Following the introduction of genetically-engineered glyphosate-resistant (GEGR) crops, commercially known as Roundup Ready (RR), no pesticide’s active principle has been used as much as glyphosate; yet its safety measures have been sternly disputed. After its classification by the International Agency for Research on Cancer (IARC) as probably carcinogenic to humans in 2015, scientists, activists, regulators and the general public revisited voluminous studies that outweighed the risk of this herbicide and raised ferocious concerns that warranted serious attention. Recently published studies on glyphosate established at least four toxicological principles. First, glyphosate exhibited severe mammalian toxicity at concentrations orders of magnitude lower than its regulatory-promulgated ‘No Observed Adverse Effect Level’ (NOAEL) or even its ‘Chronic Reference Dose’ (cRfD) and ‘Acceptable Daily Intake’ (ADI). Second, even though not transparently scrutinized or officially required for toxicological testing and risk assessment, glyphosate co-formulants and glyphosate-based herbicides (GBHs) are orders of magnitude more toxic than the principle active ingredient alone. Third, glyphosate and GBHs are cytotoxic and endocrine disruptors, and the latter explains why ultra low concentrations - yet environmentally relevant-cause severe chronic toxicity. Fourth, the endocrine disruption likely leads to epigenotoxicity that may be extended to offspring and unexposed descending generations. Taken all together, it can be fairly said that confidence in the regulatory-certified ADI values is highly eroded. To resolve the paradoxical discrepancy between regulatory safety measures and elicited toxicities at concentrations far below these measures, ADI was refined using two safety or adjustment factors. Together, these two factors scale down ADI by four orders of magnitude and bring it to an Adjusted ADI (AADI) value of 2.5 ng/kg bw/day. Contrary to regulatory ADI, the new AADI successfully explains many research findings which demonsted severe mammalian toxicity at concentrations in the neighborhood of nanograms a.i./kg bw/day. This distills confidence in the new AADI value, as well as the magnitude of the proposed safety factors. Glyphosate uses as per human capita, in two countries representing the extremes of adopting RR crops (the USA) or not-adopting these crops (Egypt), were compared. The comparison confirms the association between growing RR crops and the escalated use of glyphosate, and shows that the American public is likely exposed to glyphosate residue at forty times higher levels than the Egyptian public.
Glyphosate is the active ingredient in Monsanto’s first commercial herbicide (Roundup), and many other proprietary glyphosate-based brands (Monsanto, 2005). Worldwide, glyphosate is considered to be the most used herbicide in agriculture, horticulture, viticulture, forestry, parks, industrial and public sites, aquatic environments, gardens, sports fields, school grounds, etc. A US-patent also covers the use of glyphosate for antibiotic treatment of animal and human pathogenic infections (Organic NZ Magazine, 2015). The unprecedented use of GBHs provides uncountable exposure pathways, and increasingly raises concerns over their possible adverse outcomes in human-health and the environment. Regardless of the IARC classification of glyphosate as probably carcinogenic to humans (Guyton et al., 2015), and of the serious scientific and public concerns over its safety, pesticide industry and regulatory authorities complacently claim that when GBHs are used as recommended, the public is exposed to only ‘safe’ levels that pose no serious toxicological risks to humans (FAO/WHO, 2016).
To interpret the level of risk of any pesticide, its actual exposure is compared to a reference safety threshold, e.g., ADI; calculated for experimental animals and extrapolated to humans. ADI is the amount of a substance, expressed on a body-mass basis, daily ingested in food or drinking water over lifetime without imposing any appreciable risk to human health (The Detox Project, 2016; WHO, 1987). The calculation to set the ADI is based on one hundredth (1/100) the dose considered to be non-toxic in animal feeding trials; toxicologically known as NOAEL (Faustman and Omenn, 2001).
NOAEL-generating experimental studies are usually run by pesticide companies according to protocols set in consultation with the Organization for Economic Co-operation and Development (OECD), an agency mainly dedicated to facilitating international trade, not to shielding public health. Besides, since the data are generated by, or provided through, pesticide companies, conflict of interest may not be preventable or avoidable.
