maize grain was immune to attack by the weevil (Ivbiljaro, 2009). From the study, all the lines were undamaged for the first 10 days of storage. This showed that maize weevil damage does not commence immediately and hence maize grains can be stored for up to two weeks with minimal damage.
Maize weevil grain weight loss of inbred lines
The maize weevil Sitophilus zeamais exhibit
holometabolous type of post-embryonic development of 36 days period. This explains why after 10 days there was no grain weight loss, and this was aggravated by the fact that no damage had occurred. Later, the larvae develop and start eating the grains from the inside (Abebe et al., 2009; Wangui, 2016). The adults too immediately start aggressive feeding, resulting in increased destruction of the grains as indicated by more weight loss after 60 and 120 days of storage (Dobie, 1974; Dobie et al., 1984; Wangui, 2016). Given that both larvae and adults feed on grains, they create much dust and consequently, great maize weight losses as the storage period prolongs. The degree of weight loss has been found to be an important measure of maize grain resistance or susceptibility to the maize weevil (Derera et al., 2014), therefore its use as a key trait in discriminating genotypes in resistant categories in this study. In this study, resistant varieties had the least grain weight as it was reported before by Siwale et al. (2009). Also, from the study, grain weight loss was relatively low and was less than 40% in all storage periods. According to Dobie (1977), higher grain weight losses are expected when young weevils of particularly 0 to 3 weeks are used because they have a higher fecundity rate and increased feeding. It has been noted that a number of factors contribute to genetic resistance of varieties to stored grain insect pests attack (Adentuji, 1998; Muzemu et al., 2013). It is therefore recommended that the identified resistant lines KEN2/TZL2-2-5 and LEPOOL-1/TZ2 2-2-1 should be evaluated to determine the specific factors causing resistance to weevil attack. Such factors will then be selected when developing resistant inbred lines. The selected resistant lines should be regarded as potential sources of weevil resistance and thus be utilized in breeding resistant maize varieties.
Number of live weevils in inbred lines
Number of live weevils remained constant even after 10 days of storage. This was attributed to the fact that the development stage of most weevils is 36 days therefore no new insects had emerged within 10 days. It was expected that the number of live weevils will be more in lines which had most damages. For instance, Katumani 11-2-1, PIP2ENTRY 143 and ZIMLINE/MORO/BC18 had the highest number of live weevils at 60 days and TZL2/MUG1-2# and KEN2/TZL2-1-2# at 120. Their percent damage was also high. This increased insect multiplication resulted into enormous damages in the grains of inbred lines. The resistant lines had lesser weevils indicating antibiosis kind of resistance among the inbred lines.
Number of dead weevils in inbred lines
The numbers of dead weevils were fewer at 10 and 60 days of storage while more weevils died after 120 days. This indicates that the host lines were unfavourable for feeding and hence reproduction was not possible. The high density population could have resulted in death of insects due to competition of limited food resource. According to Sori and Keba (2013) and Abebe et al. (2009), numbers of dead weevils or weevil mortality rates are generally low in most maize varieties. They also reveal that adult weevils can survive without food for more than 10 days indicating that number of dead weevils is not a good indicator of weevil resistance in maize varieties.
Heritability of maize weevil resistance
Heritability was found to be below 50% at 10 and 120 days of storage. At 60 days, heritability was moderately high at 56%. High heritability at 60 days reveals that selection for weevil resistance in these inbred lines is effective at this stage. This also shows that resistance can easily be transferred to the inbred lines through selection procedures which then enhance development of resistant varieties (Aminu et al., 2014). High heritability estimates indicates the preponderance of additive gene action. The variation of weevil resistance at the 3 stages also affirms that weevil resistance is controlled by additive and non-additive genetic effects (Derera et al., 2001).
Correlations
There was significant positive correlation among studied traits. The results revealed a strong association between weevil damage and grain weight loss. Also a strong association of live weevils and % grain weight loss was recorded. These results were in conformity with reports of Dari et al. (2010) and Zunjare et al. (2016) who also found strong correlation in these factors indicating that they are key indicators of weevil resistance in maize.
The present study reports here the existence of sufficient variation for weevil resistance among inbred lines, thereby providing immense opportunity to impart weevil resistance through genetic means. Percent damage, grain weight loss and number of live insects are the important characters for resistance to weevil. Also this study showed that resistance against weevil attack is heritable since resistant inbred lines were selected. The selected resistant lines should be regarded as potential sources of weevil resistance and thus be utilized in breeding resistant maize varieties. Moderate heritability estimates exhibited in this study indicated considerable potential for development of lines which are resistant to weevil attack through selection of desirable plants in succeeding generation.
The authors have not declared any conflict of interests.
