Full Length Research Paper
ABSTRACT
Phytochemicals are chemical compounds formed during the plants normal metabolic processes. These chemicals are often referred to as secondary metabolites. The objective of this research was to determine the chemical composition of leaves extract from methanol. The phytochemical compound screened by gas chromatography-mass spectrometry (GC-MS) method. Fifteen bioactive phytochemical compounds were identified in the methanolic extract of Urtica dioica. The identification of phytochemical compounds is based on the peak area, retention time molecular weight, molecular formula, mass spectrometry (MS) fragment-ions and pharmacological actions. GC-MS analysis of U. dioica revealed the existence of the Oxime- methoxy-phenyl, 2, 6,-Nonadienal, 3, 7-dimethyl, 1, 2, 3-Butanetriol, Silane, triethyl(2-phenylethoxy), Benzofuran, 2,3,-dihydro, 2,5,5,8a-Tetramethyl-1,2,3,5,6,7,8, 8a-octahydronaphthalen-1-ol, 2H-Indeno[1,2-b]furan-2-one, 3,3a, 4,5,6,7,8, 8b-octahydro-8,8-dimet, 1-Dodecanamine, N, N-dimethyl, 2(3H)-Naphthalenone, 4, 4a,5,6,7,8-hexahydro-1-methoxy, D-Fructose, diethyl mercaptal, pentaacetate, [1,1-Bicyclopropyl-2-octanoic acid 2hexyl-methyl ester, Estra-1,3,5(10)-trien-17B-ol, Cyclopropaneoctanoic acid, 2-[2-pentylcycloproyl)methyl]-methyl, 1-Hydroxy-2-(2,3,4,6-tetra-O-acetyl-beta-d-glucopyranosyl)-9H-xanthe and Ethyl iso- allochlate. The FTIR analysis of U. dioica leaves proved the presence of aromatic rings, alkenes, aliphatic fluoro, alcohols, ethers, carboxlic acids, esters, nitro compounds, hydrogen bonded alcohols and phenols. It contain chemical constitutions which may be useful for various herbal formulation as anti-inflammatory, analgesic, antipyretic, cardiac tonic and antiasthamatic.
Key words: GC-MS analysis, fourier-transform infrared, phytochemicals, Urtica dioica.
INTRODUCTION
Phytochemicals are defined as bioactive non-nutrient plant compounds in fruits, vegetables, grains, and other plant foods that have been linked to reducing the risk of major chronic diseases (Hai, 2004; Magee and Rowland, 2004; Altameme et al., 2015; Hameed et al., 2015a). General description of Urtica dioica erect perennial, 50 to 300 cm tall with 4-sided stems, armed with stinging hairs, opposite leaves, 7 to 15 cm long, the stalks from about 1/10 as long to nearly 1/2 as long as the blades, depending on variety. The stipules prominent, mostly 10 to 15 mm long. Fruits are achenes, lens-shaped, flattened, about 1.5 mm long, enclosed by the 2 inner sepals. U. dioica has many hollow stinging hairs called trichomes on its leaves and stems, which act like hypodermic needles that inject histamine and other chemicals that produce the stinging sensation when contacted by humans and other animals (Kavalali, 2003; Petlevski et al., 2003; Gulcin, 2004).
The other compounds isolated are derivatives of the terpenoids previously isolated from the roots and flowers of U. dioica (Gozum et al., 2003; Luo, 2009), and they include stigmasterol derivative, sitosterol derivative and ethyl cholestanol (Belyakova et al., 2002; Benkeblia, 2004; Golalipour et al., 2009).
This study aims to analyze the chemical compounds of U. dioica leaves by fourier-transform infrared (FT-IR) spectroscopy and gas chromatography-mass spectrometry (GC-MS).
MATERIALS AND METHODS
Collection and preparation of plant material
The leaves were dried at room temperature for seven days and when properly dried then powdered using clean pestle and mortar, and the powdered plant was size reduced with a sieve (Hameed et al., 2015). The fine powder was then packed in airtight container to avoid the effect of humidity and then stored at room temperature (Hussein et al., 2015).
Preparation of sample
About 9 g of the plant sample powdered were soaked in 100 ml methanol individually. It was left for 72 h so that alkaloids, flavonoids and other constituents if present will get dissolved. The methanol extract was filtered using Whatman’s No.1 filter paper and the residue was removed (Jasim et al., 2015).