Glyphosate, which was ironically considered to be as safe as caffeine and table salt (Charry, 1997; Preston, 2014; The Credible Hulk, 2015) for four decades, was recently classified by IARC/WHO and added to the A2-carcingenic category (Guyton et al., 2015). This paradigm shift in glyphosate toxicology is due to many reasons including: (i) the escalated use of GBHs and obviously the subsequent high residues and elevated human and environmental exposures (Benbrook, 2016;Myers et al, 2016), especially after the first adoption of RR crops in 1996 (Monsanto, 2015), that is, the post-era of RR biotechnology; (ii) a growing body of solid evidence indicating that experimental animals and humans face serious risks as a result of their exposure to concentrations far below the regulatory-claimed-to-be safety thresholds (Jayasumana et al., 2015; Mesnage et al., 2015); (iii) safety thresholds or limits are set for the active ingredient ‘alone’ which is generally less toxic than the formulation blends actually polluting the environment and affecting human life. The third reason (iii) implies two things that are strongly supported by research findings: (a) the safety thresholds are erroneously overestimated; (b) the mammalian toxicity of glyphosate is bestowed by co-formulants. The ultimate result is that what is assessed to be safe in laboratory testing is not actually safe under field conditions. Therefore, one cannot use regulatory-adopted safety measures as a reference for the interpretation of risk under real-life situations of human and environmental exposures.
It is generally accepted that pesticide formulations are up to three orders of magnitude acutely or chronically more toxic than their active principles (Mesnage et al., 2014; Defarge et al., 2016) due to the toxic and/or synergistic effect of co-formulant(s). The co-forumlant effect factor can be further complicated by the diversity of used glyphosate-based generic brands. For example, over 750 formulations are registered for glyphosate use in the USA alone (Henderson et al., 2010), and more than 500 adjuvant/co-formulant substitutes are commonly used in glyphosate end-use products (The Greens-EFA-EU, 2016). Unfortunately, most of these co-formulants earn commercial confidentiality rights and are not totally scrutinized or accessible to scientists or even regulatory agents, let alone the lack of studies regarding their hazard to human health and the environment. It is surprising that regulatory authorities are sometimes misled or deceived by pesticide industry and accept the notion of co-formulants as toxicologically-inert materials that pose no toxicological risk to human health and the environment. This notion is not only inaccurate; it is also misleading and extremely dangerous if we consider that levels of GBHs for which the active principle is claimed to be safe are not actually safe over the long term or for recently-discovered toxicological endpoints, e.g., endocrine-mediated epigenetic toxicity review by Ibrahim (2016).
For example, disturbances of functional genes were observed in kidney and liver of rats treated with glyphosate at as low as 4.0 ng/kg bw/day (Mesnage et al., 2015). This dosage level is five orders of magnitude lower than the regulatory-held safe exposures or ADI levels (0.30 to 1.75 mg/kg bw/day) for this herbicide (Center for Food Safety, 2015).The fact that regulatory ADI values fail to explain recent findings which demonstrated serious animal-health outcomesat ultra-low concentrations, far below the ADI levels (Defarge et al., 2016) indicates that these levels lack the criteria and qualification of being used as a reference safety threshold. More importantly is that the public health cannot afford the adoption of what is claimed and clamored by ‘professional’ pesticide regulatory authorities or agencies to be an acceptable exposure level when in fact five orders of magnitude lower concentrations can induce serious human-health defects (Bonn, 2005). Let alone, the spread of epidemiological incidences of chronic diseases thought to be causally related to GBHs (Jayasumana et al., 2015). Gasnier et al. (2009) found that GBHs presented DNA damages and carcinogenic-mutagenic-reprotoxic (CMR) effects on human cells and in vivo. Exposure to low doses of GBHs may result in reproductive and hormonal problems, miscarriages, low birth weights, pre-term deliveries, and birth defects. It is strange that the safety of public health can sometimes be in the hands of individuals rather than professional pesticide regulatory authorities, e.g., US-EPA and EU-EFSA. This statement applies perfectly to Glyphosate; as for the time these regulatory authorities maintain glyphosate re-registration for weed control, the newly elected president of Sri Lankan, Maithripala Sirisena, announced in one of his first decisions that the country’s importation of glyphosate was to be banned immediately and that the release of any stocks already present in the country was to be halted as well (Heyes, 2015). Due to all the discrepancies between the regulatory-certified safety measures (e.g., ADI values) and reputable scientific research findings, as well as the epidemiological incidences that greatly contradict and challenge these measures, it was the intent of the author to reexamine these measures and find ways to adjust them within the scope of published research, reports and observations.
