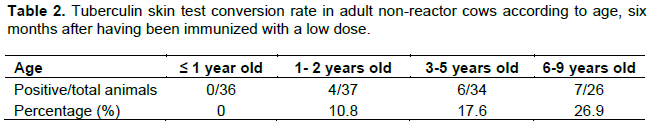ABSTRACT
Bovine tuberculosis (bTB) has a direct impact in the productive and reproductive efficiency of dairy cattle. Nowadays, disease control programs based on tuberculin testing and removal of infected cattle are unaffordable for the developing countries, since there is no program of financial compensation, especially in high bTB-prevalence herds. Thus, control strategies based on vaccination are considered the best alternative. The aim of this study was to evaluate whether a low dose of BCG-Phipps vaccine induces reactivity to tuberculin skin test and its duration in neonatal calves and adult cows. For the analysis of the former, 69 TB-free Holstein-Friesian calves less than one-month old were used; of which, 54 calves were subcutaneously inoculated with 104 CFU of the BCG-Phipps vaccine, while the rest remained without vaccination. Under similar conditions of immunization, 133 single intradermal comparative cervical (SICCT) reactors and 133 non-reactors Holstein-Friesian cows of different age were also analyzed. In calves, the SICCT-reactivity was evaluated periodically in the first 14 months post-vaccination (mpv) while, in adult cows, the effect of vaccination on the test was evaluated at six months post-vaccination. A comparative ELISA was used by measuring the antibody levels in the groups. In calves, reactivity frequencies of 7.4 and 3.7% at 3 and 5 mpv, respectively were recorded. This reactivity disappeared at six months. None calves in the control group were reactor during the study. There were no variations in the degree of reactivity in the group adult reactor cows. However, in the non-reactor group, a conversion of 12.8% at six mpv was recorded. In addition, the conversion percentage was higher in older cows than in younger cows (p<0.05). The specific antibody levels did not increase in the vaccinated groups. Data indicate that the low dose of BCG-Phipps vaccine used had a reduced effect on the development of a delayed type hypersensitivity to tuberculin in neonatal calves, and heifers lesser than one-year-old.
Key words: Bacillus Calmette-Guérin, bovine tuberculosis, M. bovis, single intradermal comparative tuberculin test.
Bovine tuberculosis (bTB) is one of the main diseases causing economic losses to dairy industry, and its incidence has been increasing in recent years. The severity of disease is more in immunosuppressed animals or in concurrent infections (Gupta et al., 2009). Therefore, it is required to develop effective control strategies. Hence, the use of Bacillus Calmette-Guérin (BCG) Mycobacterium bovis vaccine could be one of the most viable tools for this purpose. In this regard, it has been pointed out that vaccination with BCG in cattle at an early age diminishes pathological scores, so it could be an approach to decrease bacterial loads and reduce the transmission risk both from bovine to bovine and bovine to human (Lienhardt et al., 2012; Waters et al., 2012; Kaufmann et al., 2014).
However, field trials investigating protection have reported variable efficacies; furthermore, revaccination did not improve protective effect. Potential explanations of the variable levels of field protection includes the use of different BCG strains, administration of very high doses of BCG, different routes of inoculation, and previous exposure to M. bovis in maternal milk from infected cows, circumstances that diminish the effective protective immunity (González et al., 2007; Hope et al., 2011; Thom et al., 2012).
With respect to the optimum age of vaccination against tuberculosis, it has been determined that vaccine efficacy is higher in very young calves, lesser than one month old, compared to calves between five and six months old (Buddle et al., 2003; Hope et al., 2005). Why calves lesser than one month old develop higher levels of protection, may be that they have been lesser exposed to environmental mycobacteria which interferes with development of a protective immune response against bTB, compared to older calves (Siddiqui et al., 2012). It is therefore incontrovertible that eventual future application of vaccines will be used to improve the control of bTB. Thus, it is relevant to improve the knowledge of factors determining the development of a delayed type hypersensitivity (DTH) response, if it really develops when vaccine is given under a number of different modalities and stages (Cockle et al., 2002; Logan et al., 2005). Few studies talk about the development of a DTH response or conversion of tuberculin skin test (TST); as a result of the vaccine application, one of this studies demonstrated that DTH reaction is developed in the first months and lost in 90% of the vaccinated animals at nine months post-vaccination (Whelan et al., 2011; Buddle et al., 2013).
However, the theoretical framework of the BCG vaccine application indicates that it primes the animals to respond to the tuberculin test, and it is an impediment to use the BCG as a vaccine since this test is the primary tool for epidemiological surveillance used in many countries as a main strategy of bTB control, in México (Mexican Official Norm NOM-031-ZOO-1995; Good and Duignan, 2011; OIE, 2012). Today, the rate of conversion induced by the BCG, the correlation of DTH with protective immunity, and the factors determining the development of protection are unknown. These observations could have useful implications at some point in the future, for a solid improvement of BCG vaccines designed for the bovine cattle. The objective of this study was to determine whether the BCG-Phipps vaccine at low dose (104 CFU) induces reactivity to tuberculin skin test and its duration in neonatal calves as well as evaluate its effect on reactors and non-reactors adult dairy cows.
Ethics statement
Animals used in this study belong to a commercial farm and have been submitted only to the standard clinical practices specifically regulated by the Mexican legislation on tuberculosis control program (Mexican Official Norm NOM-031-ZOO-1995), which were vaccination and blood sampling.
