Full Length Research Paper
ABSTRACT
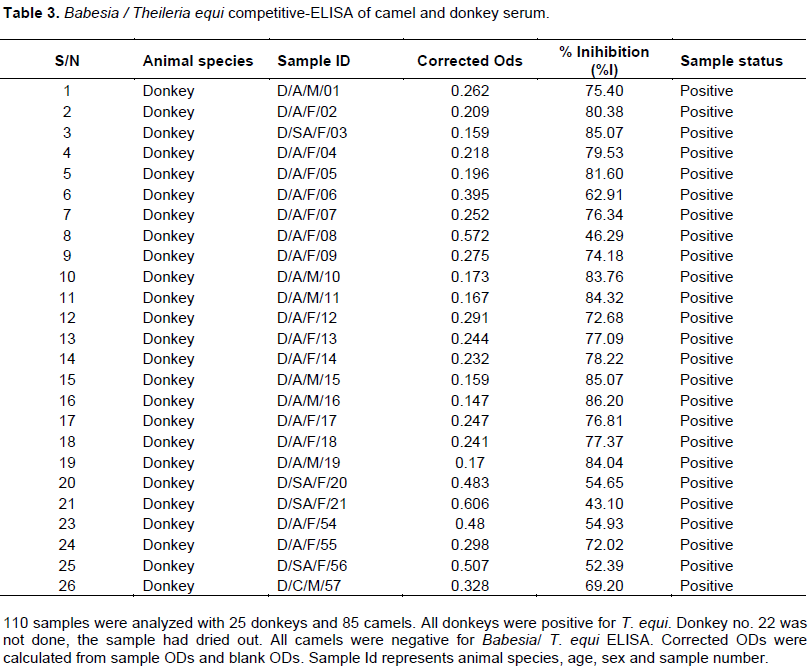
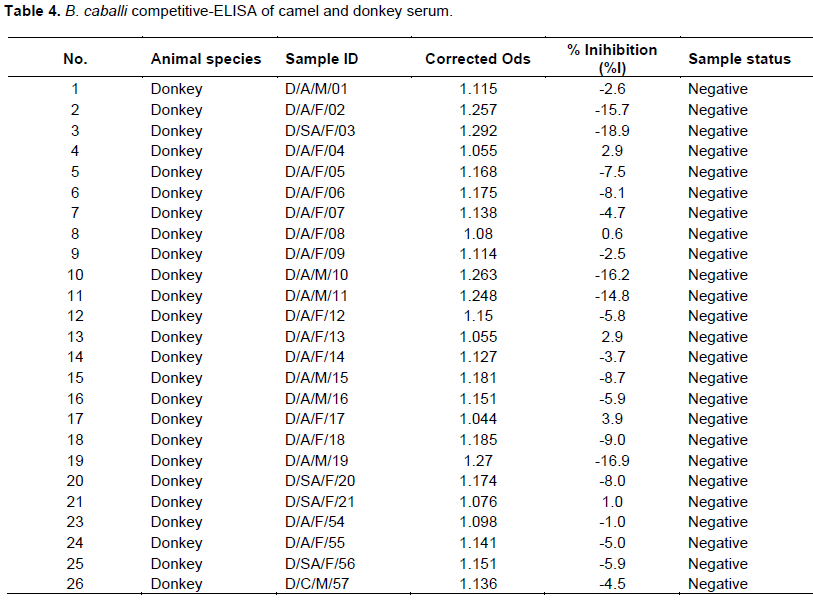
Equine piroplasmosis is a severe disease of horses caused by the intra-erythrocyte protozoan, Theileria equi and Babesia caballi. T. equi and B. caballi infections were assessed in serum from camels and donkeys using competitive- enzyme-linked immunosorbent assay (cELISA) assay. A total 110 animals were studied including 25 donkeys and 85 camels from two districts viz. Moroto and Amudat in Karamoja sub-region, North-eastern Uganda. All the (100%) donkeys tested were positive for Babesia/T. equi while none of the camels had been exposed to the infection. All animals were negative to B. caballi cELISA. Our findings indicated that all donkeys sampled in Karamoja sub-region have been exposed to T. equi and this could be prevalent in equine population in Uganda. No exposure status to B. caballi was reported. This study represents the first report on the status of T. equi and B. caballi infection in Uganda.
Key words: Donkey, Camel, Theileria equi, Babesia caballi, Seroprevalence, cELISA, Uganda.
