ABSTRACT
A comparative study of the volatile terpene fraction isolated from the leaves of Eucalyptus camaldulensis attacked by the gall wasp (Leptocybe invasa) and from the healthy leaves of the plant was carried out using gas chromatography-mass spectrometry. A total of 59 components representing 89.13 and 88.6%, respectively, of their total volatile fraction contents were analyzed. Of these volatiles, 26 compounds with concentrations greater than (0.1± 0.02%) have been used for statistical comparison. A number of 21of these compounds were identified, in different concentrations, in the leaves volatile fraction of both healthy plants and plants attacked by the wasps. The other 5 volatiles: p-mentha-2-4(8) diene, δ-elemene, β-elemene, E-caryophyllene and bicyclogermacrene, were exclusively found in the volatile fraction of the attacked leaves. The newly produced compounds in the attacked leaves or the change in concentration of those commonly found in that fraction, could be part of the plant defense mechanisms, or/and an element of the plant allelopathic and communication mechanisms. Identifying the components of the gall wasp-damaged leaves can help in their recycling for different physiological, pharmacological and medicinal uses.
Key words: Eucalyptus, gall wasp, plant defense mechanisms, plant recycling, terpene fraction.
Eucalyptus camaldulensis (Myrtaceae) (Figure 1) is one of the best known species of the genus Eucalyptus in the Middle East, its importance relay on the high production of its unique volatiles composition that exhibits medicinal and pharmacological activity. The volatile constituents in Eucalyptus have been extensively studied (Zrira et al., 2004; Barra et al., 2010; Elaissi et al., 2011, 2012; Akolade et al., 2012). All these investigations revealed the major constituents to be qualitatively more or less the same; however, quantitatively they are very different. The minor constituents show qualitative and quantitative variation depending on the soil, climate and degree of maturity (Giamakis et al., 2001; Ben Marzoug et al., 2010). Generally, 1,8-cineole is one of the most abundant and unique volatile constituent in Eucalyptus. However, in E. camaldulensis, the aromatic cyclic monoterpene p-cymene is more abundant than the 1,8-cineole (Begum et al., 2000; Barra et al., 2010). E. camaldulensis offers a broad spectrum of physiological, pharmacological and medicinal properties, which are ascribed to its volatile constituents that enable the use of E. camaldulensis oil as antimicrobial and antifungal (Sartorelli et al., 2007; Barra et al., 2010; Gilles et al., 2010; Panahi et al., 2011), antioxidant (Akolade et al., 2012), antispasmodic (Begum et al., 2000), inseticide, larvicide, pesticide and antimalarial (Batish et al., 2008; Cheng et al., 2009; Alzogaray et al., 2011)agent.

The gall forming wasp (Leptocybe invasa Fisher and La Salle, Hymenoptera: Eulophidae), commonly referred to as the blue gum chalcid, has recently come into sight as the main pest attacking E. camaldulensis and causing severe injury. It was first identified in Northern and Eastern Africa, the Middle East and Mediterranean countries, then the pest has expanded to all over the world (Mendel et al., 2004). The wasp lays its eggs in the petiole and midrib of leaves and stems of young shoots, which leads to gall formation. Gall formation by L. invasa damages the growing shoot and leaves of Eucalyptus, resulting in the abscission of leaves and drying up of shoots (Karunaratne et al., 2010), as shown in Figure 1. Because L. invasa completes its development within the gall, chemical control is not applicable. Possible control measures would include breeding of less susceptible Eucalyptus species as well as biological control represented by the natural enemies of the wasp (Kelly et al., 2012).
Plant defense mechanisms protect the plant against any biotic or abiotic attacks. One of the most common plant defense mechanism is the production of specific secondary metabolites. These chemicals can either have an anti-microbial, antifungal or insecticidal activity that can affect the attacking organism, or allelochemical characteristics, thus spreading and allowing communication mechanisms with other parts of the plant or with the adjacent plants to warn against the wasp attack. Most of these allelopathic chemicals belongs to mono-or-sesqui-terpene type of secondary metabolites and are located in the volatile components of the plant metabolome (Henery et al., 2008; Troncoso et al., 2011). These compounds are made either constitutively in low concentration (constitutive defense mechanisms), or as a response to an attack on the plant (induced defense mechanism). In constitutive defense, secondary metabolites are produced and their biosynthesis is increased in case of attack. On the other hand, in induced defenses, a set of new metabolites are produced as a consequence of the attack.