Experimental design for neonatal calves
Sixty-nine female calves between 6 to 30 days of age obtained from herds with no history of bTB were used. All animals were tested and confirmed negative for M. bovis by antigen-induced IFN-gamma produced by peripheral blood mononuclear cells (PBMCs), ELISA for detection of specific antibodies to a culture filtrate protein extract (CFPE), and PCR on nasal swabs were used. Fifty-four of these calves were inoculated subcutaneously on the neck with a dose of 104 CFU of BCG-Phipps strain in PBS. To avoid prime calves to bovine Purified Protein Derivative (PPD) by repeated tuberculin tests, they were divided in two groups for alternative testing; half of the animals were tested at 0, 3, 7 and 11 months post-vaccination, the other half, at 0, 5, 9 and 14 months, and results were analyzed altogether. Fifteen control animals were inoculated with PBS. All animals were isolated to eliminate any risk of infection from the main herd.
Experimental design for adult cows
The study was performed in a dairy herd with a bTB prevalence of 49.39% located in the State of Hidalgo, México, from which, 133 single intradermal comparative cervical tuberculin (SICCT) reactors and 133 non-reactors Holstein-Friesian adult cows were analyzed to determine the effect of vaccination on tuberculin skin test. Both study populations were immunized with 104 CFU/1.5 ml of BCG-Phipps subcutaneously injected on right side of the neck, like calves. The effect on SICCT test was evaluated at six months post-vaccination (mpv) in reactors and non-reactors cows. The latter were grouped by age to evaluate the influence of this variable on development of DTH response induced by vaccination, then forming the following groups: Group 1, ≤ 1 year old (n=36); Group 2, between 1 to 2 years old (n=37); Group 3, between 3 to 5 years old (n=34); and Group 4, between 6 to 9 years old (n=26). Cows pertaining to the Groups 2, 3 and 4 remain in production stable. The cows of Group 1 were isolated from the rest of animals to avoid exposure to new infections.
Production of BCG Phipps vaccine
For elaboration of BCG Phipps vaccine (Lot number 95 001-2013-7H9), seed was cultured on Middlebrook 7H10 OADC (Oleic Albumin Dextrose Catalase) solid medium for 15 days at 37°C. Next, bacteria were transferred to Middlebrook 7H9 ADC liquid medium and they were incubated in constant swirling at 37°C. Bacilli were subcultured once in liquid medium until they reached an early exponential growth phase (OD600nm 0.3) and aliquots frozen in PBS at -70°C. For bacterial enumeration, serial dilutions were plated onto modified Middlebrook 7H10 OADC. Petri dishes were incubated for 3 to 4 weeks at 37°C, and CFU/ml were calculated by the formula UFC x DF x 10 = bacteria/ml; in which DF = dilution factor, and concentration was adjusted to 104 CFU/ml in sterile PBS.
Single intradermal comparative cervical tuberculin test
The development of a DTH response by vaccination in study populations was determined by SICCT test, which was applied according to NOM-031-1995, in the middle third of the neck. In two different shaved and cleaned spots, 0.1 ml (3,250 IU) of bovine PPD (PPD-B) strain AN5, and 0.1 ml (3,250 IU) of avian PPD (PPD-A) strain D4 (PRONAVIBE, México) were injected. After 72 h post-inoculation skin thicknesses were measured. A positive result was considered positive if the increase in skin thickness at the bovine PPD site of injection was at least 4 mm greater than the reaction at the avian PPD injection site. The reaction was suspicious, but inconclusive, when PPD-B reaction was between 2 to 3 mm greater than PPD-A reaction. A negative result was considered when PPD-B swelling was negative or, even being positive, for no more than 2 mm greater than PPD-A reaction.
Culture filtrate protein extract
To obtain mycobaterial CFPE from M. bovis AN5 and M. avium D4, these were cultured in Dorsett-Henley medium for six weeks at 37°C. At the end of the incubation period, the bacterial mass was eliminated by filtration, using cellulose filters, followed by 1.2, 0.45 and 0.22 μm Millipore filters (Bernardelli, 2007). The proteins present on the residuals liquids were precipitated with ammonium sulfate (Sigma Aldrich, St. Louis, MO, USA) at a final saturation of 80%, under constant whirling motion for 24 h at 4°C. The precipitates were centrifuged at 4°C for 60 min at 20 000 X g. The CFPE were recovered and finally dialyzed through a membrane with exclusion limits from 3000 kDa (Membrane Filtration Products, Inc. TX, USA) at 4°C for 48 h with PBS. The protein content of each CFPE was determined by the Bradford method and CFPE was stored at -70°C (Bradford, 1976).
IgG ELISA
A comparative ELISA was used for the evaluation of humoral immune response in the study populations. In this ELISA, the plates (Nunclon, Roskilde, Denmark) were coated with 1 µg/well of either M. avium or M. bovis CFPE dissolved in 0.05 M carbonate-bicarbonate buffer (pH 9.6) overnight at room temperature at 4°C. Then, plates were washed three times with 0.1% Tween 20 in 0.01 M phosphate buffer at pH 7.4 (PBS-T). Free binding sites were blocked with 3% non-fat milk in PBS-T for 1 h at 37°C. Afterward, the plates were washed with PBS-T, next incubated with PBS-diluted (1:100) sera for 1 h at 37°C. After washing with PBS-T, 100 ml/well of Protein G-Peroxidase (1:10,000, in PBS) (P-8170 Sigma) were added and incubated for 1 h at 37°C. Next, the plates were washed and subsequently the chromogenic substrate solution was added; containing 0.04% O-phenylenediamine (Sigma P-3804) and 0.04% of hydrogen peroxide in citrate buffer at 37°C for 5 min. Reaction was stopped with 50 µl/well of 2 M sulfuric acid. Optical density was read at 492 nm (OD492nm) in a microplate reader (Benchmark Plus, Bio-Rad, Hercules, CA, USA) (Estrada-Chavez et al., 2001).