INTRODUCTION
MATERIALS AND METHODS
ELISA
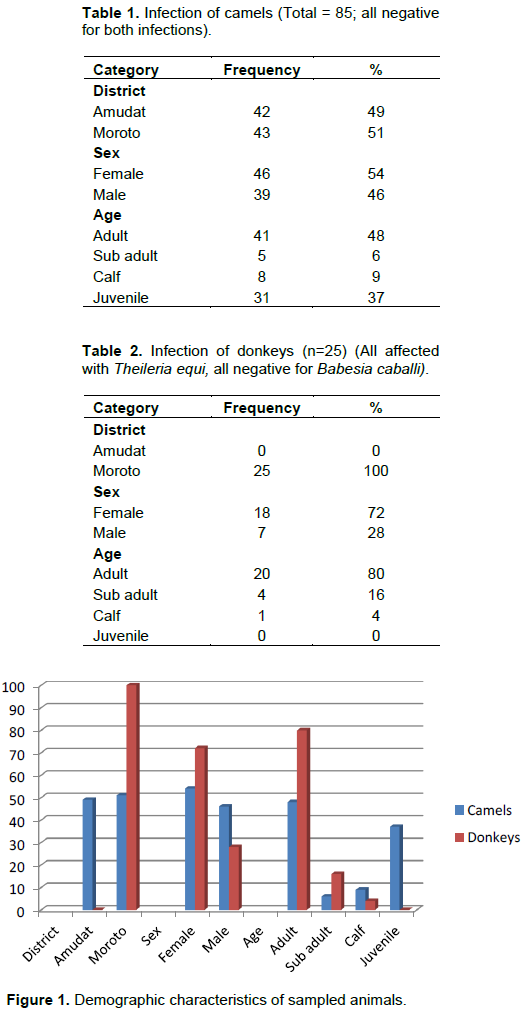
RESULTS
DISCUSSION
CONCLUSION
CONFLICT OF INTERESTS
REFERENCES
|
Abedi V, Razmi G, Seifi H, Naghibi A (2014). Molecular and serological detection of Theileria equi and Babesia caballi infection in horses and ixodid ticks in Iran. Ticks Tick Borne Dis. 5:239-244. |
|
|
Alsaad KM, Hassan SD, Al-Obaidi QT (2012). Detection of Babesia equi and Babesia caballi antibodies in horses and donkeys in Mosul, Iraq. Res. Opinions Anim. Vet. Sci. 2(4):291-294. |
|
|
Baptista C, Lopes MS, Tavares AC, Rojer H, Kappmeyer L, Mendonça D, Machado AC (2013). Diagnosis of Theileria equi infections in horses in the Azores using cELISA and nested PCR. Ticks Tick Borne Dis. 4:242-245. |
|
|
Brüning A (1996) Equine piroplasmosis an update on diagnosis, treatment and prevention. Br. Vet. J. 152:139-151. |
|
|
De Waal DT (1992). Equine piroplasmosis: a review. Br. Vet. J. 148:6-14. |
|
|
De Waal DT, van Heerden J (1994) Equine babesiosis. In: Coetzer JAW, Thomson GR, Tustin RC (eds) Infectious diseases of livestock with special reference to South Africa. vol. 1. Oxford University Press, Cape Town, South Africa. pp. 293-304. |
|
|
Donnellan CMB, Marais HJ (2009). Equine piroplasmosis.In Infectious Diseases of the Horse, ed. Mair TS, Hutchinson RE, Equine Vet. J. 333-340. |
|
|
Friedhoff KT, Soule C (1996). An account on equine babesiosis. Rev. Sci. Off. Int. Epiz.15:1191-1201. |
|
|
Friedhoff KT, Tenter AM, Muller I (1990). Haemoparasites of equines: Impact on international trade of horses. Rev. Sci. Tech. 9:1187-1194. |
|
|
Irwin PJ (2010). Canine babesiosis. Vet. Clin. North Am. Small Anim. Pract. 40(6):1141-1156. |
|
|
Kappmeyer LS, Perryman LE, Hines SA, Baszler TV, Katz JB, Hennager SG, Knowles DP (1999). Detection of equine antibodies to Babesia caballi by recombinant B. caballi rhoptry-associated protein 1 in a competitive-inhibition enzyme-linked immunosorbent assay. J. Clin. Microbiol. 37(7): 2285-2290. |
|
|
Kouam MK, Kantzoura V, Gajadhar AA, Theis JH, Papadopoulos E, Theodoropoulos G (2010). Seroprevalence of equine piroplasms and host-related factors associated with infection in Greece. Vet. Parasitol. 169: 273-278. |
|
|
Kumar S, Kumar R, Gupta AK, Dwivedi SK (2008). Passive transfer of Theileria equi antibodies to neonate foals of immune tolerant mares. Vet. Parasitol. 151:80-85. |
|
|
Kuttler KL (1988). Chemotherapy of babesiosis. In: Ristic M, (Eds.). Babesiosis of domestic animals and man. Boca Raton, USA; CRS Press. 227-243. |
|
|
Levine ND (1971). Taxonomy of the piroplasms. Trans. Am. Microsc. Soc. 90(1):2-33. |
|
|
Mehlhorn H, Schein E (1998). Redescription of Babesia equi Laveran, 1901 as Theileria equi. Parasitol. Res. 84:467-475. |
|
|
Moretti A, Mangili A, Salvatori R, Maresca C, Scoccia E, Torina A, Moretta I, Gabrielli S, Tampieri MP, Pietrobelli M (2010). Prevalence and diagnosis of Babesia and Theileria infections horses in Italy: A preliminary study. Vet. J.184:346-350. |
|
|
Nakayima J, Kabasa W, AleperD, Okidi D (2017).Prevalence of endo- parasites in donkeys and camels in Karamoja sub-region, North-eastern Uganda. J. Vet. Med. Anim. Health 9(1):11-15. |
|
|
Oncel T, Vural G, Gicik Y, Arslan MO (2007).Detection of Babesia (Theileria) equi (Laveran, 1901) in horses in the Kars Province of Turkey. Türkiye Parazitoloji Dergisi 31(3):170-172. |
