Full Length Research Paper
ABSTRACT
A cross-sectional study was carried out in Nyangatom wereda of South Omo zone, Southern Nation and Nationalities People Region (SNNPR), Ethiopia with the general objectives to find out the prevalence of bovine trypanosomiasis and the risk factors associated with its prevalence from January to June 2015. To identify the protozoa blood samples (n =384) collected from the marginal ear vein of indigenous zebu cattle of more than one year age and both the sexes from three kebeles were examined by buffy coat technique, direct blood smear, thick blood smear and thin blood smear after staining. The overall prevalence of bovine trypanosomaisis was 26.3%. On peasant associations (PA’S) basis Lebere kebele has the highest prevalence 39(30.5%) followed by Shenkora kebele 34 (26.6%) and Ayipa kebele 28 (21.9%). Trypanosoma congolense is the most prevalent species (14.3%) followed by Trypanosoma vivax (5.70%) and Trypanosoma brucei (5.50%). A significant association was observed (P<0.05) between the disease positivity and age, sex and body condition score. The prevalence of trypanosomiasis was 16.20 and 31.50% in young and adult respectively. The prevalence 42.80 and 16.30 % in poor and good body condition score respectively. There was significant association between the risk factors and the species of trypanosomiasis (P<0.05). The result of the present study revealed that trypanosomiasis is the most important problem for animal production in the study area. Strategic control of bovine trypanosomiasis should be strengthened to improve livestock production and agricultural development in the area.
Key words: Bovine, buffy coat, Nyangatom, prevalence, trypanosomiasis.
Abbreviation: ALC, Annual loss from liver condemnation; DACA, Disease Administration and Control Authority; FAO, Food and Agricultural Organization; HAT, Human African Trypanosomiasis; P, Prevalence rate of the disease at the study area; PA, Peasant Association; SNNPR, Southern Nation and Nationalities People Region; SOFEDD, South Omo zone Finance and Economy Development Department; STEP, Southern Tsetse Eradication Program.INTRODUCTION
MATERIALS AND METHODS
RESULTS

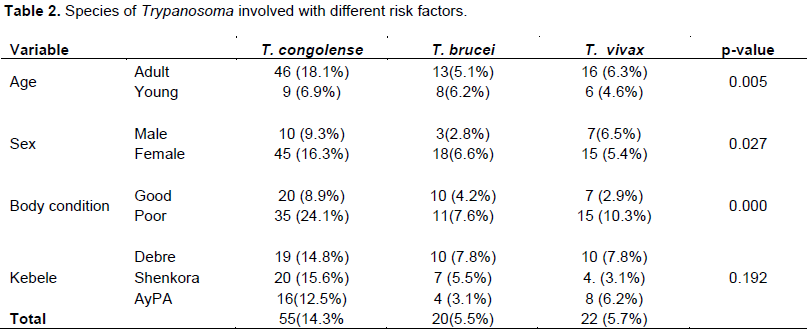

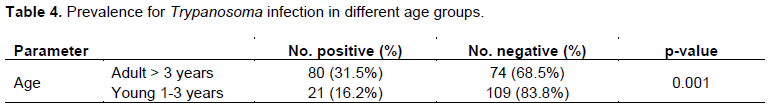
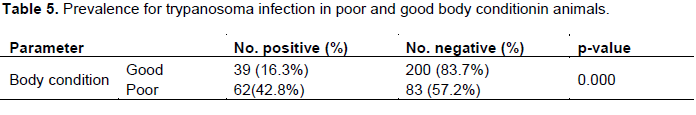
DISCUSSION
CONFLICT OF INTERESTS
REFERENCES
|
Abiy M (2002). Prevalence Of bovine trypanosomosisinGoroWoreda, southwest Ethiopia, DVM Thesis.Abstract, DebreZeit. pp. 342-344. |
|
|
Abraham Z, Tesfaheywet Z (2012). Prevalence of Bovine Trypanosomosisin Selected District of Arba Minch, Snnpr, Southern Ethiopia. Glob. Vet. 8(2):168-173. |
|
|
Afework Y (2001).Field investigation on theappearance of drug resistant population of trypanosomes in Metekel District, North-West Ethiopia.Msc thesis, Addis Ababa University and Freiuniverstat Berlin, Faculty of veterinary medicine, Ethiopia. pp. 32-36. |
|
|
Aulakh GS, Singla LD, Singh J (2005) Bovine trypanosomosis due to Trypanosoma evansi: Clinical, haemato biochemical and therapeutic studies. In: New Horizons in Animal Sciences, Sobti RC and Sharma VL(Eds), Vishal Publishing and Co., Jalandhar. pp. 137-144. |
|
|
Black J, Seed R (2002). The African Trypanosomes.KluwerAcademi Publishers Institute), Nairobi, Kenya. pp. 146-148. |
|
|
Central Statistical Agency (CSA) (2013). Federal democratic republic of Ethiopia Central Statistical Agency, Agricultural Sample Survey 2013/14 Volume II, Report on livestock and livestock characteristics (private peasant holdings), Addis Ababa. Stat. bull. 573:13-14. |
|
|
DACA (2006). standard veterinary Treatment Guidelines for veterinary practice 1Sted.Drug Administration and Control Authority of Ethiopia. |
|
|
Getachew A, Jobre Y (1996).Trypanosomiasis.A threat to cattle production in Ethiopia.Revue de Médecine Vétérinaire 147:897-902. |
|
|
Jahnke E, Tacher G, Keil P, Rojat D (1988).Livestock production in tropical Africa, with special reference to the tsetse-affected zone. In Livestock production in tsetse affected areas of Africa. International Livestock Centre for Africa/International Laboratory for Research on Animal Diseases, Nairobi, Kenya. pp. 430-432. |
|
|
Juyal PD, Singla LD, Kaur P (2005). Management of surra due to Trypanosoma evansi in India: an overview. Infectious diseases of domestic animals and zoonosis in India. 75:109-120. |
|
|