Gas chromatography-mass spectrum analysis
The GC-MS analysis of the plant extract was made in a (Agilent 7890 A) instrument under computer control at 70 eV. About 1 μL of the methanol extract was injected into the GC-MS using a micro syringe and the scanning was done for 45 min. As the compounds were separated, they eluted from the column and entered a detector which was capable of creating an electronic signal whenever a compound was detected (Mohammed and Imad, 2013; Kareem et al., 2015; Imad et al., 2014). The greater the concentration in the sample, bigger was the signal obtained which was then processed by a computer. The time from when the injection was made (Initial time) to when elution occurred is referred to as the retention time (RT). While the instrument was run, the computer generated a graph from the signal called chromatogram. Each of the peaks in the chromatogram represented the signal created when a compound eluted from the gas chromatography column into the detector. The X-axis showed the RT and the Y-axis measured the intensity of the signal to quantify the component in the sample injected. As individual compounds eluted from the gas chromatographic column, they entered the electron ionization (mass spectroscopy) detector, where they were bombarded with a stream of electrons causing them to break apart into fragments. The fragments obtained were actually charged ions with a certain mass .The mass/charge (M/Z) ratio obtained was calibrated from the graph obtained, which was called the Mass spectrum graph which is the fingerprint of a molecule (Imad et al., 2014).
Before analyzing the extract using GC-MS, the temperature of the oven, the flow rate of the gas used and the electron gun were programmed initially. The temperature of the oven was maintained at 100°C. Helium gas was used as a carrier as well as an eluent. The flow rate of helium was set to 1 ml per min. The electron gun of mass detector liberated electrons having energy of about 70eV. The column employed here for the separation of components was Elite 1 (100% dimethyl poly siloxane) (Imad et al., 2014). The identity of the components in the extracts was assigned by the comparison of their retention indices and mass spectra fragmentation patterns with those stored on the computer library and also with published literatures.
RESULTS AND DISCUSSION
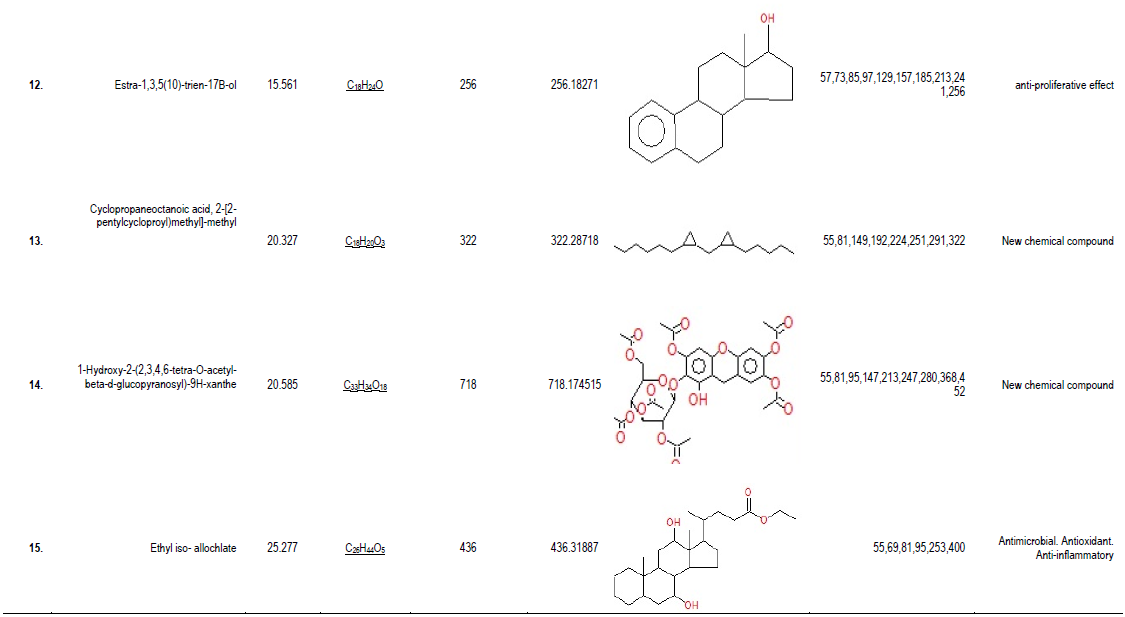
GC-MS analysis of compounds was carried out in methanolic leaves extract of U. dioica, as shown in Table 1. The GC-MS chromatogram of the 15 peaks of the compounds detected was shown in Figure 1. Chromatogram GC-MS analysis of the methanol extract of U. dioica showed the presence of fifteen major peaks and the components corresponding to the peaks were determined as follows. The first set up peak was determined to be Oxime- methoxy-phenyl (Figure 2). The second peak indicated to be 2, 6,-Nonadienal, 3, 7-dimethyl (Figure 3). The next peaks considered to be 1, 2, 3-Butanetriol, Silane, triethyl(2-phenylethoxy), Benzofuran, 2,3,-dihydro, 2,5,5,8a-Tetramethyl-1,2,3,5,6,7,8, 8a-octahydronaphthalen-1-ol, 2H-Indeno[1,2-b]furan-2-one, 3,3a, 4,5,6,7,8, 8b-octahydro-8,8-dimet, 1-Dodecanamine, N, N-dimethyl, 2(3H)-Naphthalenone, 4, 4a,5,6,7,8-hexahydro-1-methoxy, D-Fructose, diethyl mercaptal, pentaacetate, [1,1-Bicyclopropyl-2-octanoic acid 2hexyl-methyl ester, Estra-1,3,5(10)-trien-17B-ol, Cyclopropaneoctanoic acid, 2-[2-pentylcycloproyl)methyl]-methyl, 1-Hydroxy-2-(2,3,4,6-tetra-O-acetyl-beta-d-glucopyranosyl)-9H-xanthe and Ethyl iso- allochlate. (Figure 4-16).
