ABSTRACT
Knowledge on the epidemiology of hepatitis C virus (HCV) has implicit significance for the diagnosis, duration and treatment response of infected patients, as some genotypes are more responsive to therapy than others. In this paper, an examination of the possible association of hepatitis C virus (HCV) genotypes with demographic and risk factors for transmission was described. The study utilized routinely collected data of all persons diagnosed with HCV in Scotland and multivariate logistic regression was used to analyze genetic variability. The genotype variation was roughly distributed among genotypes 1 (45.6%) and 3 (47.9%), though genotype 2 (5.6%) and ‘other’ genotypes (0.89%) were also present. Furthermore, age less than 34 years, year of diagnosis between 2004 and 2008, Greater Glasgow and Clyde, Grampian, Lanarkshire and Lothian Health boards were sufficient to predict HCV status in Scotland, with Injecting Drug Use (IDU) behaviour being the most prevalent risk factor. These results will assist in the management of HCV infection in Scotland.
Key words: Epidemiology, genotypes, hepatitis C virus, risk factor, Scotland.
Hepatitis C Virus (HCV) is a notable public health challenge. Globally, the prevalence rate is reported to be 2 to 3%, with 3-4 million newly infected cases worldwide, and an estimated 130 - 150 million individuals are infected with chronic HCV (Averhoff et al., 2012; Perz et al., 2006; WHO, 2015). The virus belongs to the family of single-stranded RNA viruses of the Flaviviridae family and is the sole member of the Hepacivirus genus (Ansaldi et al., 2014; Smith et al., 2016). At least, seven genotypes (1 to 7) and roughly 84 subtypes (Smith et al., 2014) have been identified. Individuals infected with HCV virus at the acute phase have no obvious symptoms and 15 to 20% spontaneously recover. Nonetheless, nearly 65 to 80% of infected individuals become chronically infected with the possibility of developing acute liver diseases like cirrhosis and hepatocellular carcinoma (HCC) (Davis and Lau, 1997; Lauer and Walker 2001; Seeff, 2002; Jauncey et al., 2004). HCV is the prime cause of hepatic transplantation in liver failure patients in the USA and several Western nations (Bhamidimarri et al., 2017), accounting for 700 000 mortalities per annum, resulting from decompensated cirrhosis or hepatocellular carcinoma (Kanwal et al., 2011; Beste et al., 2015; Benvegnù et al., 2004).
The burden of infection of HCV in the West is established to differ across regions and nations, and is predominantly acquired through serologic surveys from the populace (Averhoff et al., 2012). The highest rate of HCV infection worldwide is in Egypt, estimated at >10%. Similarly, in the United States, like in Australia and other Western European countries, the burden is less, (<2%) (Alter, 2007; Sievert et al., 2011). On the contrary, in Eastern European countries, Latin America, former Soviet Union, certain African countries, Middle East and South Asia, the infection rates are ≥3% (Kershenobich et al., 2011; Qureshi et al., 2010; Madhava et al., 2002; Shepherd et al., 2005).
In advanced countries, injecting drug use (IDU) accounts for the majority of HCV infections (Alter, 2007; Williams et al., 2011). In less developed countries, although IDU is also known to transmit HCV, healthcare-associated practices such as unsafe injections account for the majority of HCV transmission (Alter, 2007; Hauri et al., 2004; Prati, 2006). Across the globe, China tops the countries with the highest numbers of estimated HCV associated IDU infections (Nelson et al., 2011), greater than all of Europe or America (Sievert et al., 2011), while in Western European countries such as Scotland, the high occurrence of IDUs, account for the high proportion of individuals living with HCV in the country. Accordingly, 85 to 90% of all individuals that tested positive to HCV in Scotland have injected drugs (Hutchinson et al., 2006). As at 2006, an estimated one percent of the Scottish population (50 000 persons) were known to be infected (Hutchinson et al., 2006; Roy et al., 2007), whereas it is at least 200 000 cases in UK from 1986 to 2001 (Balogun et al., 2009).
Besides collecting epidemiological information, HCV genotyping and serotyping have clinical implications, and viral genotypes have been identified to predict therapeutic response to antiviral therapy assessed as the level at which HCV RNA is undetected following 24 weeks of treatment (Toyoda et al., 2017; Yee et al., 2015). In addition, the length of time to treat for, and the choice of antiviral agents to initiate are also dependent on genotype (Josephson F, Swedish Consensus Group 2016, Lagging et al., 2001; Yasin et al., 2011). Therefore, the knowledge on HCV infecting genotype is essential in tailoring treatment regimen. The aim of this study was to examine possible associations of hepatitis C virus genotypes with demographic characteristics and risk factors (for transmission) of any individual diagnosed with hepatitis C in Scotland.