The presented study is an attempt to investigate the effect of the gall wasp (L. invasa) attack on the volatile constitutes of E. camaldulensis. This could help in the control of this wasp through stimulating plant defense or production of new allelochemicals or insecticides. Studying and comparing the volatiles fraction of Eucalyptus prior to and post plant attack by the wasp can help the re-use and recycle of the infected Eucalyptus trees for new pharmacological and medicinal activities.
Plant
The fresh gall wasp (L. invasa)-attacked and healthy leaves of E. camaldulensis (Myrtaceae) were collected from the Medicinal Plants Experimental Garden of the Faculty of Pharmacy, Zagazig University, Egypt in March 2014. The identification of the plants was confirmed by Dr. A. H. Abdel-Baset, plant taxonomist, Egyptian Agricultural Museum, Agriculture Research Centre, Cairo, Egypt. Identification of the insect species and wasp gall disease was done in the Ornamental, Medicinal and Aromatic Plants Diseases Department, Plant Pathology Research Institute, Agricultural Research Centre, Cairo, Egypt.
Isolation of the volatile terpene fraction
The fresh gall wasp-attacked and healthy leaves (100 g each) were cut and immediately subjected to hydrodistillation using Clevenger-type apparatus for 3 h (Leicach et al., 2010). The volatile fraction was recovered by decantation and dried over anhydrous sodium sulphate and stored at -20°C until analysis. The E. camaldulensis leaves attacked by the gall wasp L. invasa terpene fraction (ALTF) and the healthy leaves of the same plant terpene fraction (HLTF) gave a yield of 1.2 and 1.3% (v/fresh weight), respectively. Terpene fraction samples were kept in brown vials in the refrigerator at 4°C until further analyses. Six ALTF and HLTF samples from different trees were prepared as mentioned earlier. 20 μl of terpene fractions were diluted with n-hexane (HPLC-grade, Fisher chemicals) to 500 μl and used for the gas chromatography analysis.
Gas chromatography analysis
Gas chromatography/flame ionization (GC-FID) analysis was done using GC-2010 Plus, Shimadzu Corporation, Kyoto, Japan, gas chromatograph equipped with FID-2010 Plus detector. The following conditions were applied; column, RTX-5MS®fused silica capillary (30 m × 0.25 mm i.d and 0.25 μm film thickness); carrier gas He (2 ml/min); detector temperature 300°C, injection temperature 250°C; oven temperature program: initial temperature 45°C, 2 min isothermal, 300°C, 4°C/1 min, then 20 min isothermal; split ratio was 1:15, injection volume was 5 μl. Gas chromatography/mass spectrometry (GC-MS) data were recorded on GCMS-QP2010 Plus, Shimadzu corporation, Kyoto, Japan. The ionization energy for the mass spectrometer was 70 eV. Split ratio was 1:30; full scan mode was used, other conditions were identical to those mentioned for GC-FID. GC-MS was used to identify the compounds, which were then quantified using GC-FID.
Components identification and percentage area calculation
Kovat’s retention indices (RI) were calculated with respect to a set of co-injected standard hydrocarbons (C10-C28). The analytes were identified by comparing their spectral data and retention indices with Wiley Registry of Mass Spectral Data 10th edition (April 2013), NIST 11 Mass Spectral Library and by comparing with
published data (Adams, 2007). Calculations of peak percentage areas, based on FID response, are as follow:
Percentage area of peak= (The FID peak area / The sum of all the FID peaks areas) × 100
Most of non-identified components are present as traces with relative abundances of less than 0.1%. The most important constituents identified in the volatiles fractions analysed are listed in Table 1.