The main objective of this manuscript is to create a quasi-mechanistic model to possibly adjust the pesticide safety measures (NOAEL, ADI, cRfD, etc.) that are routinely calculated from the empirical risk assessment model. The empirical model uses data collected from experimental studies that, unfortunately, use low-resolution tools and endpoints to calculate these measures. According to the empirical model, risk assessment of any pesticide to human health and the environment relies on two principal factors: (1) its innate or potential hazard of the active ingredient; and (2) its actual level of exposure to humans and the environment. The first factor is more or less based on fixed and experimentally-defined toxicological safety measures (e.g., NOAEL or ADI), while the second one depends on actual human and environmental exposure stemming from how much pesticide is being applied in a region on a given crop, collectively across all crops, and in other places. If perfectly determined, the potential hazard is static for each toxicological endpoint, while the experienced exposure is momentarily dynamic. In line with these two factors, the results and discussion section is divided into two subsections (I & II). The first subsection contains a literature-based justification approach for the importance of refining ADI values measured for the active ingredient ‘alone’ using glyphosate as an exemplary model. This subsection is supported by two novel figures that clearly show how erroneously overestimated ADI value leads to enormously underrated risk, especially in the era of RR biotechnology. The second subsection is dedicated to comparing some data for glyphosate use in the USA and Egypt, as representatives of countries adopting or not adopting RR crops, respectively. This comparison allows the author to see how much of the escalated use of glyphosate can be attributed to growing RR crops, and how this escalated use can seriously threaten the safety reputation this herbicide with reference to an adjusted or miniaturized ADI value. In this subsection, the global use of glyphosate is also included.
The underlying principles of adjusting glyphosate ADI values
There are several reasons that led the author to question and challenge the reliability and validity of the currently-known and regulatory-certified ADI values of glyphosate and its formulations (GBHs). The same and other reasons have encouraged the author to seek ways to refine the currently-accepted but evidently-overestimated ADI values. The six reasons that create the underlying principles of this manuscript are as discussed in the following:
First, ADI values have been determined by testing the active principle ‘alone’ on laboratory animals; yet the regulatory authorities enforce these values on all used GBHs; barely known for the identity and toxicity of their individual components. That is in spite of the fact that people and the environment are genuinely exposed to formulations, not just their isolated active ingredient. Several Studies confirmed that glyphosate formulations administered to rats and pigs at levels - deemed safe for glyphosate active ingredient alone - were extremely harmful to treated animals (Adam et al., 1997; Antoniu et al., 2012; Benedetti et al., 2004; Lee et al., 2009; Romano et al., 2010).
Second, ADI values are based on studies conducted on adult animals mostly failed to test or observe the effects of exposure during vulnerable windows of development, e.g. foetal development and unexposed descending generations. The issue of trans generational or epigenetic inheritance of adverse human-health and environmental effects of endocrine disrupting pesticides was strongly emphasized when the well-known fungicide vinclozolin was given at a single time to mice with testis in a critical period of development. As discovered by Anway et al. (2005), vinclozolin produced an adverse effect on the developing testis that was passed on to the following three generations of mice. The epigenetic inheritance was also found with other pesticides and pesticide mixtures. For example, Manikkam et al. (2012) showed clearly that the epigenotoxic effects of an insecticidal mixture (permethrin + DEET) lasted for three successive generations. A subtle endocrine disruption during early life can modify the morphologies and functions of many organs and eventually cause reprotoxicity and cancer (Vandenberg et al., 2012).