Epidemiological and demographic data of all diagnosed population of HCV in Scotland were electronically obtained and collected from the database of Health Protection Scotland (HPS). Data stored on the National Hepatitis C Diagnoses database is expected to be representative of the study population taking into account, the fact that all the health board areas in Scotland are represented. The database was established in affiliation with microbiological laboratories and hospitals across Scotland (Shaw et al., 2003). From the information held on the database, only the genotypes, age group, gender, health board of residence, risk information, year of diagnosis and vital status were extracted for statistical analysis in this study. Information stored in the database has already been anonymised to ensure patient confidentiality and ethical approval for this study was obtained from the University of Glasgow Ethics Committee.
Transformation of the data prior to statistical analysis
In order to arrive at groups of sufficient size for the purpose of statistical analysis, additional groups were either created from the dataset or existing variables were collapsed. For instance, genotypes 4 and 5 were regrouped as ‘others’ while those patients for which genotyping was not carried out were classified as not known. Data on health board area of residence was reordered representing the geographical regions in Scotland such that the North was composed of Highland, Western Isles, Orkney and Shetland; the South was composed of Dumfries and Galloway, Borders, Ayrshire and Arran; and Central Scotland was now made up of Forth valley and Fife. Information on risk factor was broken down into injecting drug use, blood products (comprising of blood haemophiliacs and blood transfusion), and not known (NK) consisting of information from unconfirmed sources such as transmission sexually by contact with an IDU or a partner infected with HCV, tattoo/body piercing, needle stick, bite, blood spillage and perinatal risk. Individuals whose area of residence were not known, the health board area from where the individual’s specimen was obtained was recorded and this occurred especially for prison convicts. A matching process was used in identification via the use of forename initial, Soundex code of surname, date of birth and gender in order to prevent duplication of records.
Statistical analysis
All analysis for this study was carried out using SPSS for Windows XP. Summary measures were utilized to describe the characteristics of the study population. Since the data are categorical, Pearson chi-square (X2) test was used to test for association between genotype and all the other factors listed, with the significance level set at P <0.05. Clustered bar charts were used to show the pictorial representation of the main response variable and other explanatory variables. Furthermore, a multivariate analysis using binary stepwise logistic regression in SPSS was carried out in order to compare HCV genotypes in HCV diagnosed individuals in Scotland by covariates. The multivariate analysis was carried out using a backward stepwise method.
A total of 24419 records were held on the National Hepatitis C Diagnoses Database as at June 30, 2008. Of the HCV diagnosed population held on the National Hepatitis C Diagnoses Database, 73.6% had no information on genotype and were excluded from all the analyses performed. The characteristics of the study population according to genotype are shown in Supplementary Table 1.
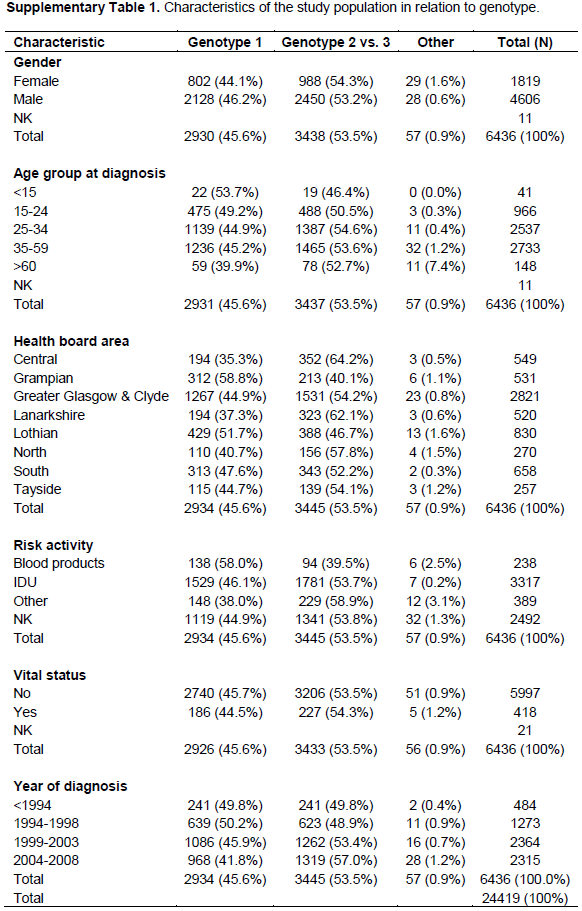
Results obtained indicate that in the study population, the genotype is roughly distributed equally into genotypes 1 and 3 (Figure 1). By gender, 44.1% of females with a known genotype were genotype 1, in comparison with 46.2% among males. For genotype 2, 5.9% were females in comparison with 5.5% males. In addition, 47.9% had a genotype of 3; 48.4% of that were females and 47.7% were males. For the ‘other’ category, there were 0.9% cases of which 1.6% were females and 0.6% males. Figure 1 shows the distribution of gender and HCV genotype. Pearson X2 test comparing these distributions found a statistically significant difference between males and females diagnosed with hepatitis C based on genotype alone (X2= 16.198, P < 0.001).