|
|
Posnett ES, Fehrsen J, de Waal DT, Ambrosio RE (1991). Detection of Babesia equi infected horses and carrier animals using a DNA probe. J. Vet. Parasitol. 39:19-32. |
|
|
Rosales R, Rangel-Rivas A, Escalona A, Jordan LS, Gonzatti MI, Aso PM, Perrone T, Silva-Iturriza A, Mijares A (2013). Detection of Theileria equi and Babesia caballi infections in Venezuelan horses using Competitive-Inhibition ELISA and PCR. Vet.Parasitol.196:37-43. |
|
|
SadeghiDehkordi Z, Zakeri S, Nabian S, Bahonar A, Ghasemi F, Noorollahi F, Rahbari S (2010). Molecular and biomorphometrical identification of ovine babesiosis in Iran. Iranian J.Parasitol. 5(4):21-30. |
|
|
Salib FA, Youssef RR, Rizk LG, Said SF (2013). Epidemiology, diagnosis and therapy of Theileriaequi infection in Giza, Egypt. Vet. World 6(2):76-82. |
|
|
Salim B, Hassan SM, Bakheit MA, Alhassan A, Igarashi I, Karanis P, Abdelrahman MB (2008). Diagnosis of Babesia caballi and Theileria equi infections in horses in Sudan using ELISA and PCR. Parasitol. Res.103: 1145-1150 |
|
|
Schein E (1988). Equine babesiosis. In: Ristic M. (Eds.). Babesiosis of Domestic Animals and Man. Boca Raton, USA; CRS Press. pp.197-208. |
|
|
Scoles GA, Ueti MW (2015). Vector ecology of equine piroplasmosis. Annu. Rev. Entomol; 60:561-580. |
|
|
Shkap V, Cohen I, Leibovitz B, Savitsky, Pipano E, Avni G, Shofer S, Giger U, Kappmeyer L Knowles D (1998). Seroprevalence of Babesiaequi among horses in Israel using competitive inhibition ELISA and IFA assays. Vet. Parasitol. 76:251-259. |
|
|
Singla LD, Sumbria D (2017). Equine piroplasmosis: Belles-lettres update with special reference to Indian scenario. In: An Update on Diagnosis and Control of Parasitic Diseases, Ananda KJ, Pradeep BS, Rakesh RL and Malatesh DS (Eds), Department of Veterinary Parasitology, Veterinary College, Shimoga, KVAFSU,Bidar, Karnataka, India. pp. 372-400. |
|
|
Steinman A, Zimmerman T, Klement E, Lensky IM, Berlin D, Gottlieb Y, Baneth G (2012). Demographic and environmental risk factors for infection by Theileria equi in 590 horses in Israel. Vet Parasitol.187:558-562. |
|
|
Sumbria D, Singla LD, Kumar S, Sharma A, Dhayia R, Setia RK (2016). Spatial distribution, risk factors and haematobiochemical alterations associated with Theileria equi infected equines of Punjab diagnosed by indirect ELISA and nested PCR. Acta Trop.155:104-112. |
|
|
Sumbria D, Singla LD, Mandhotra A, Randhawa CS (2015).Molecular Detection and Treatment of Equine Piroplasmosis. Philippine J. Vet. Med. 52(2):131-136. |
|
|
Sumbria D, Singla LD, Sharma A (2016). Theileria equi and Babesia caballi infection of equids in Punjab, India: a serological and molecular survey. Trop. Anim. Health. Prod. 48(1):45-52. |
|
|
Sumbria D, Singla LD, Sharma A, Bal MS, Randhawa CS (2017). Molecular survey in relation to risk factors and haemato-biochemical alteration in Theileria equi infection of equines in Punjab Province, India. Vet. Parasitol. Reg. Stud. Rep. 8:43-50. |
|
|
Uilenberg G (2006). Babesia-a historical overview. Vet.Parasitol.138(1-2):3-10. |
|
|
Wise LN, Kappmeyer LS, Mealey RH, Knowles DP (2013). Review of equine piroplasmosis. J. Vet. Intern. Med. 27(6):1334-1346. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0