The percentage area ratio was calculated for each component and displayed in Table 1. This ratio indicates that this component increased in concentration due to the gall wasp attack (the ratio will become than 1) or decreased due to the attack (the ratio will be less than 1). The percentage area ratio was calculated using the following formula:
Percentage area ratio for a compound= (Percentage area of this compound in ALTF/ Percentage area of this compound in HLTF)
Statistical analysis
Six samples were used for both ALTF and HLTF (n=6), respectively and each sample was injected in triplicate. Quantitative values are expressed as mean ± standard error of mean (SEM) of percentage areas and significance difference was determined using unpaired student-sample-t-test performed using SPSS statistical package version (SPSS for Windows, Version 11.5, SPSS Inc., Chicago, IL). P<0.05 was considered significant.
Volatile terpene fraction components
The gas chromatographic analysis of the volatile terpene fraction produced by E.camaldulensis leaves attacked by the gall wasp L. invasa (ALTF, Figure 2B) and the healthy leaves of the same plant (HLTF, Figure 2A) allowed the separation and identification of 59 components representing 89.13 and 88.60% of the total fraction contents respectively. The results of HLTF samples were very much in agreement of published data of the same species elsewhere (El-Ghorab et al., 2003; Cheng et al., 2009)with little qualitative and quantitative variation in minor constituents. This variation could be attributed to the stage of maturity or other ecological factors. Twenty-six major compounds at an average concentration greater than 0.1 ± 0.02% have been retained for the statistical comparison (Table 1). From these main components, twenty-one were found in both HLTF and ALTF, in different percentages, whilst five components were found exclusively in ALTF. These specific compounds were the open chain mono terpene p-mentha-2-4(8) diene and the sesquiterpenes δ-elemene, β-elemene, E-caryophyllene and bicyclogermacrene. These compounds existed in different concentration in ALTF samples varying from 0.69% for p-mentha-2-4(8) diene to 2.10% in case of bicyclogermacrene with a total of 5.58% of the total fraction components’ percentage (Table 1 and Figure 2C). The percentage areas of other volatile components showed an increase or decrease in concentration between the ALTF and