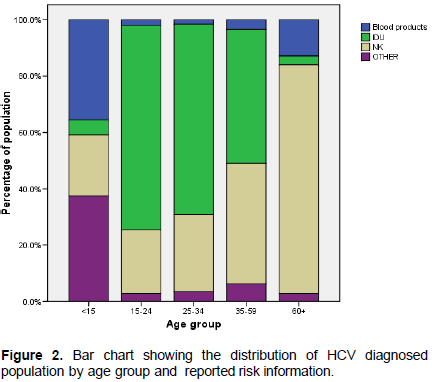
Next, the distribution of HCV diagnosed population by age group and reported risk information was evaluated. As shown in Figure 2, the 25-34 age group had a greater number of HCV diagnosed cases (42.7%), followed by the 35-59 (34.0%) and the 15-24 age groups (18.2%). The age groups with the least number of cases were the 60+ age group (3.3%) and the <15 age group (0.5%). Furthermore, 1.4% of cases had no known age group and was excluded from analyses. The number of injectors in the 25-34 groups was almost twice as much as that found in the other groups and Pearson χ2 found a highly statistically significant difference (χ2 = 3132.375, P<0.001).
Data also revealed that the reported risk information for HCV were: IDU (59.0%), blood products (2.9%), not known (33.8%) and ‘others’ (4.4%) (Figure 3). A high proportion of genotype 1 (58.0%) was found among blood products (which comprise of haemophiliacs and blood transfusions) followed by 32.4% of genotype 3, 7.1% of genotype 2 and 6.0% of ‘others’ modes of transmission. This distribution was found to differ from the other reported risk information all of which were similar to the distribution of the other genotypes. 48.7% of the diagnosed HCV positive individuals that have ever injected drugs were infected with genotype 3, when compared with 46.1% infected with genotype 1. For the ‘other’ group, genotype 3 was more prevalent, 51.7% in comparison with 38.0% of genotype 1. Pearson χ2 test was also found to be significant (χ2= 82.379, P< 0.001).
An assessment of infecting HCV genotype by year of diagnosis was also performed. Data revealed that for genotype 1, the percentage of diagnosed cases fluctuated by year of diagnosis, though there was a steady increase in the diagnoses made for all the other genotypes. Of the diagnosed cases that had a genotype test, 50.2% of genotype 1 were identified in the period between 1994 and 1998, whereas there were more cases of genotype 2, 8.5% before 1994. Diagnoses for the most prevalent genotype 3 were between 2004 and 2008 (51.0%) (Figure 4). Pearson χ2 carried out was significant (χ2= 45.115, P<0.001).
The question, what percentage of the diagnosed HCV population is dead? was asked. This was categorised by vital status. As indicated in Figure 5, the numbers of HCV diagnosed individuals who are not dead were 86.3%, and far outnumber those who are known to be dead (13.3%).
A logistic regression model was set up to identify parameters that are independently associated with HCV genotype. The results for the binary logistic regression model are displayed (Table 1). From the univariate analysis, all the factors were significantly associated with genotype and were therefore included in the model while in the multivariate analysis, gender, age group and vital status did not contribute significantly to the model and were therefore excluded.

Further results identified that in the univariate analysis, women were more likely to be diagnosed with HCV genotypes 2 and 3 than men (odds ratio-OR: 1.232, 95% CI 1.122-1.352,
P<0.001). Furthermore, infecting genotype was not influenced by sex, thus
P >0.05 for both males and females. Patients in the 25-34 years age group (unadjusted OR: 1.194, 95% CI 1.031-1.382,
P=0.018) and the 35-59 years age group (unadjusted OR: 1.162, 95% CI 1.005-1.343,
P=0.043) exhibited a high and statistically significant risk of been diagnosed with HCV 2 and 3 rather than genotype 1 in relation to the <15 and >60 age groups, whereas individuals in the greater than 60 years age group were less likely to be diagnosed with genotype 1 as compared to genotypes 2 and 3, and this result was not statistically significant (OR: 1.296, 95%CI 0.904-1.858,
P=0.158).