Third, regulatory-accepted risk assessment protocols are based on the 15
th century old adage of Philippus von Hohenheim (globably known as
Paracelsus, the father/founder of toxicology) who stated that :
“the dose makes the poison” and implied that the higher the dose, the greater the degree of toxicity (The Detox Project, 2016; Wikipedia, 2016). Although it fully applies to acute toxicity and related endpoints, this adage does not apply to some chronic toxicity, especially what is related to endocrine-disruption, wherein the dose-response relationship is not always monotonic and safe levels cannot simply be extrapolated from high doses (Heindel et al., 2013; Lagarde et al., 2015; Vandenberg et al., 2012; Zoeller and Vandenberg, 2015). Ultra-low concentrations of some endocrine-disrupting pesticides are more toxic than NOAELs which are commonly expected or extrapolated from higher concentrations. Besides, NOAEL itself may still cause serious response or damage on the same or different endpoints, if the dose matches the vulnerability window(s) and/or exhibits a biphasic or concaved relationship with its response. In the light of the endocrine-disrupting potential of glyphosate (Babalola, 2016), the author prefers to rephrase the well-known Paracelsus toxicology norm to make it applicable to any pesticide chemicals regardless of the shape of its dose-response curve (monotonic or non-monotonic). The rephrased toxicological principle states that
“the dose unfolds the actual risk of its potential or tacit hazardousness.” The dose required for some toxicological outcomes or endpoints does not have to be only in the range of high doses.
Fourth, the potential endocrine-disruption by glyphosate and its commercial formulations (Séralini et al, 2014; Séralini, 2015; Thongprakaisang et al., 2013) indicates that the standard long-term animal studies and traditional endpoints required by regulatory authorities and executed by pesticide companies are inadequate to accurately determine valid and reliable ADI values. In a comprehensive review including 314 references, Fuhrman et al. (2015) compiled and discussed the uncertainties and unknown that regulators may face when considering the risk assessment of endocrine disruptors and indicated clearly that there is no definitive risk assessment tool for these chemicals; a situation that will enforce regulators to accept data from loosely designed testing protocols and poorly defined, even distant or irrelevant, endpoints.
Fifth, several studies demonstrated additive or synergistic effects of different types of endocrine disruption, e.g., estrogenic, antiandrogenic, or thyroid-disrupting agents, when used in mixture at concentrations far below their NOAELs. A dramatic enhancement of endocrine effects not predicted from tests on individual compounds (Rajapakse et al., 2002; Silva et al., 2002, 2011) has been observed for some estrogenic chemicals. When three estrogenic test systems were used (Seeger et al., 2016), similar outcomes on mixtures of endocrine-disrupting pesticides were confirmed. The additive/synergistic behavior of endocrine disruptors is likely to be the case with glyphosate and additives in glyphosate-based formulations.
Sixth, commercially used formulations of glyphosate contain additives (adjuvants or co-formulants), which are either toxic in their own right and/or increase the toxicity of glyphosate (Mesnage et al., 2013; Séralini, 2015).
The six abovementioned reasons, along with their solid research evidence supporting them, challenge the validity and reliability of regulatory-enforced ADI values. These values seem to be highly overestimated and the risk of exposure assessed with reference to them is significantly underestimated. This has been simply and conceptually illustrated in Figure 1. Like many toxicologists from around the world, the author believes that the EPA’s cRfD or the European ADI values for glyphosate are overly estimated. The range of these values (0.30 to 1.75 mg/kg bw/day) is considered to be too high to mark any acceptable or conservative human-exposure threshold. Based on these values, the safety margin or ceiling of this herbicide is likely wider or higher than the actual case scenarios especially in the light of the highly vulnerable endocrine system and its mediated epigenetic effects or outcomes (Defarge et al., 2016; Ibrahim, 2016). The endpoints of these outcomes are likely: (a) inflicted by ultra-low doses; and (b) appeared in maternally exposed offspring or unexposed descending generations (Ibrahim, 2016).To simply explain the danger of relying on overestimated ADI value while assessing the risk of actual pesticide exposure, Figure 1 was generated. Although highly simplified, this figure superbly illustrates the risk situation of glyphosate exposure in the pre- and post-era of RR Biotechnology. It also illustrates the author’s renovated toxicological principle which states: “Once the ADI value is erroneously overestimated for any pesticide, the risk from exposure to this pesticide will always be enormously underestimated.”