The cut-off point was calculated as the media of the OD492nm from all SICCT-negative animals before the vaccination plus two standard deviations, and animals having a value greater than this cut-off point was regarded as positives.
PCR
DNA was extracted from nasal exudate samples for detecting the presence of M. bovis by PCR. Briefly, the samples contained in 5 ml of sterile PBS (phosphate buffer saline 0.01 M, pH 7.4) were centrifuged at 12,000 X g for 5 min. The pellet was incubated with 400 µl of Tris-EDTA buffer (TE) (100 mM Tris-HCl, 10 mM EDTA, pH8.0) and 50 µl of lysozyme (100 mg/ml) 1 h at 37°C. 70 µl of 10% SDS and 5 µl of proteinase K (10 mg/ml) were added and incubated for 20 min at 65°C. Then, 100 µl of 5 M NaCl, and 100 µl of CTAB/NaCl (4.1 g of NaCl, 10 g of CTAB, 80 ml of distilled water) was added, and pre-warmed at 65°C for 10 min. After adding 750 µl of chloroform-isoamyl alcohol (24:1), mix was centrifuged at 12,000 x g for 5 min. After recovering the upper phase, this was mixed with 600 µl of absolute isopropyl alcohol for 30 min at -20°C. DNA was centrifuged at 12,000 x g for 5 min, supernatant discarded, and 1 ml of cold -20°C ethanol 70% was added to wash the DNA. Samples in microcentrifuge tubes were again centrifuged at 12,875 x g for 5 min; DNA was dissolved into 50 µl of sterile water, and stored at -20°C.
For PCR, primers TB1-F, 5’GAACAATCCGGAGTTGACAA3’, and TB1-R, 5’AGCACGCTGTCAATCATGTA3’, related to the M. tuberculosis Complex, were used; these primers amplify a 372 bp sequence from the gen MPB70 codifying the secreted protein MPB70 (Cousins et al., 1992).
PCR mix for a final volume of 25 µl was prepared with 0.5 µl of each primer (0.4 µM), 13.25 µl of sterile water, 2.5 µl of 10X buffer, 0.5 µl of dNTPs (250 µM), 2.5 µl of MgCl2 (2.5 mM), 0.25 µl of Taq polimerase (0.5 U), and 5 µl of sample DNA (50 pg-250 ng). DNA obtained from M. bovis AN5, M. tuberculosis H36Rv, and from tuberculous lesions of diseased cows was used as positive controls. Sterile milli-Q water and DNA obtained from nasal swabs from IFN-γ and ELISA-negative calves were used as negative controls.
Cycling conditions were pre-warmed for 15 min at 96°C, and 35 cycles of denaturation for 30 s at 94°C, annealing for 30 s at 58°C, and extension for 60 s at 72°C; also a final step of extension for 5 min at 72°C (Gene Amp PCR System 9700, Applied Biosystems, Foster City, CA USA). 10 µl of each PCR product were analyzed in agarose 1.5% gels stained with ethidium bromide 1%, using 2.5 µl of molecular weight standards (Amresco K180-250 UL), and visualized with a Benchtop UV Transilluminator in a EpiChemi II Darkroom (BioImaging Systems, Upland California USA).
Statistical analysis
Data were analyzed with Student “t” test using the Statistical Analysis System software (SigmaStat Ver. 3.5) to determine significant differences between ELISA results before and after vaccination, and Xi2 to analyze distribution of SICCT test data. A value of p < 0.05 was regarded as significant (Wayne, 2006).