.png)
The FTIR analysis of U. dioica leaves proved the presence of aromatic rings, alkenes, aliphatic fluoro, alcohols, ethers, carboxlic acids, esters, nitro compounds, hydrogen bonded alcohols and phenols which shows major peaks at 891.11, 958.69, 1010.70, 1091.71, 1242.16, 1319.31, 2686.84 and 3363.86 (Table 2; Figure 17). Polar extract of the U. dioica contains lignans +)-neoolivil, (-)-secoisolariciresinol, Dehydrodiconiferyl alcohol, isolariciresinol, pinoresinol, and 3,4divanillyltetrahydrofuran, and has antiinflammatory effects and stimulates the proliferation of human lymphocytes (Obertreis et al.,1996; Harput et al.,2005; Kanter et al., 2005; Hameed et al., 2015c). Traditionally, it has been used for uterine hemorrhage, cutaneous eruption, infantile and psychogenic eczema, epistaxis, and melena and specifically for nervous eczema (Bandow et al., 2003; Burt, 2004; Banso and Adeyemo, 2006). Among those identified, phytocompounds have the property of antioxidant and antimicrobial activities (Silva et al., 2004; Sein et al., 2008). Plant based antimicrobials have enormous therapeutic potential as they can serve the purpose with lesser side effects. Continued further exploration of plant derived antimicrobials is needed today.
CONCLUSION
U. dioica is native plant of Iraq. It contains chemical constitutions which may be useful for various herbal formulation as anti-inflammatory, analgesic, antipyretic, cardiac tonic and antiasthamatic properties.
ACKNOWLGEMENT
The authors wish to express their deepest gratitude to Prof. Dr. Adul-Kareem for his valuable contributions and support throughout this study. They would also like to ex-press their gratitude to Dr. Ali for his valuable suggestions and comments.
CONFLICTS OF INTEREST
The authors have none to declare.
REFERENCES
|
Altameme HJ, Hameed IH, Kareem MA (2015). Analysis of alkaloid phytochemical compounds in the ethanolic extract of Datura stramonium and evaluation of antimicrobial activity. Afr. J. Biotechnol.14(19):1668-1674. |
|
|
Bandow JE, Brotz H, Leichert L (2003). Proteomic approach to understanding antibiotic action. Antimicrob. Agents Chemother. 47:948-955. |
|
|
Banso A, Adeyemo S (2006). Phytochemical screening and antimicrobial assessment of Abutilon mauritianum, Bacopamonnifera and Daturastr-amonium. Biochemistry 18(1):39-44. |
|
|
Belyakova VA, Vainshtein KV, Markova Y, Demchenko T, Chibilyaev TH (2002). Extraction of nettle leaves using synthetic esters of fatty acids. Pharm. Chem. J. 39(11):598-602. |
|
|
Benkeblia N (2004). Antimicrobial activity of essential oil extracts of various onions (Allium cepa) and garlic (Allium sativum). Lebensm-Wiss u-Technol. 37:263-268. |
|
|
Burt S (2004). Essentials oils: their antibacterial properties and potential applications in food: a review. Int. J. Food Microbiol. 94:254-259. |
|
|
Golalipour MJ, Ghafari S, Farsi MM (2009). Effect of Urtica dioica extract on quantitative morphometric alterations of liver paranchymal cells in STZ diabetic rats. Int. J. Morphol. 27(4):1339-1344. |
|
|
Gozum S, Tezel A, Koc M (2003). Complementary alternative treatments used by patients with cancer in eastern Turkey. Cancer Nurs. 26:230–236 |
|
|
Gulcin I (2004). Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J. Ethnopharmacol. 90:205-215. |
|
|
Hai LR (2004). Potential Synergy of Phytochemicals in Cancer Prevention: Mechanism of Action. J. Nutr. 134:3479-3485. |
|
|
Hameed IH, Hussein HJ, Kareem MA, Hamad NS (2015a). Identification of five newly described bioactive chemical compounds in methanolic extract of Mentha viridis by using gas chromatography-mass spectrometry (GC-MS). J. Pharmacogn. Phytother. 7(7):107-125. |