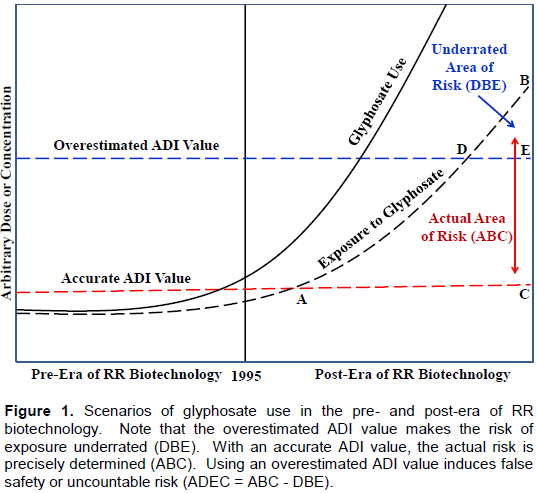
It also shows that there is a huge area of actual risk (the ABC area) when exposures are compared to an accurately-determined safety measures (accurate ADI value). To the contrary, this risk is underrated and shrunk to the DBE area when exposures are compared to overestimated regulatory safety measures or ADI values. Therefore, it is highly critical that the current ADI values of glyphosate are reassessed and refined, while taking endocrine disruption and the likely heritable epigenetic havoc into consideration. Since this has not been experimentally done yet, the author will provide some hypothetical adjustment of the acceptable exposure threshold of GBHs, specifically the ADI. It is within our understanding that the relationship between the exposure level to any pesticide and its used quantity is not perfectly straight - but certainly correlated. It is also understood that the interface of pesticide use, human and environmental exposure and observation, biologically-responsive system(s) and adverse outcomes is very complex. Obviously, the nature and severity of these outcomes vary depending on the overall health of the exposed organism, its physiological and psychological state, the level, timing and duration of exposures, the tissues exposed, their vulnerability, the consequent human health outcomes, to count just a few. In particular, the timing of pesticide exposure that temporally and spatially matches the sensitivity window is a key determinant, especially with endocrine-disruption and epigenetically-mediated outcomes (Ibrahim, 2016).
ADI-adjusting factors
Two safety factors were introduced to adjust or scale down glyphosate ADI values. The first factor (10×) is to compensate for the unlikely certainty of no harm in the light of elevated environmental and human exposure and the repeated epidemiological incidences of glyphosate-related health effects. The second factor (1000×) is to compensate for the bestowed toxicity of glyphosate in the presence of co-formulants. The introduction of the co-formulant safety factor is extremely important due to the fact that even though ADI is determined for glyphosate alone, people are exposed to the whole formulation simply because glyphosate can never be used alone and by itself for weed control.
FQPA factor
According to researchers, cell damage and/or cell death, especially, embryonic, placental and umbilical cord cells, can occur at residue concentrations commonly found on Roundup-treated crops, yards, lawns, parks and gardens for weed control (Scientific America, 2009). It is important to note that the US Food Quality Protection Act (FQPA) requires the Environmental Protection Agency (EPA) to assure that a pesticide can be used if only its residues demonstrate “A Reasonable Certainty of No Harm.” This assurance requires the EPA to introduce a tenfold (10×) safety factor when setting and reassessing tolerances unless adequate data are available to support a different factor (EPA, 1996; McDonald Jr., 2000). This factor is also used to compensate for dietary exposures and higher risk of glyphosate or any pesticide to extra-sensitive groups in the population, e.g., pregnant women, infants, children, and elderly people living in or nearby heavily exposed areas. Considering the uncertain safety of safety measures set for GBHs, and of the continual and high exposure of pesticide applicators, farm workers and bystanders in residential areas close to RR fields, one can introduce, for partial adjustment of glyphosate ADI, a safety factor of 10X, similar to that of the 1996 mandate of US-FQPA Act.
