ABSTRACT
This study was conducted to determine the prevalence and antibiotic susceptibility pattern of Salmonella species from raw beef obtained from markets and Japanese quail eggs from farms and retail outlets in Jos and environs, Nigeria, using colony morphology and biochemical tests. Of the 100 raw beef samples examined, 11 were contaminated with Salmonella given a prevalence of 11.0%. Salmonella was isolated from 3 out of the 225 quail eggs analyzed. The result revealed that only 0.8% of the quail egg contents were positive for Salmonella while the prevalence on egg shell was 1.7%. The prevalence of Salmonella on raw beef was found to be relatively higher in Tudun Wada and Rukuba Road 4(20.0%), while Terminus, Abattoir and Gada Biyu had a lower prevalence of 1 (5.0%). The relative prevalence of Salmonella on the egg shell and content base on location indicates considerable high (4.0%) levels of contamination in samples from Jos South Local Government Area while eggs collected from Jos North and Jos East revealed a prevalence of 0.0%. The antibiotic sensitivity pattern of the isolates showed varying degree of sensitivity. The isolates were moderately sensitive to Ciprofloxacin, Sulphademethoxazole, Chloramphenicol and Gentamycin recording the highest sensitivity. The isolates were completely resistant to Tetracyline, Neomycin, Oxacillin and Erythromycin. Multi-drug resistance was reported in isolates from quail eggs from Farm A. All the isolates from the three farms were resistant to Oxacillin and Erythromycin while two (from Farms A and C) were resistant to Sulphadimethoxazole. Regulatory control of antibiotics usage in livestock production, meat/poultry hygiene and pharmaco-epidemiological surveillance in food animals to ensure consumer safety were therefore recommended.
Key words: Salmonella, resistance, susceptibility, sensitivity, surveillance.
Salmonella have been isolated from nearly all vertebrates, and infection has been associated with both animal and
human disease. Human Salmonella infections in the United States have been estimated in millions
annually. In some of these cases, foods of animal origin have been implicated as the source of the pathogen (APHIS, 2001). Although,Salmonella infections are increasing worldwide, there are only few reports on Salmonella surveillance in African and other developing countries. This has made it difficult to estimate the actual burden of salmonellosis, especially in Africa (Saba et al., 2013).
Contaminated raw meat is one of the main sources of food-borne illness (Bhandare et al., 2007; Podpecan et al., 2007). Cattle have been incriminated as reservoirs of Salmonella species. In meat animals (cattle, swine and sheep), Salmonella infection arises from intensive rearing practices and the use of contaminated feeds. Cross contamination of carcasses with Salmonella can also occur during slaughtering operations which may include harvest time such as transport, lairage, slaughter and dressing. Other sources of contamination may be at post harvest time such as boning, processing, retail distribution, and preparation (Ejeta et al., 2004).
Salmonella infections have also been reported to result from the ingestion of contaminated eggs and these are associated with consumption of raw eggs and food containing eggs (Hogue et al., 1997).
Egg and egg products have been implicated in most cases of human Salmonellosis (Lepoutre et al., 1994). Infected ovaries and oviducts of the hen have been shown to be the major source of contamination of eggs with Salmonella Enteritidis (CDC, 1996). More so, eggs can also become contaminated on the surface either from feces or the environment. Contaminated poultry eggs serve as important vehicles of Salmonella infections, especially when the bacterium is in the egg contents. Hence, increased consumption of raw eggs (as with quail eggs in Jos) may increase the potential for exposure to Salmonella spp. The administration of antimicrobial agents in animals creates selection pressure that favors the survival of antibiotic resistant pathogens. Drug resistance has been shown to be the most important hazard of drug residues in the country (Dipeolu and Alonge, 2002). Resistant bacteria could cause disease that is difficult to treat in humans and may also transfer the resistant gene to some other human pathogens (Barton, 2000; Doyle, 2006).
Multidrug-resistant phenotypes have been increasingly described among Salmonella spp. worldwide (Gyels, 2008). Consumption of raw quail egg has increased over the years in Jos Plateau State of Nigeria while beef are sold in open market where flies and other dust particles can serve as source of contamination. At present, there is no documented study of prevalence of Salmonella spp. in food products especially beef and quail eggs available in Jos. For effective long-term control of Salmonella infection, assessment of safety and quality of food is important. This study was therefore conducted to determine the prevalence of Salmonella spp. in raw beef and quail eggs in Jos Plateau State Nigeria.
Study area
The study was conducted in Jos metropolis. It is located at Latitude: 10° 0' 0 N, Longitude: 9° 30' 0 E on the Jos Plateau (SCRD, 2011). The city is divided into three separate local government areas: Jos-North, Jos-South, and Jos- East with a combined population density of 391 persons/km2. The city has an altitude of 1,217 m above sea level and so enjoys a more temperate climate than most of the rest of Nigeria (SCRD, 2011).
Sampling technique
The study was cross-sectional and samples were collected using the formula of Thrusfield (1997). A total of 100 beef samples were bought from randomly selected 5 beef retail points in Jos and its environs. Twenty beef samples were collected at each retail points. Five retail outlets each in Jos North, Jos South and Jos East Local Government Areas were identified. Fifteen Japanese quail eggs were bought in each retail outlet giving a total of 225 (This comprises of 105 for egg shell and 120 for egg content).
Samples were collected aseptically into clean, dry leak-proof wide-mouth sterile bags and placed into icebox. Samples were then transported to the Bacteriology Laboratory Department of National Veterinary Research Institute, Vom.