|
|
Hameed IH, Ibraheam IA, Kadhim HJ (2015b). Gas chromatography mass spectrum and fourier-transform infrared spectroscopy analysis of methanolic extract of Rosmarinus oficinalis leaves. J. Pharmacogn. Phytother. 7 (6):90-106. |
|
|
Hameed IH, Jasim H, Kareem MA, Hussein AO (2015c). Alkaloid constitution of Nerium oleander using gas chromatography-mass spectroscopy (GC-MS). J. Med. Plants Res. 9(9):326-334. |
|
|
Harput S, Saracoglu I, Ogihara Y (2005). Stimulation of Lymphocyte Proliferation and Inhibition of Nitric Oxide Production by Aqueous Urtica dioica Extract U. Phytother. Res.19:346–348. |
|
|
Hussein AO, Hameed IH, Jasim H, Kareem MA (2015). Determination of alkaloid compounds of Ricinus communis by using gas chromatography-mass spectroscopy (GC-MS). J. Med. Plants Res. 9(10):349-359. |
|
|
Imad H, Mohammed A, Aamera J (2014a). Genetic variation and DNA markers in forensic analysis. Afr. J. Biotechnol. 13(31):3122-3136. |
|
|
Imad H, Mohammed A, Cheah Y, Aamera J (2014b) Genetic variation of twenty autosomal STR loci and evaluate the importance of these loci for forensic genetic purposes. Afr. J. Biotechnol. 13:1-9. |
|
|
Imad H, Muhanned A, Aamera J, Cheah Y (2014c). Analysis of eleven Y-chromosomal STR markers in middle and south of Iraq. Afr. J. Biotechnol. 13(38):3860-3871. |
|
|
Jasim H, Hussein AO, Hameed IH, Kareem MA (2015). Characterization of alkaloid constitution and evaluation of antimicrobial activity of Solanum nigrum using gas chromatography mass spectrometry (GC-MS). J. Pharmacogn. Phytother. 7(4):56-72. |
|
|
Kanter M, Coskun O, Budancamanak M (2005). Hepatoprotective effects of Nigella sativa L. and Urtica dioica L. on lipid peroxidation, antioxidant enzyme systems and liver enzymes in carbon tetrachloride-treated rats. World J. Gastroenterol. 42:6684–6688. |
|
|
Kareem MA, Hussein AO, Hameed IH (2015). Y-chromosome short tandem repeat, typing technology, locus information and allele frequency in different population: A review. Afr. J. Biotechnol. 14(27):2175-2178. |
|
|
Kavalali G (2003). Hypoglycemic activity of Urtica pilulifera in streptozotocin-diabetic rats. J. Ethnopharmacol. 84(2-3):241-5. |
|
|
Luo JR (2009). Paleophytochemical components from Miocene-Fossil wood of Pinus griffithii. J. Chin. Chem. Soc. 56(3):600-605. |
|
|
Magee PJ, Rowland IR (2004). Phyto-oestrogens, their mechanism of action: current evidence for a role in breast and prostate cancer. Brit. J. Nutr. 91:513-531. |
|
|
Mohammed A, Imad H (2013). Autosomal STR: From locus information to next generation sequencing technology. Res. J. Biotechnol. 8(10):92-105. |
|
|
Obertreis B, Ruttkowski T, Teucher T, Behnke B (1996). Ex-vivo in-vitro inhibition of lipopolysaccharide stimulated tumor necrosis factor-alpha and interleukin-1 beta secretion in human whole blood by extractum Urticae dioicae foliorum. Arzneimittelforschung 46(9):936. |
|
|
Petlevski R, Hadzija M, Slijepcević M, Juretić D, Petrik J (2003). Glutathione S-transferases and malondialdehyde in the liver of NOD mice on short-term treatment with plant mixture extract. Phytother. Res. 17(4):311-4. |
|
|
Sein TT, Spurio R, Cecchini C, Cresci A (2008). Screening for microbial strains degrading glass fiber acrylic composite filters. Int. Biodeterior. Biodegrad. 63:901–905. |
|
|
Silva BM, Andrade PB, Valentão P, Ferreres F, Seabra RM, Ferreira MA (2004). Quince (Cydonia oblonga Miller) fruit (pulp, peel, and seed) and jam: antioxidant activity. J. Agric. Food Chem. 52:4405–4712. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0