Kumar H, Gupta MP, Sidhu PK, Mahajan V, Bal MS, Kaur K, Ashuma, Verma S, Singla LD (2012) An outbreak of acute Trypanosomaevansi infection in crossbred cattle in Punjab, India. J. Appl. Anim. Res. 40(03):256-259. |
|
|
Langridge P (1976). A tsetse and trypanosomiasis survey of Ethiopia. UK Ministry of Overseas Development, London, 118-119. |
|
|
Leak A (1999). Tsetse biology and ecology: The role in the epidemiology and control of trypanosomosis. CAB international. Walling fored (UK). pp. 152-210. |
|
|
OIE (Office Internationale des Epizooties)(2008).Trypanosomiasis(tsetse-transmitted):TerrestrialManual.OfficeInternationale des.Epizooties (OIE), Paris, France. |
|
|
Paris J, Murray M, Mcodimba F (1982). A comparative evaluation of the parasitological technique currently available for the diagnosis of African trypanosomosis in cattle. Acta Trop. 39:307-316. |
|
|
Radostits OM, Gay CC, Hinchcliff, KW and Constable, PD (2007).Veterinary Medicine, A textbook of the disease ofcattle,sheep,goat,pigsandhorses, 10th edi. Saunders Elsevier London, New York. pp. 2047-1533. |
|
|
Rowlands GJ, Leak SG, Peregrine AS, Nagda SM, Mulatu W, Dieteren GD (2001). The incidence of new and the prevalence and persistence of recurrent trypanosome infections in cattle in south-west Ethiopia exposed to a high challenge with drug-resistant parasites. Acta Trop. 79:149-163. |
|
|
Sharma A, Singla LD, Tuli A, Kaur P, Bal MS (2015). Detection and assessment of risk factors associated with natural concurrent infection of Trypanosomaevansiand Anaplasmamarginalein dairy animals by duplex PCR in eastern Punjab. Trop. Anim. Health Prod. 47:251-257. |
|
|
Sharma P, Juyal PD, Singla LD, Chachra D, Pawar H (2012). Comparative evaluation of real time PCR assay with conventional parasitological techniques for diagnosis of evansi in cattle and buffaloes. Vet. Parasitol. 190:375-382. |
|
|
Shimelis D, Aran S, Getachew A (2005). Epidemiology of tsetse transmitted Trypanosomosis in Abay (Blue Nile) basin of North West Ethiopia. Proceedings of the 28th meeting of the International Scientific Council for Trypanosomiasis. |
|
|
Shimelis D, Aran S, Getachew A (2005). Epidemiology of tsetse transmitted Trypanosomosis in Abay (Blue Nile) basin of North West Ethiopia. Proceedings of the 28th meeting of the International Scientific Council for Trypanosomiasis. pp. 49-53. |
|
|
Singla LD, Juyal PD, Sharma NS (2009) Immune responses to haemorrhagicsepticaemia (HS) vaccination in Trypanosomaevansi infected buffalo-calves. Trop. Anim. Health Prod. 42:589-595. |
|
|
SOFEDD (South Omo zone Finance and Economy Development Department) (2012). Zonal Statistical Abstracts 2012 (2004 E C), Jinka, Ethiopia. |
|
|
Sumbria D, Singla LD, Sharma A, Bal MS, Kumar S (2015). Multiplex PCR for detection of Trypanosomaevansiand Theileriaequiin equids of Punjab, India. Vet. Parasitol. 211(3-4):293-299. |
|
|
Terzu D (2004).Season dynamics of TseTse and trypanosomiasis in selected sites of Southern Nations and Nationality People Region (SNNPRG).Mscthesis.AAU.Debrezeit Ethiopia. |
|
|
Tewelde N, Abebe G, Eisler M, Mcdermott J, Greiner M, Afework Y, Kyule M, Munstermann S, ZessinH, Clausen H (2004).Application of field methods to assess isomethamidium resistance of trypanosomes in cattle in Western Ethiopia. Acta Trop. 90:163-170. |
|
|
Thrusfield M (2005). Veterinary Epidemiology.3rd edition. UK, Blackwell science Ltd. pp. 233-250. |
|
|
Uilenberg G (1998). A field guide for diagnosis, treatment and prevention of African animal trypanosomosis. Adapted from the original edition by Boyt.W. P. Food and Agriculture organization of United Nations (FAO), Rome. pp. 43-135. |
|
|
Urquhart M, Armour J, Duncan L, Dunn M and Jennings W (1996). parasitology 2nd edition. Black Well Science Ltd., London UK. pp. 212-219. |
|
|
Woldeyes G, Aboset G (1997). Tsetse and trypanosomosis distribution, identification and assessment of socio-economic viabilities of the new vector control approaches in Arbaminch ZuriaWoreda. EVA. Proceedings of the 11th conference 143-154. |
|
|
Wondewosen T, Dechasa T, Anteneh W (2012). Prevalence study of bovine trypanosomosis and tsetse density in selected villages of Arbaminch, Ethiopia. J. Vet. Med. Anim. Health 4(3):36-41. |
|
|
Wondwosen A (1986). Tsetse andTrypanosomosis in Bunnoprovince. DVM Thesis.F.V.M: A.A.U. Debre Zeit. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0