Adjuvant factor
Based on a diversity of recent studies, a second safety factor of 1000X was introduced in the present study to further adjust the thought- and also found-to-be overestimated ADI values. This factor possibly compensates for the bestowed toxicity of glyphosate induced by adjuvants or co-formulants which are mistakenly believed to be inert additives. It has been recently mentioned (Mercola.com, 2016) that certain GBH adjuvants cause human cell toxicity, adding to the hazards inherent in the active principle (glyphosate). In a study of the effects of glyphosate and its adjuvants on hepatic (HepG2), embryonic (HEK293) and placental (JEG3) cell lines, Mesnage et al (2013) found that the toxicity of commercial formulations was due to adjuvants rather than the active ingredient itself, and the toxicity was in fact proportional to the concentration of these adjuvants. Mesnage et al. (2014) found out that this has also been the case with other herbicides, as well as some insecticides and fungicides. The formulations in almost all the tested pesticides were up to 1000 times more toxic than their active ingredients to human cells in vitro. Polyethoxylated tallow amine (POEA), a major adjuvant/surfactant in Roundup formulations, has been shown to be 1,200 and 2,000 times more cytotoxic than glyphosate (Defarge et al., 2016). The bestowed toxicity of the formulated vs. active principle of glyphosate is emphasized not only for human-health outcomes but also for environmental disruption (Martini et al., 2016; Székács et al., 2014). For example, glyphosate at 50 ppb was shown to have significant negative impacts on the aquatic invertebrate, Daphnia magna (Cuhra et al., 2013; Myers et al., 2016). This concentration is orders of magnitude lower than the range of the Maximum Contaminant Level or eco-toxicological threshold (700-27000 ppb) assigned by regulatory authorities in the USA and Canada (Canadian Council of Ministers of the Environment, 2012). Based on the aforementioned studies, the author chose to use a safety factor of 1000X to compensate for the bestowed toxicity of glyphosate induced by co-formulants.
Adjusted ADI (AADI) value
A group of scientists has compiled evidence supporting a miniaturized ADI value of 0.025 mg/kg bw/day (Antoniu et al., 2012). Although this value is 12 to 70 times lower that the EU and EPA reference values, it is still four orders or magnitude higher than what was found to inflict gene disturbance or epigenetic disorder in rats (Mesnage et al., 2015). Therefore, Antoniu’s ADI value requires further refinement. When this value was taken as a baseline for adjustment, and divided by the combined safety factors of 104X, as proposed in the present study, an Adjusted ADI (AADI) value of only 0.0000025 mg/kg bw/day or 2.50 ng/kg bw/day was obtained for glyphosate in the context of its formulated blends. A recent finding by Mesnage et al. (2015) clearly showed that genes in kidney and liver of rats treated with glyphosate at 4.0 ng/kg bw/day were functionally disturbed. The fact that this dose is only 1.6 times that of the AADI value from the present study indicates that this value is reasonably calculated and conservatively adjusted and refined. After rationally adjusting the ADI value based on this manuscript’s quasi-mechanistic model, the danger of relying on an overestimated glyphosate ADI value as a yardstick for risk assessment of GBHs deserves further emphasis. Figure 2 compares the calculated risk of exposure to GBHs when an overestimated and adjusted ADI values of glyphosate are taken into consideration.
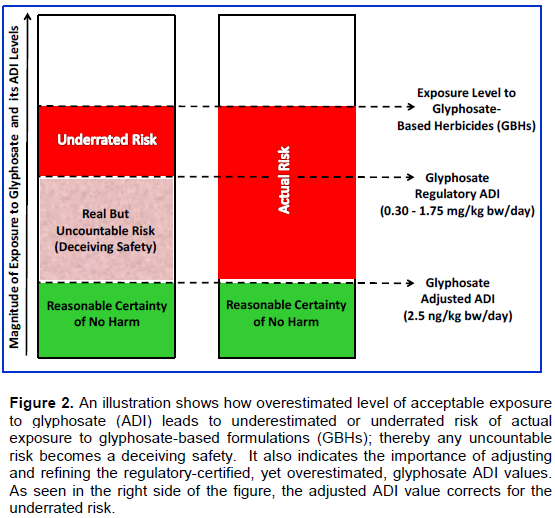
By looking at Figure 2, one can easily extract two intimately related points: (1) the higher the magnitude of overestimation, the bigger the chance of missing the assessment of a significant part of the actual risk; (2) the bigger the difference between the inaccurate and accurate ADI values, the bigger the area of deceiving safety. Obviously a result like this one erodes confidence in regulatory-promulgated ADI values, at least in the case of GBHs. With this conception in mind, it appears that levels of GBHs, for which the active principle is claimed to be safe, may in fact pose serious risk to humans over the long term. It is, therefore, believed that people are misled by the current safety measures (ADI values) of pesticides’ active ingredients when these measures are applied to interpret and assess the risk of end-use products or formulations. Even if the safety thresholds or measures adopted by regulatory authorities for glyphosate were accurate, the overuse of this herbicide in the past two decades and after the introduction of RR crops may have driven its exposure levels far above these measures, thereby the certainty of no harm is becoming foggy or uncertain.