SICCT test reactivity after BCG vaccination in calves
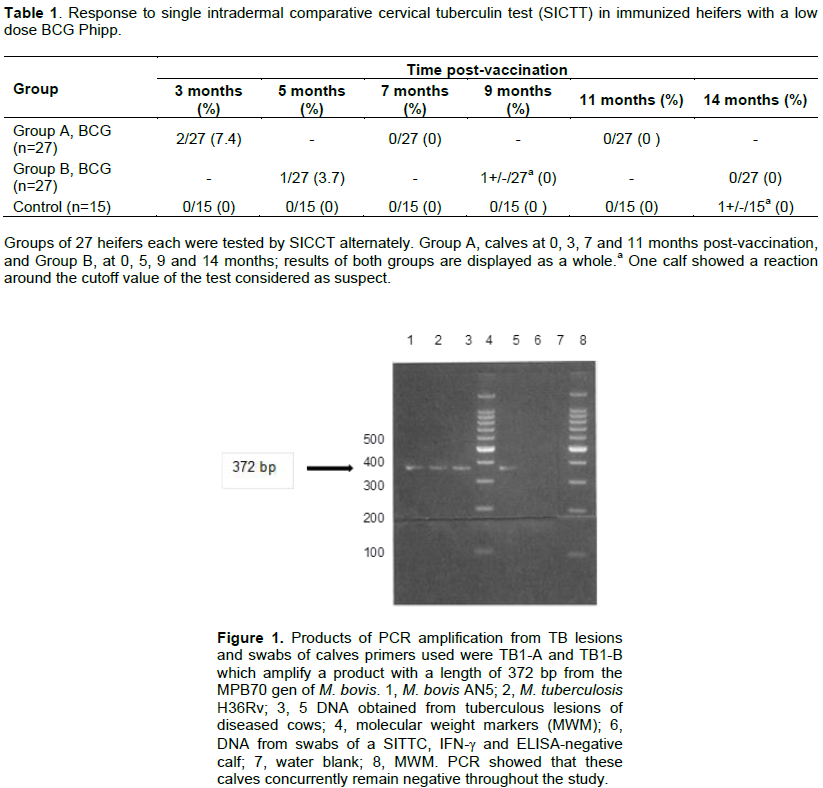
BCG vaccination in calves induced SICCT test reactivity in 2/27 (7.4%) at 3 months post-vaccination, and 1/27 (3.7%) at five months post-vaccination (Table 1); one animal alone was suspicious at 9 months; at 14 months post-vaccination, none of the animals showed reactivity. None of the control animals (n=15) was reactor at that time; however, one control animal showed a value around the cut-off point of the test. These data showed that at three months post-vaccination, the young female calves showed the greater frequency of reactivity, which diminished during the following months (Table 1). PCR analysis showed that both groups of calves concurrently remained negative all over the study (Figure 1).
ELISA in calves
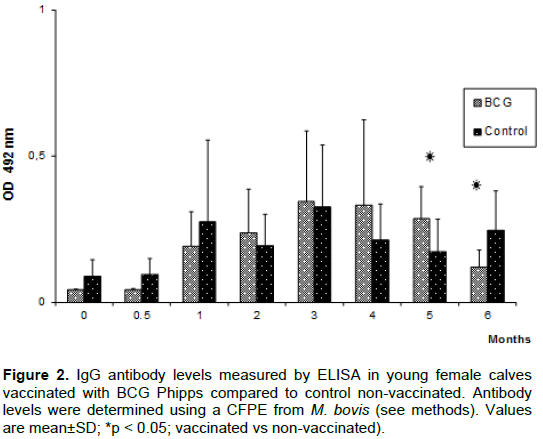
The baseline levels of IgG antibodies to CFPE from M. bovis were similar between groups; these increased lightly in both groups without being significant at the first months post-vaccination; in the course of time, reaching a peak value of 0.34±0.24 vs 0.32±0.21 (OD492nm ± standard error) for vaccinated group and control, respectively, at 3 months. In the subsequent two months, levels remained without much variation for BCG vaccinated group. However, at 5 months post-vaccination the vaccinated group was higher than the control group (P<0.05); and at the end of the monitoring period of IgG antibodies, the values for the vaccinated group were even lower than from control group (P<0.05). For which, the evaluation of the humoral immune response was limited only to this period (Figure 2).
SICCT test reactivity after BCG vaccination in cows
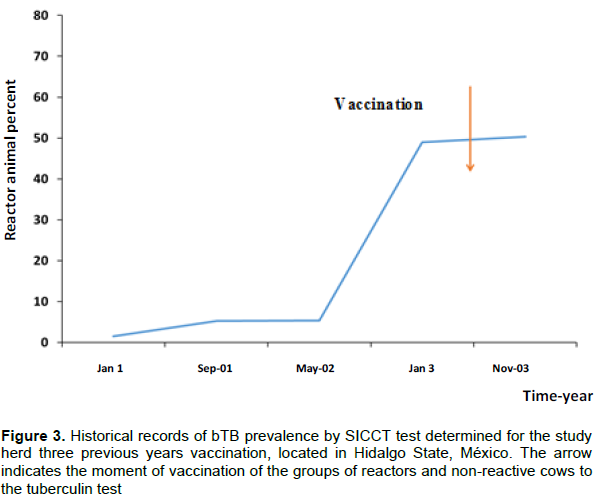
We had the SICTT reactivities over the last three years for the dairy herd participating in this study. Figure 3 shows the bTB prevalence for 1 to 3 years in adult cows. The percentage of reactors at the beginning of the first year was 1.5% and at the end of the same year was 5.2% while for the second year, before vaccination of adult calves, it was 49.39%. As shown in Figure 3, bTB prevalence after vaccination increased from 0.9 to 50.5%, which is an insignificant increase. Because of an urgent requirement of alternative measures of bTB control, it was necessary to employ the BCG Phipps vaccine altogether with other biosafety measures to give response for the stated objectives.
Related to the SICCT-non-reactor animals (n = 133), 17 animals converted to positive ones in a 12.7%. Further, a more detailed analysis showed that in older animals conversion rate was greater, as described in Table 2; conversion rates were: in animals ≤ 1 year old, 0%; between 1 to 2 years old, 10.8%; 3 to 5 years old, 17.6% and 6 to 9 years old, 26.9%. Thus, a greater conversion was observed in older cows, which could be related to a greater exposition to environmental mycobacteria considered a risk factor for bTB. These data complement those for calves, in which percentages of SICCT positive animals lesser than one month old after vaccination were low, and reactivity was diminishing during the studied period.