HLTF samples. Some compounds dramatically increased in ALTF samples such as myrecene, α-phellandrene, sabinene and β-phellandrene (increased by 10.56, 7.91, 3.37 and 5.45 folds, respectively). Interestingly, the main component in HLTF, p-cymene, decreased in ALTF samples to reach 0.61 of its percentage area in HLTF samples (12.8% of the total components’ percentage). Moreover, all oxygenated monoterpenes component in ALTF, such as 1,8-cineole, cryptone and 1-terpeniol decreased to 0.55, 0.60 and 0.14 of their percentage area values in HLTF samples, respectively (Table 1).
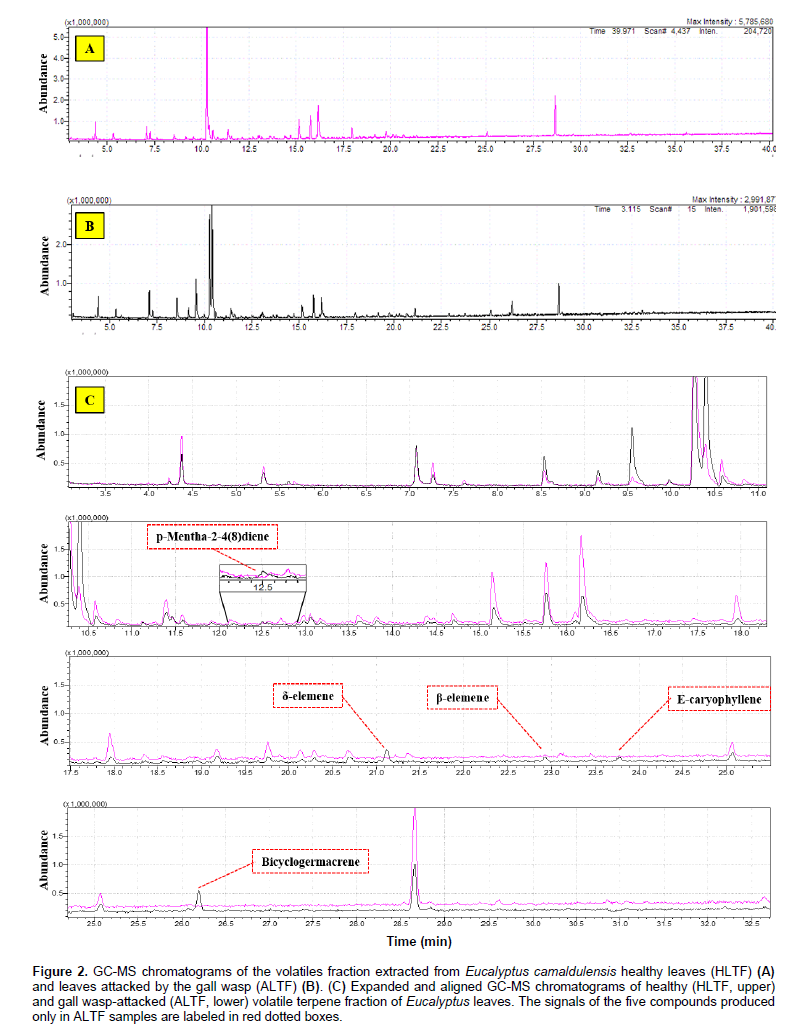
The sesquiterpene fraction increased from 8% in HLTF to reach 11% of the total percentage area of components in ALTF samples and nearly all the newly produced components were found in this fraction. The amount of spathulenol decreased by gall wasp attack to reach 0.70 of its percentage area in HLTF samples.
Biosynthesis of volatile components
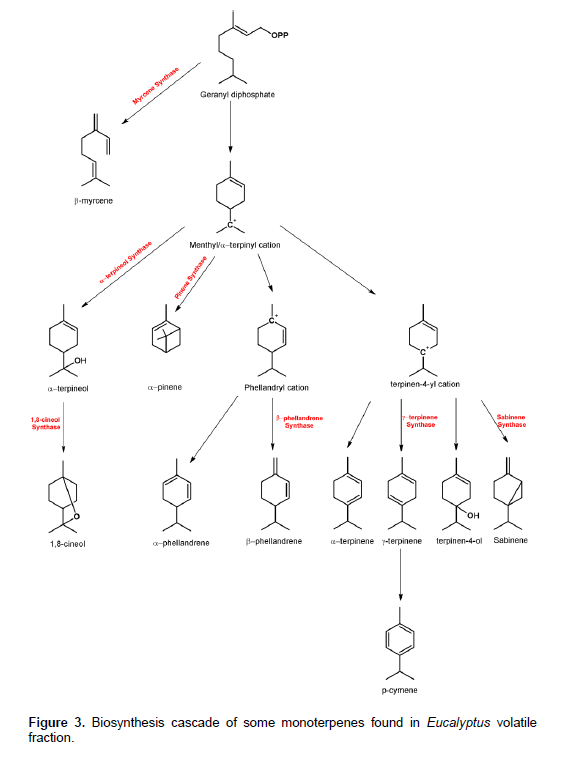
The attack of gall wasp induced the production of aliphatic non-oxygenated monoterpenes as in the case of myrcene and phellandrenes. Oxygenation process was generally inhibited and thus the production of compounds such as 1,8-cineol, cryptone and terpinen-4-ol was reduced. Furthermore, production of aromatic compounds such as p-cymene was also diminished; consequently, the production of p-cymene-7-ol and carvacrol was affected (Table 1). The biosynthesis of monoterpenes in Eucalyptus could go through four different metabolic pathways as shown in Figure 3. These pathways produce α-terpineol, pinenes, phellandrenes and terpinen-4-yl cation. The attack with gall wasp might have induced enzymes in the pinene and phellandrene pathways and some enzymes in the terpinen-4-yl cation pathway; however, it could have inhibited enzymes in the α-terpineol pathway (Figure 3). These induction processes might have resulted in the increase of phellandrenes on the expense of oxygenated and aromatic monoterpenes such as 1,8 cineol and p-cymene. The gall wasp attack could have induced the myrcene synthase enzyme resulting in the 10-fold increase in the open chain monoterpene, myrcene. The biosynthesis of p-mentha-2-4(8)diene could be achieved through the phellandryl cation by satisfaction of carbon 1 cation from carbon 4 and formation of a double bond between carbons 4 and 8, however, this pathway needs more investigation.