REFERENCES
|
Abebe F, Tefera T, Mugo S, Beyene Y, Vidal S (2009). Resistance of maize varieties to the maize weevil Sitophilus zeamais (Motsch.) (Coleoptera: Curculionidae). Afr. J. Biotechnol. 8(21).
|
|
|
|
Adentuji JF (1998). A study of the resistance of some sorghum seed cultivars to Sitophilus oryzae (L.) Coleoptera: Curculionidae. J. Stored Prod. Res. 24:67-71.
|
|
|
|
|
Aminu D, Muhammed SG, Kabir BG (2014). Estimates of combining ability and heterosis for yield and yield traits in maize population (Zea mays L.) under drought conditions in the Northern Guinea and Sudan savanna zones of Borno State, Nigeria. Int. J. Agric. Innov. Res. (5):824-830.
|
|
|
|
|
Baba-Tierto N (1994). Ability of powder and slurries from ten plant species to protect stored grains from attack by Prostephanusus truncatus (Horn) (Coleoptera: Bostrichidae) and Sitophilus oryzae (L). (Coleoptera: Curculionidae). J. Stored Prod. Res. 30:297-301.
Crossref
|
|
|
|
|
Bilgami KS, Sinha KL (1987). Aflatoxin in India IM: Zuber .M.S,Lilleho,E.B and Renfro.B.L.ed. Aflatoxin in maize proceedings of a workshop, CIMMYT, Mexico, D.F 7-11April,1986,349-358.
|
|
|
|
|
Cherry AJ, Bantino A, Djegui D, Lomers C (2005). Suppression of thestem borer Sesamia calamistis (Lepidoptera: Noctuidae) in maize following seed dressing, topical application and stem injection with African isolates of Beauveria bassiana. Int. J. Pest Manage. 50:67-73.
Crossref
|
|
|
|
|
Dari S, Pixley KV, Setimela (2010). Resistance of early generation maize inbred lines and their hybrids to maize weevil (Sitophilus zeamais (Motsch)). J. Crop Sci. 50:1310-1317.
Crossref
|
|
|
|
|
Demianyk CJ, Sinha RN (1987).Effect of infestation by the large grain borer and the lesser grain borer (Coleoptera: Bostrichidae) on stored corn. J. Environ. Entomol. 16:614-624.
Crossref
|
|
|
|
|
Demissie G, Tefera T, Tadesse A (2008). Efficacy of Silicon, filter cake and wood ash against the maize weevil, Sitophilus zeamais Motschulsky (Coleoptera: Curculinoidae) on three maize genotypes. J. Stored Prod. Res. 44:227-231.
Crossref
|
|
|
|
|
Derera J, Pixley KV, Giga D (1999). Inheritance of maize weevil resistance in maize hybrids among lines in Southern Africa, Mexico and CIMMYT –production technology for the future: Challenges and opportunities. Proceedings of the South Eastern African Regional Maize Conference, 21- 25.
|
|
|
|
|
Derera J, Pixley KV, Giga DP, and Makanda I (2014). Resistance of maize to the maize weevil: III. Grain weight loss assessment and implications for breeding. J. Stored Prod. Res. 59:24-35.
Crossref
|
|
|
|
|
Derera, J, Pixley KV, Giga D (2001). Resistance of maize to the maize weevil. I. Antibiosis. J. Afr. Crop Sci. 9:431- 440.
|
|
|
|
|
Dhliwayo T, Pixley KV (2002). Breeding for resistance to the maize weevil (Sitophilus Zeamais Motsch.): Is it feasible? In: Seventh Eastern and Southern Africa Regional Maize Conference 11th 15th February, 2001. pp. 134-138.
|
|
|
|
|
Dobie P (1974). The laboratory assessment of the inherent susceptibility of maize varieties to post-harvest infestation by Sitophilus zeamais (Motshulski) (Coleoptera: Curculionidae). J. Stored Prod. Res. 10:179-183. Elsevier Science Ltd., England.
Crossref
|
|
|
|
|
Dobie P (1977). The contribution of the tropical stored products centre to the study of insect resistance in stored maize. Trop. Stored Prod. Information 34:722.
|
|
|
|
|
Dobie P, Haines P, Hodges CP, Prevett PF (1984). Insects and Arachnids of Tropical Stored Products, their Biology and Identification (A Training Manual). TDRI. UK.
|
|
|
|
|
García-Lara S, Bergvinson DJ, Burt AJ Ramputh AI, Díaz-Pontones DM, Arnason JT (2004).The role of pericarp cell wall components in maize weevil resistance. J. Crop Sci. 44 (5):1546-1552.
Crossref
|
|
|
|
|
Gerema G, Bogale T, Mangitsu G, Lule D (2017). Resistance for Sorghum genotypes to the rice weevil, Sitophilus oryzae (L) (Coleoptera Curculionidae). Int. J. Food Sci. 7(1):1-10.
|
|
|
|
|
Gwinner J, Harnisch R, Muck O (1996). Manual on the prevention of post-harvest seed losses, post-harvest project, GTZ, D-2000, Hamburg, FRG, P 294.
|
|
|
|
|
Ivbiljaro MF (2009). The resistance of new varieties of maize to post harvest infestation by Sitophilus zeamais Motchulsky and Sitophilus oryzae (L.). J. Agric. Sci. 96:479-48.1.
Crossref
|
|
|
|
|
Johnson HW, Robinson HF, Comstock RE (1955). Estimation of genetic and environmental variability in soybeans. J. Agron. 47:314-318.