ELISA in cows
The prevalence of bTB was 49.39% before vaccination and antibody level was 3.15±1.01 (OD492nm ± SD). Six months later, antibody level was 3.45±0.95. We found no significant differences by t Student test. Similarly, for isolated positive animals any differences were found.
Bovine tuberculosis is an important disease of dairy cattle, which has been difficult to control and eradicate. Incidence of this disease represents a significant problem to livestock production, because of a fast dissemination of the disease. Due to multiple factors, such as defective management, poor feeding, new infected animals in the herd, and mainly lack of adequate biosafety measures, bTB represents a risk of disease for humans (Thoen et al., 2006; Schiller et al., 2010; El-Sayed et al., 2016).
For many years, BCG vaccine effectiveness to reduce the bTB has been called into question, and a trouble that prevents its application is the interference of vaccination on tuberculin tests. However, the fundamentals of this interference and the involved factors are unknown. Considering that BCG vaccine represent an alternative for controlling bTB, and that it primes animals to give a DTH to tuberculin reaction, this results in an obstacle for using the BCG, since the TST is the primary tool in epidemiological surveys used in many countries as support for controlling bTB (OIE, 2012).
Today, conversions percentages to TST and protection effectiveness induced by BCG are unknown, as well as factors that intervene in both events. BCG strain, dose, age of vaccinated animals, role of environmental mycobacteria, and idiosyncrasy of each animal, among a number of factors, could influence in both events; conversion and protection. So our first objective was to determine the effect of BCG-Phipps vaccine in a reduced dose (104 CFU) on TST reactivity in bTB-free young calves of ≤ 1 month of age. The results showed a percentage of reactivity to the test in vaccinated calves of 7.4% at three months post-vaccination, and 3.7% at five months, then wane at seven months post-vaccination. In non-vaccinated calves, there was no reactivity to TST in the first year, up to 14 months after the study started in which one of them was suspicious. The percentages of reactivity observed in vaccinated calves in this study were much lower than that reported by Whelan et al. (2011). These authors, reported an 80% of TST conversion in calves vaccinated with BCG-Danish during the first six months post-vaccination, with a decay of 8% reactivity at nine months. This difference in reactivity percentages with our results could be related to the dose and strain used. Similar percentages of TST conversion have been reported in other studies using high vaccine doses between 106-108 CFU in humans, wildlife animals, and mouse model (Buddle et al., 2013; Tree et al., 2004). In addition, the protective efficacy reported in those studies was lower in vaccinated animals that showed a strong DTH response to bovine PPD than those animals that did not develop it (Skinner et al., 2001; Ritz et al., 2008). In follow-up of the possible establishment of M. bovis infections in vaccinated and control calves, PCR reactions performed from nasal exudates showed that both groups of calves were negative throughout this study. Even though we found no differences between vaccinated and unvaccinated groups in the detection of the DNA of M. bovis during time evaluated, there was concordance between TST and PCR tests after vaccination. Regarding the tuberculin conversion in vaccinated non-reactive cows, heifers less than one year old belonging to this group showed no reactivity. However, conversion percentages increased according to aging of cows, since the cows of 6 to 9 years showed a conversion percentage of 26.9% (7/26). This can be explained because cows during his long life were largely exposed to M. bovis, since they belonged to a herd with high prevalence of the disease. Consequently, the previous sensitizations in conjunction with the application of BCG vaccine favored the development of delayed type hypersensitivity in these animals while in the reactor group, there were no differences in the degree of reactivity or development of ulcerations and/or necrosis, at least with the dose of vaccine used. It has been mentioned that in infected cattle confirmed with at least two consecutive tests of tuberculin, as in humans, could developed an adverse reaction to vaccination with BCG (Koch phenomenon) in case of having a pathology advanced or generalized (Cardona, 2006). In this regard, Buddle et al. (2016) showed that vaccination of cattle with a high dose of BCG vaccine applied after an experimental infection with M. bovis increased the inflammatory response, but not tuberculosis pathology. However, all these observations should be taken with reserve and to carry out more studies, which show the benefit or prejudice resulting in the use of the BCG vaccine in infected cows. On the other hand, it has been suggested that the BCG vaccine is capable of preventing bacilli excretion. Thus, taking into account that airborne route is the most important transmission route for bovine tuberculosis, the BCG vaccine could reduce the risk of contagion among animals (López-Valencia et al., 2010).
In relation to the BCG vaccine, it is widely documented that because of the in vitro passage, the genome of the original strain has been modified giving rise to a series of heterogeneous strains, which show clear variations in their genomes and protective immune properties (Castillo-Rodal et al., 2006; Abdallah et al., 2015). With regards to the BCG-Phipps the main regions of differentiation (RD) lost are RD1, RD2 and nRD18. RD1 is a DNA segment comprising 9.5 kb, which was deleted in all other BCG strains, that encodes T-lymphocyte epitopes such as ESAT-6, CFP-10, Rv3873, and PPE protein among others (Okkels et al., 2003). RD2 is a 10.7 kb DNA segment that encodes many proteins including Mpt-64 and CFP-21 (Joung and Ryoo, 2013) and nRD18 is a 1.5 kb segment containing genes encoding SigI, an alternative RNA polymerase sigma factor that was only lost in the strains BCG Pasteur, Phipps, Frappier, Connaught and Tice (Da Costa et al., 2014).