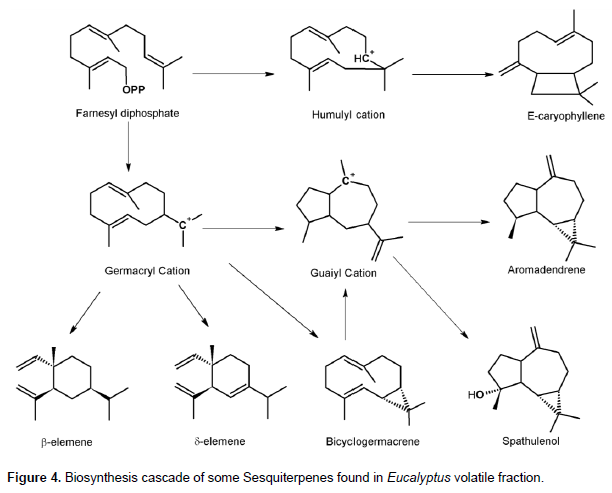
Similar scenarios can be implemented in sesquiterpenes biosynthesis; however, the situation is more interesting. The humulyl cation pathway might have been induced after the gall wasp attack, resulting in the production of E-caryophyllene. Furthermore, germacrene A and C beside bicyclogermacrene were produced after the attack and during GC analysis and due to high temperature, germacrene A and C undergo cope rearrangement to produce β- and δ-elemene, so the latter compounds are consider as artefacts of germacrene A and C (Adio, 2009). Both β- and δ-elemene were produced in concentrations of 0.7 and 1.3% of the total components’ percentage areas, respectively. Bicyclogermacrene was produced, which is the precursor of guaiyl derivatives such as aromadandrene and spathulenol (Mulyaningsih et al., 2010)(Figure 4). Therefore, it is better to say that bicyclogermacrene has accumulated due to inhibition in the production of the oxygenated sesquiterpene spathulenol, however, no trace for bicyclogermacrene can be found in HLTF samples. These compounds present only in ALTF samples, were not reported in previous studies of Eucalyptus volatiles, except for the analysis of the oil produced from immature Eucalyptus flowers (Giamakis et al., 2001). This study reports on the mechanisms for the production of such compounds in Eucalyptus, although they are not produced unless needed. In the case of immature flowers, these compounds are needed for the protection of flowers in this sensitive stage of plant growth. Similarly, the wasp attack induces in the plant, the production of such defense compounds.
Plant defense and allelopathy
The present study reveals that, due to the wasp attack, the concentrations in the Eucalyptus leaves were increased for several volatile components, such as myrecene, α-phellandrene, sabinene and β-phellandrene. These components are assumed to represent the plant constitutive defense mechanisms or could be a part of the allelopathic mechanisms. Eucalyptus volatile components are known for their insecticidal and larvicidal activities (Batish et al., 2008; Cheng et al., 2009; Alzogaray et al., 2011; Gomes and Favero, 2013). Unfortunately, almost all these studies concentrate on the effect of total fraction, not the individual components. Cheng et al. (2009) demonstrated that α-phellandrene, γ-terpinene and α-terpinene exhibited good larvicidal activity against the mosquitoes’ larvae, A. aegypti and A. albopictus (Cheng et al., 2009). These compounds’ concentrations increased due to the gall wasp’s attack on Eucalyptus leaves in the study hereby. The newly produced sesquiterpenes can also be a part of the plant-induced defense mechanism or they can be involved in allelopathy. Germacrenes are known for their wide spectrum of biological activities, including antibacterial, antifungal, insect pheromones and phytotoxic activities (Adio, 2009; Vandermoten et al., 2011). The allelopathic effect of Eucalyptus is well known (Nishimura et al., 1984; Inderjit et al., 1999; Verdeguer et al., 2009; Zhang and Fu, 2009), however, these published studies do not identify the compounds that determine this effect of Eucalyptus.