Crossref
|
|
|
|
|
Kang'ethe E (2011). Situation analysis: improving food safety in the maize value chain in Kenya. Report prepared for FAO. College of Agriculture and Veterinary Science, University of Nairobi, Nairobi.
|
|
|
|
|
Kumar D, Kalita P (2017). Reducing postharvest losses during storage of grain crops to strengthen food security in developing countries. Foods 6(1):8.
Crossref
|
|
|
|
|
Mejia D (2007). Food and Agriculture Organization Corporate Document Repository (www.fao.org/docrep) (Internet Information Accessed on 17 thJuly, 2009).
|
|
|
|
|
Muzemu S, Chitamba J, Goto S (2013). Screening of stored maize (Zea mays L.) varieties grain for tolerance against maize weevil, Sitophilus zeamais (Motsch.). Int. J. Plant Res. 3(3):17-22.
|
|
|
|
|
Mwololo JK, Mugo S, Okori P, Tefera T, Otim M, Munyiri SW (2012). Sources of resistance to the maize weevil Sitophilus zeamais in tropical maize. J. Agric Sci. 4:1916-9752.
|
|
|
|
|
Nicoloupolou-Stamati P, Maipas S, Kotampasi C, Stamatis P, Hens L (2016). Chemical pesticides and human health: the urgent need for a new concept in Agriculture. Frontiers in public health 4.
Crossref
|
|
|
|
|
Ranjan KS, Sahay SS, Sinha AK (1992).The influence of storage structure on aflatoxin contamination in wheat and mustard. J. Stored Prod. Res. 28:221-224.
Crossref
|
|
|
|
|
Serratos A, Arnason JT, Nozzolillo C, Lambert JDH, Philogene BJR, Fulcher G, Davidson K, Peacock L, Atkinson J, Morand P (1987). Factors contributing to resistance of exotic maize populations to the maize weevil, Sitophilus zeamais. J. Chem. Ecol. 13:751-761.
Crossref
|
|
|
|
|
Siwale J, Mbata K, Mcrobert J, Lungu D (2009). Comparative resistance of improved maize genotypes and landraces to maize weevil Sitophilus zeamais (Motchulski). Afr. Crop Sci. J. 17(1):1-116.
|
|
|
|
|
Sori W, Keba T (2013). Differential resistance of maize varieties to maize weevil (Sitophilus zeamais Motschulsky) (Coleoptera: Curculionidae) under laboratory conditions. J. Entomol. 10:1-12.
Crossref
|
|
|
|
|
Subramanyam B, Hagstrum DW (Eds.). (2012). Alternatives to pesticides in stored-product IPM. Springer Science & Business Media.
|
|
|
|
|
Suleiman RA, Kurt RA (2015). Current maize production, postharvest losses and the risk of mycotoxins contamination in Tanzania. In 2015 ASABE Annual International Meeting. American Society of Agricultural and Biological Engineers. P 1.
|
|
|
|
|
Tefera T, Mugo S, Beyene Y, Karaya H, Gakunga J, and Demissie G (2013). Postharvest insect pest and foliar disease resistance and agronomic performance of new maize hybrids in East Africa.
|
|
|
|
|
Tefera T, Mugo S, Likhayo P (2011). Effects of insect population density and storage time on grain damage and weight loss in maize due to the maize weevil Sitophilus zeamais and the Larger Grain Borer Prostephanus truncatus. Afr. J. Agric. Res. 6(10):2249-2254.
|
|
|
|
|
Tipping PW, Legg DE, Rodriguez JG, Poneleit CG (1988). Influence of maize pericarp surface relief on resistance to the maize weevil (Coleoptera: Curculionidae). J. Kansas Entomol. Society 237-241.
|
|
|
|
|
Togola A, Seck PA, Glitho IA, Diagne A, Adda C, Toure A, Nwilene FE (2013). Economic losses from insect pest infestation on rice stored on-farm in Benin. J. Appl. Sci. 13:278-285.
Crossref
|
|
|
|
|
Wangui KA (2016). Utilization of lighted candle and sealing methods in metal silos for management of the larger grain borer, prostephanus truncatus (horn) (Coleoptera; bostrichidae) in stored maize. (Doctoral dissertation, Department of Plant Science and Crop Protection, University of Nairobi).
|
|
|
|
|
War AR, Murugesan S, Boddepalli VN, Srinivasan R, Nair RM (2017). Mechanism of Resistance in Mungbean [Vigna radiata (L.) R. Wilczek var. radiata] to bruchids, Callosobruchus spp. (Coleoptera: Bruchidae). Front. Plant Sci. 8:1031.
Crossref
|
|
|
|
|
Zunjare R, Hossain F, Muthusamy V, Jha SK Kumar P, Sekhar JC, Gupta HS (2016). Genetic variability among exotic and indigenous maize inbreds for resistance to stored grain weevil (Sitophilus oryzae L.) infestation. Cogent Food Agric. 2(1):1137156.
Crossref
|
|