In a study realized by Zhang et al. (2016) assessing the virulence and efficacy of 13 BCG strains in SCID and BALB/c, showed that BCG strains of the DU2 group IV to which BCG-Phipps belongs, showed the highest levels of virulence among strains studied. They reported that these distinct levels of virulence could be explained by strain-specific duplications and deletions of genomic DNA. There appears to be a general trend that more virulent BCG strains are also more effective in protection against challenge. Similar results were reported by Castillo-Rodal et al. (2006) whom showed that BCG-Phipps immunized mice developed lesser pneumonia areas and had lesser bacterial loads than animals vaccinated with other strains, indicating resistance establishment against disease. In addition, reactivity to tuberculin was lower compared to animals vaccinated with other BCG strains and control groups. Considering the above, our results suggest that the BCG-Phipps vaccine can be applied to neonatal calves because we observed a low and transitory DTH reaction in these animals. Besides, the results obtained in a previous study showed that the use of low dose induced the development of good immunity against M. bovis in vaccinated calves under field conditions (González-González et al., 2012). Thus, BCG-Phipps vaccine constitutes a good candidate for use in disease control.
On the other hand, we have not observed significant difference in antibody levels to M. bovis for vaccinated calves and cows compared to respective controls at first months post-vaccination which may be related to the dose of vaccine used, such as previously reported by Lyashcehnko et al. (2004), whom with a similar dose of the BCG-Pasteur strain did not observe the development of antibodies to defined antigens of M. bovis in vaccinated animals.
In recent years, research has expanded on the development of new vaccines in livestock and wildlife, with encouraging results for potential in the control of the disease. However, so many questions remain without response. Biomarkers to predict a protective immunity against tuberculosis is required to be studied, in order to evaluate vaccine effectiveness and reduce challenge experiments, because of controversial issues related to bioethical principles. Additional studies are needed to put into test whether cattle vaccination extends protection in function of time, or affect the results of traditional diagnostic tests. Considering progress reached in the last decade, we optimistically think answers to these questions will be found, and TB vaccination will become a valuable control measure to control this disease in human beings, livestock and wildlife.
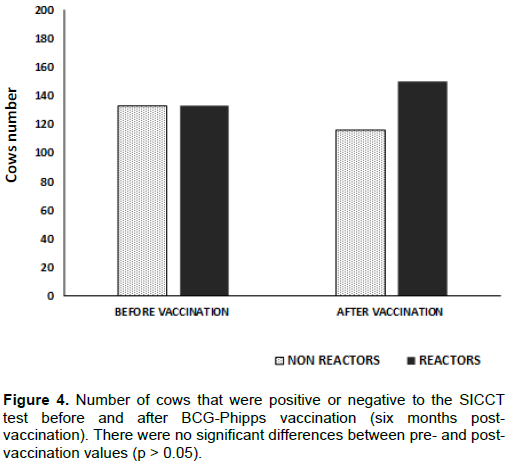
Assessment of bTB prevalence in the studied dairy herd, by the SICCT test before BCG vaccination was needed with the aim to establish a reference point and from there to consider the impact of vaccination on the control of bTB prevalence. To determine the influence level of the BCG-Phipps vaccine on reactivity to the TST in adult animals, a high prevalence herd (49.39%) was chosen to analyze its effect in both reactors and non-reactors cows. Results indicate that the number of reactor cows increased slightly after BCG vaccination, and that the negative animals showed a reduction equivalent to the increase in positive ones. However, the increase in positive animals and the decrease in negative animals were not statistically significant according to the Chi-square test (Figure 4).
BCG-Phipps vaccination with a single reduced (UFC) dose, had a minimal transient effect on development of DTH to tuberculin in neonatal calves and heifers lesser than one year, and declined in the first six months post-vaccination. In contrast, under these conditions a greater number of older cows became reactors. In addition, PCR analysis used throughout the study did not show the establishment of M. bovis infections in vaccinated and control groups. In vaccinated non-reactive cows, the tuberculin conversion percentages increased according to aging of cows. For additional studies, age of vaccination should be considered since bTB is a chronic disease and the exposition risk increases as the age of animals increase, hence this parameter will be a critical factor in stables where bTB vaccines are considered as a control measure. Furthermore, it remains to be tested if protective immunity and long life is induced by low doses of BCG-Phipps vaccine.
The authors have not declared any conflict of interests.
REFERENCES
|
Abdallah AM, Hill-Cawthorne GA, Otto TD, Coll F, Guerra-Assunção JA, Gao G, Naeem R, Ansari H, Malas TB, Adroub SA, Verboom T, Ummels R, Zhang H, Panigrahi AK, McNerney R, Brosch R, Clark TG, Behr MA, Bitter W, Pain A (2015). Genomic expression catalogue of a global collection of BCG vaccine strains show evidence for highly diverged metabolic and cell-wall adaptations. Sci. Rep. 5:15443.
Crossref
|
|
|
|
Bernardelli A (2007). Producción y control de tuberculina bovina y aviar. Derivado Proteico Purificado (DPP). Servicio Nacional de Sanidad y Calidad Agroalimentaria. Buenos Aires. Argentina.
|
|
|
|
|
Bradford MMA (1976). Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254.
Crossref
|
|
|
|
|
Buddle BM, Hewinson RG, Vordermeier HM, Wedlock DN (2013). Subcutaneous administration of a 10-fold-lower dose of a commercial human tuberculosis vaccine, Mycobacterium bovis Bacillus Calmette-Guérin Danish, induced levels of protection against bovine tuberculosis and responses in the tuberculin intradermal test similar to those induced by a standard cattle dose. Clin. Vaccine Immunol. 20(10):1559-1562.