Reuse and recycle of attacked Eucalyptus
The gall wasp attacks thousands of Eucalyptus trees every year and the insect is not easy to be controlled. The leaves of the plants affected by this condition are inappropriate for medicinal use. The present study identifies the components of volatile fraction after the wasp attack. Many of these have increased concentration, such as myrcene. Myrcene has a good effect in gastric ulcers (Bonamin et al., 2014), so the elevation of this compound concentration in ALTF samples (to 1.6% of the total components percentage areas, a 10 fold) could make this fraction more suitable to treat ulcers. Germacrenes are known for their cytotoxic and anti-cancer, anti-parasites and anti-inflammatory activities (Adio, 2009; Vandermoten et al., 2011). Phellandrenes are recognized for their antimicrobial, pain-inhibition and promotion of immune responses (Iscan et al., 2012; Lima et al., 2012; Lin et al., 2013). The presence of these new compounds or the change of other volatiles’ concentration could add more medicinal and pharmacological properties for Eucalyptus. Comparing the major terpene constituents of Eucalyptus attacked by the gall wasp and of the healthy plant leaves, might help in finding new uses and medicinal applications of the attacked plants.
The volatile terpene fraction of E. camaldulensis leaves has been analysed after the attack of the gall wasp (L. invasa) and compared to the same fraction obtained from healthy leaves of the same plant. The insect attack has triggered changes in the production of many phyto-volatile constituents, and also induced the synthesis of five new components: one open chain monoterpene and four sesquiterpenes. The insect switched on some metabolic pathways, and inhibited others, especially those for the production of the oxygenated and aromatic compounds. This regulation could be attributed to the plant induced or constitutive defense mechanisms against the insect attack, or to the plant communication to other plants or insects through allelochemicals production. A way to reuse and recycle the attacked Eucalyptus plants is to get benefit from their volatile constituents. This study reported some metabolic changes in the terpenes concentrations, plus the production of new compounds in the affected plants, thus suggesting that the medicinal uses and value of Eucalyptus can be further diversified.
The author thanks the College of Clinical Pharmacy, University of King Faisal for the financial support.
The author has not declared any conflict of interests.
REFERENCES
|
Adams RP (2007). Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. Chicago, Illinois, USA, Allured Publishing corporation.
|
|
|
|
Adio AM (2009). Germacrenes A - E and related compounds: thermal, photochemical and acid induced transannular cyclizations. Tetrahedron 65(8):1533-1552.
Crossref
|
|
|
|
|
Akolade J, Olajide O, Olutayo O, Afolayan M, Akande S, Idowu D (2012). Chemical composition, antioxidant and cytotoxic effects of Eucalyptus globulus grown in north-central Nigeria. J. Nat. Prod. Plant Resour. 2:1-8.
|
|
|
|
|
Alzogaray RA, Lucia A, Zerba EN, Masuh HM (2011). Insecticidal activity of essential oils from eleven Eucalyptus spp. and two hybrids: lethal and sublethal effects of their major components on Blattella germanica. J. Econ. Entomol. 104(2):595-600.
Crossref
|
|
|
|
|
Barra A, Coroneo V, Dessi S, Cabras P, Angioni A (2010). Chemical variability, antifungal and antioxidant activity of Eucalyptus camaldulensis essential oil from Sardinia. Nat. Prod. Commun. 5(2):329-335.
|
|
|
|
|
Batish DR, Singh HP, Kohli RK, Kaur S (2008). Eucalyptus essential oil as a natural pesticide. For. Ecol. Manage. 256(12):2166-2174.
Crossref
|
|
|
|
|
Begum S, Farhat F, Sultana I, Siddiqui BS, Shaheen F, Gilani AH (2000). Spasmolytic constituents from Eucalyptus camaldulensis var. obtusa leaves. J. Nat. Prod. 63(9):1265-1268.