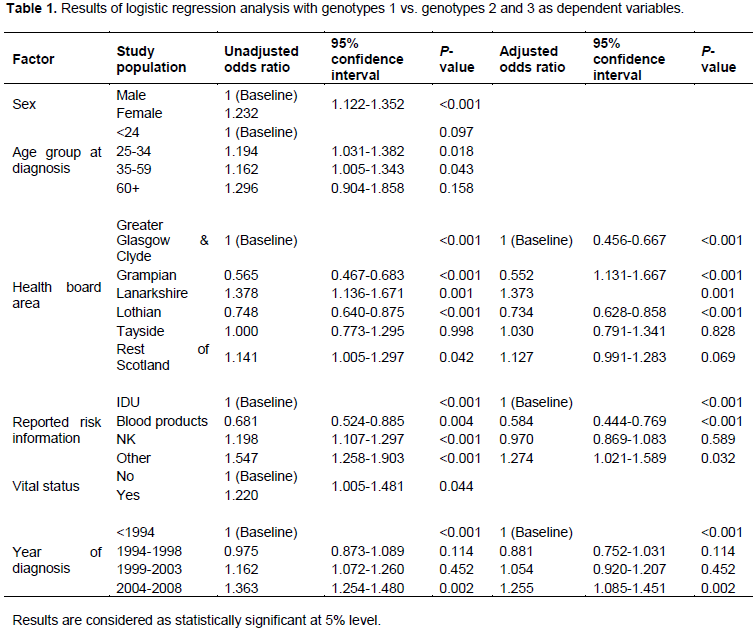
All the health boards of residence posed a great risk of acquiring HCV. However patients residing in the Grampian HB had a lower odds of been diagnosed with genotype 1 than genotypes 2 and 3 (unadjusted OR: 0.565, 95% CI 0.467-0.683, P<0.001; Adjusted OR: 0.552, 0.456-0.667, P<0.001). There are higher odds and a statistically significant association between genotypes 2 and 3 and patients residing in Lanarkshire (unadjusted OR: 1.378, 95%CI 1.136-1.671, P<0.001; Adjusted OR: 1.373, 95%CI 1.131-1.667, P<0.001), while there is a significant association between genotype 1 and patients residing in Lothian (unadjusted OR: 0.748, 95%, CI 0.640-0.875, P<0.001; adjusted OR: 0.734, 95% CI 0.628-0.858, P<0.001). In Tayside, there was no statistically significant association and patients were more likely to be infected with genotypes 2 and 3 than 1 (unadjusted OR: 1.000, 95% CI 0.773-1.295; adjusted OR 1.030, 95% CI 0.791 -1.341, P value = 0.828). There were increased odds for patients residing in the rest of Scotland to be diagnosed with genotypes 2 and 3 than 1. This result was statistically significant in the univariate analysis (unadjusted OR: 1.141, 95%CI 1.005-1.297, P=0.042) but was not significant when adjusted for confounders in the multivariate analysis (adjusted OR: 1.127, 95% CI 0.991-1.283, P=0.069).
Both IDU and blood products carried a significant risk of anti-positive HCV status in the population. However, the lowest odds was found among the blood products and there was a significant association for patients who reported blood products as risk information and genotype 1 (unadjusted OR: 0.681, 95% CI 0.524-0.885, P=0.004; Adjusted OR: 0.584, 95% CI 0.444-0.769, P<0.001). Patients whose risk information was not known were significantly more likely to be infected with genotypes 2 and 3 than genotype 1 (unadjusted OR: 1.198, 95% CI 1.107-1.297, P<0.001), whereas in the multivariate analysis, the result was not statistically significant (adjusted OR: 0.970, 95% CI 0.869-1.083, P=0.589). There was however increased odds of been infected with genotypes 2 and 3 in patients who belonged to the ‘other’ risk category (unadjusted OR: 1.547, 95%CI 1.258-1.903, P <0.001; adjusted OR: 1.274, 95% CI 1.021-1.589, P= 0.032). Patients who were known to be dead were significantly more likely to have been diagnosed with genotypes 1 than 2 and 3 (unadjusted OR: 1.220, 95% CI 1.005-1.481, P=0.044). There was a statistically significant association for patients diagnosed with HCV between 2004 and 2008, to be infected with genotypes 2 and 3 than genotype 1 (unadjusted OR: 1.363, 95%CI 1.254-1.480, P=0.002; adjusted OR: 1.255, 95% CI 1.085-1.451, P=0.002).