Crossref
|
|
|
|
|
Buddle BM, Shu D, Parlane NA, Subharat S, Heiser A, Hewinson RG, Vordermeier HM, Wedlock DN (2016). Vaccination of cattle with a high dose of BCG vaccine 3 weeks after experimental infection with Mycobacterium bovis increased the inflammatory response, but not tuberculous pathology. Tuberculosis (Edinb). 99:120-127.
Crossref
|
|
|
|
|
Buddle BM, Wedlock DN, Parlane NA, Corner LAL, de Lisle GW, Skinner MA (2003). Revaccination of neonatal calves with Mycobacterium bovis BCG reduced the level of protection against tuberculosis induced by a single vaccination. Infect. Immun. 71(11):6411-6419.
Crossref
|
|
|
|
|
Cardona PJ (2006). RUTI: A new chance to shorten the treatment of latent tuberculosis infection. Tuberculosis (Edinb). 86(3):273-289.
Crossref
|
|
|
|
|
Castillo-Rodal AI, Casta-ón-Arreola M, Hernández-Pando R, Calva JJ, Sada-Díaz E, López-Vidal Y (2006). Mycobacterium bovis BCG substrains confer different levels of protection against Mycobacterium tuberculosis infection in a BALB/c model of progressive pulmonary tuberculosis. Infect. Immun. 74(3):1718-1724.
Crossref
|
|
|
|
|
Cockle PJ, Gordon SV, Lalvani A, Buddle B.M, Hewinson RG, Vordenmeier HM (2002). Identification of novel Mycobacterium tuberculosis antigens with potential as diagnostic reagents or subunit vaccine candidates by comparative genomic. Infect. Immun. 70:6996-7003.
Crossref
|
|
|
|
|
Cousins DV, Wilton SD, Francis BR, Gow BL (1992).Use of polymerase chain reaction for rapid diagnosis of tuberculosis. J. Clin. Microbiol. 30(1):255-258.
|
|
|
|
|
Da Costa AC, Nogueira SV, Kipnis A, Junqueira-Kipnis AP (2014). Recombinant BCG: innovations on an old vaccine. Scope of BCG strains and strategies to improve long-lasting memory. Front. Immunol. 5:152.
Crossref
|
|
|
|
|
El-Sayed A, El-Shannat S, Kamel M, Casta-eda-Vazquez MA, Casta-eda-Vazquez H (2016). Molecular epidemiology of Mycobacterium bovis in humans and cattle. Zoonoses Public Health. 63(4):251-264.
Crossref
|
|
|
|
|
Estrada-Chavez C, Mancilla R, Arriaga-Díaz C, Pérez-González R, Díaz-Otero F (2001). Determination of anti-PPD antibodies in dairy herds with different prevalence of bovine tuberculosis in Mexico. Vet. Méx. 32:207-211.
|
|
|
|
|
González-González XE, Jaramillo-Meza L, Lascurain-Ledezma R, Torres-Barranca JI, Quevillon CE, Díaz-Otero F (2012). Evaluation of T lymphocyte subpopulations in cattle vaccinated against bovine tuberculosis: longitudinal comparative study. Rev. Mex. Cienc. Pecu. 3(2):137-154.
|
|
|
|
|
González-Salazar D, Díaz-Otero F, Jaramillo-Meza L, Pérez-González R, Padilla-Urbina J, Santillán-Flores MA, Arriaga-Díaz C, Bojórquez-Narváez L (2007). Evaluación de diferentes inmunógenos contra la tuberculosis bovina mediante la presencia de lesiones a la necropsia. Vet. Méx. 38(3):271-284.
|
|
|
|
|
Good M, Duignan A (2011). Perspectives on the history of bovine TB and the role of tuberculin in bovine TB eradication. Vet. Med. Int. 2011:410470.
Crossref
|
|
|
|
|
Gupta MP, Kumar H, Singla LD (2009) Trypanosomosis concurrent to tuberculosis in black bucks. Indian Vet. J. 86:727-728.
|
|
|
|
|
Hope JC, Thom ML, McAulay M, Mead E, Vordermeier HM, Clifford D, Hewinson RG, Villarreal-Ramos B (2011). Identification of surrogates and correlates of protection in protective immunity against Mycobacterium bovis infection induced in neonatal calves by vaccination with M. bovis BCG Pasteur and M. bovis BCG Danish. Clin. Vac. Immunol. 18(3):373-379.
Crossref
|
|
|
|
|
Hope JC, Thom ML, Villarreal-Ramos B, Vordermeier HM, Hewinson RG, Howard CJ (2005). Vaccination of neonatal calves with Mycobacterium bovis BCG induces protection against intranasal challenge with virulent M. bovis. Clin. Exp. Immunol. 139:48-56.
Crossref
|
|
|
|
|
Joung SM, Ryoo S (2013). BCG vaccine in Korea. Clin. Exp. Vaccine Res. 2(2):83-91.
Crossref
|
|
|
|
|
Kaufmann SHE, Lange C, Rao M, Balaji KN, Lotze M, Schito M, Zumla AI, Maeurer M (2014). Progress in tuberculosis vaccine development and host-directed therapies a state of the art review. Lancet Respir. Med. 2:301-320.