Crossref
|
|
|
|
|
Ben Marzoug HN, Bouajila J, Ennajar M, Lebrihi A, Mathieu F, Couderc F, Abderraba M, Romdhane M (2010). Eucalyptus (gracilis, oleosa, salubris, and salmonophloia) essential oils: their chemical composition and antioxidant and antimicrobial activities. J. Med. Food 13(4):1005-1012.
Crossref
|
|
|
|
|
Bonamin F, Moraes TM, dos Santos RC, Kushima Hl, Faria FM, Silva MA, Junior IV, Nogueira L, Bauab TM, Souza Brito ARM, da Rocha LRM, Hiruma-Lima ClA (2014). The effect of a minor constituent of essential oil from Citrus aurantium: The role of β-myrcene in preventing peptic ulcer disease. Chem. Biol. Interact. 212(0):11-19.
Crossref
|
|
|
|
|
Cheng SS, Huang CG, Chen YJ, Yu JJ, Chen WJ, Chang ST (2009). Chemical compositions and larvicidal activities of leaf essential oils from two eucalyptus species. Bioresour. Technol. 100(1):452-456.
Crossref
|
|
|
|
|
El-Ghorab AH, El-Massry KF, Marx F, Fadel HM (2003). Antioxidant activity of Egyptian Eucalyptus camaldulensis var. brevirostris leaf extracts. Nahrung 47(1):41-45.
Crossref
|
|
|
|
|
Elaissi A, Bel Haj Salah K, Mabrouk S, Chemli R, Harzallah-Skhiri F (2011). Antibacterial activity and chemical composition of 20 Eucalyptus species essential oils. Food Chem. 129:1427-1434.
Crossref
|
|
|
|
|
Elaissi A, Rouis Z, Mabrouk S, Salah KB, Aouni M, Khouja ML, Farhat F, Chemli R, Harzallah-Skhiri F (2012). Correlation between chemical composition and antibacterial activity of essential oils from fifteen Eucalyptus species growing in the Korbous and Jbel Abderrahman arboreta (North East Tunisia). Molecules 17(3):3044-3057.
Crossref
|
|
|
|
|
Giamakis A, Kretsi O, Chinou I, Spyropoulos CG (2001). Eucalyptus camaldulensis: volatiles from immature flowers and high production of 1,8-cineole and beta-pinene by in vitro cultures. Phytochemistry 58(2):351-355.
Crossref
|
|
|
|
|
Gilles M, Zhao J, An M, Agboola S (2010). Chemical composition and antimicrobial properties of essential oils of three Australian Eucalyptus species. Food Chem. 119(2):731-737.
Crossref
|
|
|
|
|
Gomes SP, Favero S (2013). Assessment of the insecticidal potential of Eucalyptus urograndis essential oil against Rhodnius neglectus Lent (Hemiptera: Reduviidae). Neotrop. Entomol. 42(4):431-435.
Crossref
|
|
|
|
|
Henery ML, Wallis IR, Stone C, Foley WJ (2008). Methyl jasmonate does not induce changes in Eucalyptus grandis leaves that alter the effect of constitutive defences on larvae of a specialist herbivore. Oecologia 156(4):847-859.
Crossref
|
|
|
|
|
Inderjit, Keating KI, Donald LS (1999). Allelopathy: Principles, Procedures, Processes, and Promises for Biological Control. Adv. Agron. 67:141-231.
|
|
|
|
|
Iscan G, Kirimer N, Demirci F, Demirci B, Noma Y, Baser KH (2012). Biotransformation of (-)-(R)-alpha-phellandrene: antimicrobial activity of its major metabolite. Chem. Biodivers. 9(8):1525-1532.