These factors, age less than 34, IDU behaviour and blood products, year of diagnosis 2004- 2008 and the Greater Glasgow and Clyde, Grampian, Lanarkshire and Lothian health boards were sufficient to predict HCV status.
In this report, the description of the epidemiology of HCV in Scotland with respect to demographic characteristics and risk factors was carried out. The results obtained showed the most prevalent HCV genotypes as 1 and 3, and this distribution is found to be similar to what is obtained in Europe (Messina et al., 2015; Petruzziello et al., 2016). Data obtained from the Scottish population also revealed a complete absence of genotype 6, which was not surprising as this genotype is restricted to South China and Southeast Asia (Thong et al., 2014; Bunchorntavakul et al., 2013). However, genotype 6 has been identified to have a very low prevalence (0.1%) in Central and Western Europe (Petruzziello et al., 2016).
The percentage of HCV diagnosed males in the Scottish population is twice as much as that for females (Supplementary Table 1) but from the general population, it appears that there are more females to males. This difference could be as a result of more males indulging in risky behaviours like IDU that are known to transmit HCV. Correspondingly, of the participants that were found to attend the Needle Exchange Surveillance Initiative in 2007, less than 25% were females (HPS, UWS, 2008). Recent reports from 2008 to 2010 also suggest that only 28% of females attended the Needle Exchange Surveillance Initiative (HPS, UWS, 2012).
Similarly, although the distribution of HCV genotype is roughly equal among gender, females had a higher proportion (1.6%) of the ‘others’ genotype in comparison with males. However, this group has been found to be prevalent in the Middle East and Africa, especially, in Egypt; however, there is evidence of spread to several European countries possibly due to the transit of persons from endemic regions and also the movement of injectors (Nguyen and Keeffe 2005; Kamal and Naseer, 2008).
The distribution of HCV genotypes varies with the age of individuals in the Scottish population. HCV genotype 3 was predominant in young people <34. The strong association of genotype 3 infections with IDU was found in the population. This may be the reason for the age dependence considering the fact that IDU is common amongst young adults. The increased prevalence of HCV genotype 3 in IDU has been reported from various countries (Wiessing et al., 2014; Salemovic et al., 2017; Ücbïlek et al., 2016) and this is expected, as Scotland is one of the countries in Western Europe that are battling a drug problem.
Most historical infection of HCV infection in the UK was attributed to blood products. Thus genotype 1 particularly, subtype 1a, is found to be associated with hemophiliacs and receipt of blood transfusion (Watson et al., 1996; Harris et al., 1999). Apparently, IDU is the major source of acquiring HCV in the United Kingdom with an increased likelihood of transmission through sharing of injecting paraphernalia (Balogun et al., 2003; Palmateer et al., 2014). Tattooing has also been revealed to increase the chances of acquiring HCV and this is more frequent in the community (Carney et al., 2013; Tohme and Holmberg, 2012). Genotype 1 accounted for 58.0% in the blood products category and this coincides with 53.7% in the 15 years age group where blood products alone accounted for 35.7%. All these occurred in the 1994 diagnosis when Scotland had not introduced the treatment of clotting factor by heat for hemophiliacs, as such, many patients with clotting disorder were at increased risk of contracting HCV (Lowe 1987). Furthermore, it is possible that such people have transfused blood or blood products abroad (Patel et al., 2006). There was a significant decrease in the percentage of people contracting HCV by blood products from 19.8% in 1994 to 1.1% in 2004-2008. This suggests therefore that genotype 1 is more likely associated with blood products in the Scottish population than any other genotypes. The association between HCV and blood transfusion is likely to diminish with time due to a decreasing number of patients who received transfusion before blood screening and improved diagnostic methods. In comparison with the other genotypes, there is evidence to suggest that genotype 1 is correlated with a higher level of cirrhosis or chronic active hepatitis and this is particularly worrying because a large proportion of this genotype was seen among Scottish HCV diagnosed population (Harris et al., 2007; Dusheiko et al., 1994). Multivariate analysis revealed a statistically significant risk related to blood transfusion.
Treatment of patients who are chronically infected together with the rollover of preventive programs will assist in the reduction of HCV infection. It is thus essential to continually monitor the genotype distribution
of HCV in order to identify drug-resistant genotypes and risk factors for transmission.
The author has not declared any conflict of interests.
This work was carried out as part of Master of Public Health at the University of Glasgow. The author recognizes the contributions of David Goldberg and Allan McLeod by providing guidance and supervision, and also granting access to the hepatitis C virus database. The author is also grateful to Harper Gilmour, who provided assistance with regards to data analysis. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sector.