Crossref
|
|
|
|
|
Lienhardt C, Fruth U, Greco, M (2012). The blueprint for vaccine research and development: Walking the path for better TB vaccines. Tuberculosis (Edinb). 92(S1):33-35.
Crossref
|
|
|
|
|
Logan KE, Chambers MA, Hewinson RG, Hogarth PJ (2005). Frequency of IFN-γ producing cells correlates with adjuvant enhancement of bacilli Calmette-Guérin induced protection against Mycobacterium bovis. Vaccine 23(48-49):5526-5532.
Crossref
|
|
|
|
|
López-Valencia G, Rentería-Evangelista T, Williams JJ, Licea-Navarro A, Mora-Valle AL, Medina-Basurto G (2010). Field evaluation of the protective efficacy of Mycobacterium bovis BCG vaccine against bovine tuberculosis. Res. Vet. Sci. 88(1):44-49.
Crossref
|
|
|
|
|
Lyashchenko K, Whelan AO, Greenwald R, Pollock JM, Andersen P, Hewinson RG, Vordermeier HM (2004). Association of tuberculin-boosted antibody responses with pathology and cell-mediated immunity in cattle vaccinated with Mycobacterium bovis BCG and infected with M. bovis. Infect. Immun. 72(5):2462-2467.
Crossref
|
|
|
|
|
Norma Oficial Mexicana NOM-031-ZOO-1995. 27 de agosto 2008. Campa-a Nacional Contra la Tuberculosis Bovina (Mycobacterium bovis). Secretaria de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación. Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria. Avalable at:
View
|
|
|
|
|
Office International des Epizooties (OIE) (2012). Manual of diagnostic tests and vaccines for terrestrial animal. Office International des Epizooties (OIE). Chapter 2.4.7. Bovine tuberculosis, pp. 674-689. Available at:
View
|
|
|
|
|
Okkels LM, Brock I, Follmann F, Agger EM, Arend SM, T. H. M. Ottenhoff THM, Oftung F, Rosenkrands I, Andersen P (2003). PPE protein (Rv3873) from DNA segment RD1 of Mycobacterium tuberculosis: strong recognition of both specific T-cell epitopes and epitopes conserved within the PPE family. Infect. Immun. 71(11):6116-6123.
Crossref
|
|
|
|
|
Ritz N, Hanekom WA, Robins-Browne R, Britton WJ, Curtis N (2008). Influence of BCG vaccine strain on the immune response and protection against tuberculosis. FEMS Microbiol. Rev. 32(5):821-841.
Crossref
|
|
|
|
|
Schiller I, Oesch B, Vordermeier HM, Palmer MV, Harris BN, Orloski KA, Buddle BM, Thacker TC, Lyashchenko KP, Waters WR (2010). Bovine Tuberculosis: A review of current and emerging diagnostic techniques in view of their relevance for disease control and eradication. Transbound. Emerg. Dis. 57(4):205-220.
Crossref
|
|
|
|
|
Siddiqui N, Price S, Hope J (2012). BCG vaccination of neonatal calves: potential roles for innate immune cells in the induction of protective immunity. Comp. Immunol. Microbiol. Infect. Dis. 35(3):219-226.
Crossref
|
|
|
|
|
Skinner MA, Wedlock DN, Buddie BM (2001). Vaccination of animals against Mycobacterium bovis. Rev. Sci. Technol. Off. Int. Epiz. 20(1):112-132.
Crossref
|
|
|
|
|
Thoen C, Lobue P, de Kantor I (2006). The important of Mycobacterium bovis as a zoonosis. Vet. Microbiol. 112(2-4):339-345.
Crossref
|
|
|
|
|
Thom ML, McAulay M, Vordermeier HM, Clifford D, Hewinson RG, Villarreal-Ramos B, Hope JC (2012). Duration of immunity against Mycobacterium bovis following neonatal vaccination with Bacillus Calmette-Guérin Danish: significant protection against infection at 12, but Not 24, Months. Clin. Vaccine Immunol. 19(8):1254-1260.
Crossref
|
|
|
|
|
Tree JA, Williams A, Clark S, Hall G, Marsh PD, Ivanyi J (2004). Intranasal bacilleCalmette–Guérin (BCG) vaccine dosage needs balancing between protection and lung pathology. Clin. Exp. Immunol. 138(3):405-409.
Crossref
|
|
|
|
|
Waters WR, Palmer MV, Buddle BM, Vordermeier HM (2012). Bovine tuberculosis vaccine research: Historical perspectives and recent advances. Vaccine 30:2611-2622.
Crossref
|
|
|
|
|
Wayne DW (2006). Bioestadística: base para el análisis de las ciencias de la salud. Fourth Ed., Limusa Editorial S. A. Mexico. ISBN: 978-968-18-6164-3
|
|
|
|
|
Whelan AO, Coad M, Upadhyay BL, Clifford DJ, Hewinson RG, Vordermeier HM (2011). Lack of correlation between BCG-induced tuberculin skin test sensitization and protective immunity in cattle. Vaccine 29(33):5453-5458.
Crossref
|
|
|
|
|
Zhang L, Ru HW, Chen FZ, Jin CY, Sun RF, Fan XY, Guo M, Mai JT, Xu WX, Lin QX, Liu J (2016). Variable virulence and efficacy of BCG vaccine strains in mice and correlation with genome polymorphisms. Mol. Ther. 24(2):398-405.
Crossref
|
|