Crossref
|
|
|
|
|
Karunaratne W, Edirisinghe J, Ranawana K (2010). Rapid survey of damage due to gall wasp infestation in a coppiced Eucalyptus camaldulensis plantation in Maragamuwa, Naula in the Matale district of Sri Lanka.
|
|
|
|
|
Kelly J, La Salle J, Harney M, Dittrich-Schroder G, Hurley B (2012). Selitrichodes neseri n sp a new parasitoid of the eucalyptus gall wasp Leptocybe invasa Fisher & La Salle (Hymenoptera: Eulophidae: Tetrastichinae). Zootaxa 3333:50-57.
|
|
|
|
|
Leicach SR, Garau AM, Guarnaschelli AB, Yaber Grass MA, Sztarker ND, Dato A (2010). Changes in Eucalyptus camaldulensis essential oil composition as response to drought preconditioning. J. Plant Interact. 5(3):205-210.
Crossref
|
|
|
|
|
Lima DF, Brandao MS, Moura JB, Leitao JM, Carvalho FA, Miura LM, Leite JR, Sousa DP, Almeida FR (2012). Antinociceptive activity of the monoterpene alpha-phellandrene in rodents: possible mechanisms of action. J. Pharm. Pharmacol. 64(2):283-292.
Crossref
|
|
|
|
|
Lin JJ, Lin JH, Hsu SC, Weng SW, Huang YP, Tang NY, Lin JG, Chung JG (2013). Alpha-phellandrene promotes immune responses in normal mice through enhancing macrophage phagocytosis and natural killer cell activities. In Vivo 27(6):809-814.
|
|
|
|
|
Mendel Z, Protasov A, Fisher N, La Salle J (2004). Taxonomy and biology of Leptocybe invasa gen. & sp. n. (Hymenoptera: Eulophidae), an invasive gall inducer on Eucalyptus. Aust. J. Entomol. 43:101-113.
Crossref
|
|
|
|
|
Mulyaningsih S, Sporer F, Zimmermann S, Reichling J, Wink M (2010). Synergistic properties of the terpenoids aromadendrene and 1,8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine 17:1061-1066.
Crossref
|
|
|
|
|
Nishimura H, Nakamura T, Mizutani J (1984). Allelopathic effects of p-menthane-3,8-diols in Eucalyptus citriodora. Phytochemistry 23(12):2777-2779.
Crossref
|
|
|
|
|
Panahi Y, Sattari M, Pour Babaie A, Beiraghdar F, Ranjbar R, Hedaiat Joo A, Bigdeli M (2011). The Essential Oils Activity of Eucalyptus polycarpa, E. largiflorence, E. malliodora and E. camaldulensis on Staphylococcus aureus. Iran J. Pharm. Res. 10(1):43-48.
|
|
|
|
|
Sartorelli P, Marquioreto A, Amaral-Baroli A, Lima M, Moreno P (2007). Chemical composition and antimicrobial activity of the essential oils from two species of Eucalyptus. Phytother. Res. 21:231-233.
Crossref
|
|
|
|
|
Troncoso C, Becerra J, Bittner M, Perez C, Sãez K, Sãnchez-olate M, Rãos D (2011). Chemical Defense Responses In Eucalyptus Globulus (Labill) Plants. J. Chil. Chem. Soc. 56:768-770.
Crossref
|
|
|
|
|
Vandermoten S, Mescher MC, Francis Fdr, Haubruge E, Verheggen FoJ (2011). Aphid alarm pheromone: An overview of current knowledge on biosynthesis and functions. Insect Biochem. Molecular Biol. 42(3):155-163.
Crossref
|
|
|
|
|
Verdeguer M, Blazquez MA, Boira H (2009). Phytotoxic effects of Lantana camara, Eucalyptus camaldulensis and Eriocephalus africanus essential oils in weeds of Mediterranean summer crops. Biochem. Systematics Ecol. 37(4):362-369.
Crossref
|
|
|
|
|
Zhang C, Fu S (2009). Allelopathic effects of Eucalyptus and the establishment of mixed stands of Eucalyptus and native species. Forest Ecol. Manage. 258(7):1391-1396.
Crossref
|
|
|
|
|
Zrira S, Bessiere J, Menut C, Elamrani A, Benjilali B (2004). Chemical composition of the essential oil of nine Eucalyptus species growing in Morocco. Flav. Fragr. J. 19:172-175.
Crossref
|
|