REFERENCES
|
Alter MJ (2007). Epidemiology of hepatitis C virus infection. World Journal of Gastroenterology, 13:2436-2441.
Crossref
|
|
|
|
Ansaldi F, Orsi A, Sticchi L, Bruzzone B, Icardi G (2014). Hepatitis C virus in the new era: perspectives in epidemiology, prevention, diagnostics and predictors of response to therapy. World Journal of Gastroenterology, 20(29):9633-9652.
Crossref
|
|
|
|
|
Averhoff FM, Glass N, Holtzman D (2012). Global Burden of Hepatitis C: Considerations for Healthcare Providers in the United States. Clinical Infectious Diseases, 55 Suppl 1:S10-15. doi: 10.1093/cid/cis361.
Crossref
|
|
|
|
|
Balogun MA, Murphy N, Nunn S, Grant A, Andrews NJ, Teo CG, Ramsay ME, Parry JV (2009). Prevalence and incidence of hepatitis C in injecting drug users attending genitourinary medicine clinics. Epidemiology and Infection 137:980-987.
Crossref
|
|
|
|
|
Balogun MA, Ramsay ME, Parry JV, Donovan L, Andrews NJ, Newham JA, McGarrigle C, Harris KA, Teo CG (2003). A national survey of genitourinary medicine clinic attenders provides little evidence of sexual transmission of hepatitis C virus infection. Sexually Transmitted Infections 79(4):301-306.
Crossref
|
|
|
|
|
Benvegnù L, Gios M, Boccato S, Alberti A (2004). Natural history of compensated viral cirrhosis: a prospective study on the incidence and hierarchy of major complications. Gut 53(5):744-749.
Crossref
|
|
|
|
|
Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN (2015). Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology, 149(6):1471-1482.
Crossref
|
|
|
|
|
Bhamidimarri KR, Satapathy SK, Martin P (2017). Hepatitis C Virus and Liver Transplantation. Journal of Gastroenterology and Hepatology, 13(4):214-220.
|
|
|
|
|
Bunchorntavakul C, Chavalitdhamrong D, Tanwandee T (2013). Hepatitis C genotype 6: A concise review and response-guided therapy proposal. World Journal of Hepatology, 5(9):496-504.
Crossref
|
|
|
|
|
Carney K, Dhalla S, Aytaman A, Tenner CT, Francois F (2013). Association of tattooing and hepatitis C virus infection: a multicenter case-control study. Hepatology, 57(6):2117-2123.
Crossref
|
|
|
|
|
Davis GL, Lau JY (1997). Factors predictive of a beneficial response to therapy of hepatitis C. Hepatology, 26(3 Suppl 1):122S-127S.
Crossref
|
|
|
|
|
Dusheiko G, Schmilovitz-Weiss H, Brown D, McOmish F, Yap PL, Sherlock S, McIntyre N, Simmonds P (1994). Hepatitis C virus genotypes: an investigation of type-specific differences in geographic origin and disease. Hepatology 19(1):13-18.
Crossref
|
|
|
|
|
Harris HE, Eldridge KP, Harbour S, Alexander G, Teo CG, Ramsay ME, HCV National Register Steering Group (2007). Does the clinical outcome of hepatitis C infection vary with the infecting hepatitis C virus type? Journal of Viral Hepatitis, 14(3):213-220.
Crossref
|
|
|
|
|
Harris KA, Gilham C, Mortimer PP, Teo CG (1999). The most prevalent hepatitis C virus genotypes in England and Wales are 3a and 1a. Journal of Medical Virology 58(2):127-31.
Crossref
|
|
|
|
|
Hauri AM, Armstrong GL, Hutin Y (2004). The global burden of disease attributable to contaminated injections given in health care settings. International Journal of STD & AIDS, 15:7-16.
Crossref
|
|
|
|
|
Health Protection Scotland (HPS), University of the West of Scotland (UWS) (2008) The Needle Exchange Surveillance Initiative (NESI):
|
|
|
|
|
Health Protection Scotland (HPS), University of the West of Scotland (UWS) (2012) Needle Exchange Surveillance Initiative (NESI): Prevalence of HCV and injecting risk behaviours among people who
|
|
|
|
|
Hutchinson SJ, Roy KM, Wadd S, Bird SM, Taylor A, Anderson E, Shaw L, Codere G, Goldberg DJ (2006). Hepatitis C Virus in Scotland: epidemiological review and public health challenges. Scottish Medical Journal 51(2):8-15.