RR crops: Glyphosate overuse and risk concerns
Successes in developing RR crops allow farmers to overuse glyphosate either forcibly, voluntarily or even irresponsibly. Statistics have shown that no pesticide in the history of plant protection has been used as widely as glyphosate (Benbrook, 2016;
Van Hoesen, 2016), especially after the introduction of RR crops to agriculture. It is no wonder why voluminous research studies indicate that glyphosate is predominantly found in the air, the water, the soil, the food, the feed and the human body (Myers et al., 2016 for citations), sometimes at levels far-exceeding the regulatory-allowed thresholds. To make the theme of this study clear and intact, the 2014 consumption of glyphosate has been compared in the USA, wherein RR crops are heavily cultivated; in Egypt, wherein these genetically-engineered crops have never been introduced to the Egyptian agriculture; and worldwide, wherein these crops are adopted in some countries and are not adopted in others. Table 1 shows glyphosate use in these three comparisons, along with the corresponding populations to calculate this use as per human capita. The arbitrary human exposure (the last column in Table 1) was calculated according to the following equation:Human Exposure (ng glyphosate a.i./kg bw/ day) = [(mg a.i. used as per capita x 10
6)/ (365 x 70 x 10
6)] (1) Wherein 365 is the number of days in the year; 70 is an assumed average weight of working adults (kg/adult) whoare either fractionally at risk or directly exposed to glyphosate; the 10
6 in the numerator is for the conversion of mg to ng; and the 10
6 in the denominator is a hypothetically suggested fraction of glyphosate that may find its way to human body or a hypothetical fraction of population that may receive an exposure above the average population in a normal distribution. Even though the exposure levels were mostly arbitrary, comparing the data of Egypt and the USA to examine the effect of RR adoption on glyphosate use and human exposure still holds. In this regard, just by looking at the amount of glyphosate used as per capita (Table 1), one can easily find that this amount in the USA is 40.8 times that of Egypt’s amount and 3.4 times that of the global amount. In short, the comparison implies that: (1) the overuse of glyphosate, especially in the USA, is concomitant with heavily growing RR crops; (2) it is legitimately accepted to raise concerns over glyphosate overuse; (3) reassessment of the actual risk of glyphosate in areas heavily growing RR crops is highly justifiable and irresponsibly overdue; (4) countries not growing RR crops and do not experience the spread of resistant weed biotypes, like Egypt, may still use glyphosate with some
‘severe’ label restrictions as previously suggested by Ibrahim (2015). Comparing the arbitrary exposure levels in Table 1 with the AADI value (2.50 ng a.i./kg bw/day) shows that the US person in the highest sector of glyphosate exposure receives daily concentration 6.2 times higher than the AADI value (15.39/2.50).
This indicates that this sector of the population is at actual glyphosate risk, and may explain the recently documented correlation between the application of GBHs in the USA and the spread of several human diseases. In their study, Swanson et al. (2014) found positive and highly significant correlation between annual glyphosate use and the spread of hypertension, stroke, diabetes prevalence, diabetes incidence, obesity, lipoprotein metabolism disorder, Alzheimer’s, senile dementia, Parkinson's, multiple sclerosis, autism, inflammatory bowel disease, intestinal infections, end stage renal disease, acute kidney failure, cancers of the thyroid, the liver, the bladder, the pancreas, the kidney and myeloid leukemia. On the extreme end of the comparison, the exposure of the Egyptian person in the highest glyphosate exposure sector is only 0.148 times that of the ADDI value (0.37/2.50). Contrary to the US, the Egyptian person in this sector is 6.8 times further down the acceptable daily threshold or AADI value. The average person in the highest exposure sector in the world is exposed to almost twice (4.50/2.50 = 1.8X) as much as the reference ADDI dose. The world exposure is lower than that of the USA due to the fact that some countries in the world are still growing traditional crops, that is, not genetically-engineered for glyphosate-resistance. The total amount of glyphosate used is not expected to be evenly distributed among: days of the year; cropland areas; or population. To the contrary, people exposed to glyphosate either occupationally (farm-workers, that is, applicators and pickers), or by virtue of their rural residence in areas heavily cultivated with RR crops, are expected to incur relatively higher exposure levels than the average arbitrary values calculated in Table 1. Besides, the risk to pregnant woman, infants, children, and elderly people may be actually higher.