Crossref
|
|
|
|
|
Jauncey M, Micallef JM, Gilmour S, Amin J, White PA, Rawlinson W, Kaldor JM, van Beek I, Dore GJ (2004). Clearance of hepatitis C virus after newly acquired infection in injection drug users. The Journal of Infectious Diseases 190(7):1270-1274.
Crossref
|
|
|
|
|
Josephson F, Swedish Consensus Group (2016). Treatment of hepatitis C virus infection for adults and children: Updated Swedish consensus recommendations. Infectious Diseases (Lond) 48(4):251-261
Crossref
|
|
|
|
|
Kamal SM, Naseer IA (2008). Hepatitis C Genotype 4: What we know and don't yet know (Reviews). Hepatology, 47(4).
Crossref
|
|
|
|
|
Kanwal F, Hoang T, Kramer JR, Asch SM, Goetz MB, Zeringue A, Richardson P, El–Serag HB (2011). Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology 140(4):1182-1188. doi: 10.1053/j.gastro.2010.12.032.
Crossref
|
|
|
|
|
Kershenobich D, Razavi HA, Sánchezâ€Avila JF, Bessone F, Coelho HS, Dagher L, Gonçales FL, Quiroz JF, Rodriguezâ€Perez F, Rosado B, Wallace C (2011). Trends and projections of hepatitis C virus epidemiology in Latin America. Liver International, 31 Suppl 2:18-29.
Crossref
|
|
|
|
|
Lagging M, Wejstål R, Norkrans G, Karlström O, Aleman S, Weiland O, Castedal M, Lauer GM, Walker BD (2001). Hepatitis C virus infection. New England Journal of Medicine 345(1):41-52.
Crossref
|
|
|
|
|
Lowe GD (1987). Haemophilia, blood products and HIV infection. Scottish Medical Journal 32(4):109-11.
Crossref
|
|
|
|
|
Madhava V, Burgess C, Drucker E (2002). Epidemiology of chronic hepatitis C virus infection in sub-Saharan Africa. The Lancet Infectious Diseases 2:293-302.
Crossref
|
|
|
|
|
Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, Barnes E (2015). Global distribution and prevalence of hepatitis C virus genotypes. Hepatology 61(1):77-87.
Crossref
|
|
|
|
|
Nelson PK, Mathers BM, Cowie B, Hagan H, Des Jarlais D, Horyniak D, Degenhardt L (2011). Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet 378:571-583.
Crossref
|
|
|
|
|
Nguyen MH, Keeffe EB (2005). Prevalence and treatment of hepatitis C virus genotypes 4, 5 & 6. Clinical Gastroenterology and Hepatology 3 Suppl 2: S97-S101.
Crossref
|
|
|
|
|
Palmateer NE, Taylor A, Goldberg DJ, Munro A, Aitken C, Shepherd SJ, McAllister G, Gunson R, Hutchinson SJ (2014). Rapid decline in HCV incidence among people who inject drugs associated with national scale-up in coverage of a combination of harm reduction interventions. PLoS One 11;9(8):e104515. doi: 10.1371/journal.pone. 0104515.
|
|
|
|
|
Patel K, Muir AJ, McHutchison JG (2006). Diagnosis and treatment of chronic hepatitis C infection. British Medical Journal 332(7548):1013-1017.
Crossref
|
|
|
|
|
Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP (2006). The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. Journal of Hepatology 45(4):529-38. https://doi.org/10.1016/j.jhep.2006.05.013.
Crossref
|
|
|
|
|
Petruzziello A, Marigliano S, Loquercio G, Cacciapuoti C (2016). Hepatitis C virus (HCV) genotypes distribution: an epidemiological up-date in Europe. Infectious Agents and Cancer (1):53.
Crossref
|
|
|
|
|
Prati D (2006). Transmission of hepatitis C virus by blood transfusions and other medical procedures: a global review. Journal of Hepatology 45:607-616.
Crossref
|
|
|
|
|
Qureshi H, Bile KM, Jooma R, Alam SE, Afridi HUR (2010). Prevalence of hepatitis B and C viral infections in Pakistan: findings of a national survey appealing for effective prevention and control measures. Eastern Mediterranean Health Journal 16 suppl: S15-23.
Crossref
|
|
|
|
|
Roy KM, Hutchinson SJ, Wadd s, Taylor A, Cameron SO, Burns S, Molyneaux P, McIntyre PG, Goldberg DJ (2007). Hepatitis C virus infection among injecting drug users in Scotland: a review of prevalence and incidence data and the methods used to generate them. Epidemiology and Infection 135(3):433-442.
Crossref
|
|
|
|
|
Salemovic D, Pesic-Pavlovic I, Jevtovic D, Bojovic K, Ranin J, Brmbolic B, Stanojevic M (2017). Intravenous drug use - an independent predictor for HCV genotypes 3 and 4 infection among HIV/HCV co-infected patients. Archives of Medical Science 13(3):652-658
Crossref
|
|
|
|
|
Seeff LB (2002). Natural history of chronic hepatitis C. Hepatology 36(5 Suppl 1):S35-46.
|
|
|
|
|
Shaw L, Taylor A, Roy KM, Cameron SO, Burns S, Molyneaux, McIntyre P, Codere G, Goldberg DJ (2003). Establishment of a database of a diagnosed HCV-infected persons in Scotland. Communicable Disease and Public Health 6:305-310.
|
|
|
|
|
Shepard CW, Finelli L, Alter MJ (2005). Global epidemiology of hepatitis C virus infection. The Lancet Infectious Diseases 5(9):558-67.
Crossref
|
|
|
|
|
Sievert W, Altraif I, Razavi HA, Abdo A, Ahmed EA, AlOmair A, Amarapurkar D, Chen CH, Dou X, El Khayat H, Elshazly M (2011). A systematic review of hepatitis C virus epidemiology in Asia, Australia and Egypt. Liver International 31(s2):61-80. doi:10.1111/j.1478-3231.2011.02540.x.
Crossref
|
|
|
|
|
Smith DB, Becher P, Bukh J, Gould EA, Meyers G, Monath T, Muerhoff AS, Pletnev A, Rico-Hesse R, Stapleton JT, Simmonds P (2016). Proposed update to the taxonomy of the genera Hepacivirus and Pegivirus within the Flaviviridae family. Journal of General Virology 97(11):2894-2907.
Crossref
|
|
|
|
|
Smith DB, Bukh J, Kuiken C, Muerhoff AS, Rice CM., Stapleton JT., et al (2014). Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology 59: 318-327.
Crossref
|
|
|
|
|
Thong VD, Akkarathamrongsin S, Poovorawan K, Tangkijvanich P, Poovorawan Y. Hepatitis C virus genotype 6: virology, epidemiology, genetic variation and clinical implication (2014). World Journal of Gastroenterology 20(11):2927-2940.
Crossref
|
|
|
|
|
Tohme RA, Holmberg SD (2012). Transmission of hepatitis C virus infection through tattooing and piercing: a critical review. Clinical Infectious Diseases 54(8):1167-78.
Crossref
|
|
|
|
|
Toyoda H, Kumada T, Tada T, Yama T, Mizuno K (2017). Significance of day-1 viral response of hepatitis C virus in patients with chronic hepatitis C receiving direct- acting antiviral therapy. Journal of Gastroenterology and Hepatology 33(6):1264-1270.
Crossref
|
|
|
|
|
Watson JP, Brind AM, Chapman CE, Bates CL, Gould FK, Johnson SJ, Burt AD, Ferguson J, Simmonds P, Bassendine MF (1996). Hepatitis C virus: epidemiology and genotypes in the north east of England. Gut 38(2):269-276.
Crossref
|
|
|
|
|
Wiessing L, Ferri M, Grady B, Kantzanou M, Sperle I, Cullen KJ, Hatzakis A, Prins M, Vickerman P, Lazarus JV, et al (2014). Hepatitis C virus infection epidemiology among people who inject drugs in Europe: a systematic review of data for scaling up treatment and prevention. PLoS One.9:e103345.
Crossref
|
|
|
|
|
Williams IT, Bell BP, Kuhnert W, Alter MJ (2011). Incidence and transmission patterns of acute hepatitis C in the United States, 1982–2006. Archives of Internal Medicine 171:242-248.
Crossref
|
|
|
|
|
World Health Organization (2015). Hepatitis C Fact sheet N°164. Available online at http://www.who.int/en/news-room/fact-sheets/detail/hepatitis-c.
|
|
|
|
|
Yasin T, Riley TR, Schreibman IR (2011). Current treatment of choice for chronic hepatitis C infection. Infection and Drug Resistance 4:11-18.
|
|
|
|
|
Yee BE, Nguyen NH, Zhang B, Lin D, Vutien P, Wong CR, Lutchman GA, Nguyen MH (2015). Sustained virological response and its treatment predictors in hepatitis C virus genotype 4 compared to genotypes 1, 2, and 3: a meta-analysis. BMJ Open Gastroenterology, 2(1):e000049.
Crossref
|
|