Bacterial culture, isolation and identification
Samples were analyzed as described by Wallace and Thomas (2003). Briefly, 25 g of each sample of beef was thoroughly homogenized with 15 ml of buffer peptone water and incubated at 37°C for 24 h. Swab technique was used to sample the surface of the intact eggs. The swabs were directly inoculated into 10 ml buffered peptone water for pre-enrichment, in screw capped bottles and incubated at 37°C for 24 h.
In order to collect the egg content, the egg shell was aseptically broken and the content of each egg was transferred into 10 ml of buffered peptone water, homogenized and incubated at 37°C for 24 h. A portion was then inoculated into an enrichment media (Rappaport Vasiliadis broth) and incubated for 24 h.
Bacteria culture
A wire loop full was then cultured into an enriched selective media for Salmonella (XLD agar) and incubated at 37°C for 24 h. Typical colonies of Salmonella based on cultural and morphological characteristics (that is, transparent colonies with black centre on SSA and pink colonies surrounded by a red medium on BGA, and small red translucent and or dome-shaped colonies, which may have central black spot due to hydrogen sulphide (OIE, 2012) production were picked.
Purification of isolates
The isolates were sub cultured onto SSA and nutrient agar for isolation of pure culture and subsequent biochemical characterization.
Biochemical characterization of Salmonella
Isolation and identification of organisms was carried out as described by ISO (2002), Habtamu et al. (2011) and OIE (2012). A 24 h pure culture of each isolate was used to determine their gram stain reaction. The following biochemical tests were carried out on each isolate in individual test tubes: Indole test (Yellow color-Negative), triple sugar iron test (H2S production-black color along stab line), citrate test (green color-negative), methyl-red test (Red color-positive), Voges-Proskauer test (yellow brown color-negative), lysine decarboxylase test (purple color-positive), ornithine decarboxylase test, urease test (No change in color-negative), sugar (trehalose, sucrose (negative), inositol, glucose (positive), lactose (negative) dulcitol, maltose, mannitol (positive), maltose (positive), melibiose, salicin, rhamnose and arabinose fermentation test and motility test.
Determination of antibiotic sensitivity patterns of the isolates
The Salmonella isolates were further subjected to the antibiotic sensitivity test using the agar disc-diffusion method according to the guidelines of the Clinical Laboratory Standard Institute (CLSI) (2008). Briefly, the antibiotic discs (Oxoid, UK) were evenly dispensed unto the surface of the inoculated agar plate using a disc dispenser and were gently pressed down to ensure complete contact with the agar surface. The plates were inverted and incubated at 37°C for 18 h. The following 8 antibiotic discs were used: sulphamethoxazole 25 μg, ciprofloxacin (CIP) 5 μg, chloramphenicol (C) 30 μg, gentamycin (CN) 30 μg, tetracycline (TE) 30 μg, oxacillin (OX) 5 μg, and Erythromycin 15 μg, Neomycin 30 μg were applied in the test. The diameters of the zone of inhibition were measured in millimeters with a ruler and compared with a zone interpretation chart (Muragkar et al., 2004). McFarlland scale was used and the result was reported as sensitive, intermediate or resistant based on the size of zone of inhibition table 5.
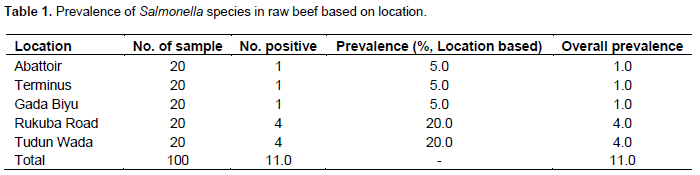
Of the 100 raw beef samples examined, 11 (11.0%) were positive for Salmonella. Prevalence of Salmonella in raw beef was higher in Tudun Wada and Rukuba Road 20.0% (4/100) than in Terminus, Abattoir and Gada Biyu 5% (1/100) as shown in Table 1.
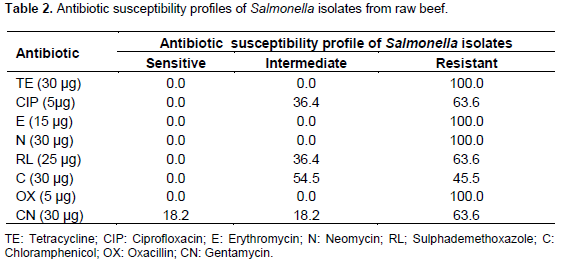
The antibiotic susceptibility profile of the Salmonella isolates from beef characterized in this study displayed resistance to one or more antibiotics (Table 2). The antibiotic sensitivity pattern of the isolates showed varying degree of sensitivity with Ciprofloxacin, Chloramphenicol and Gentamycin recording the highest sensitivity. The isolates were moderately sensitive to Sulphademethoxazole, Tetracyline and completely resistant to Oxacillin (Table 2). The overall prevalence of Salmonella in eggs was 1.3% (3/235). Of this, 1.7 and 0.8% represented prevalence rates of egg shell and egg content, respectively as shown in Table 3. Based on location, Jos South recorded prevalence rates of 5.7 and 2.5% for egg shell and content, respectively. However, both Jos North and Jos East recorded prevalence rates of 0.0% (Table 4).
A total of three (3/235) Salmonella isolates from Japanese quail eggs and content were tested for their susceptibility to 8 different antibiotics. Overall resistance or susceptibility among Salmonella from quail eggs is given in Table 3. The isolate from farm A was only susceptible to Gentamycin and was resistant to Chloramphenicol, Neomycin, Erythromycin, Ciproflaxacin, Tetracycline, and Sulphademethoxazole. All the isolates from the three farms were susceptible to Gentamycin and resistant to Oxacillin while isolates from Farm B was susceptible to almost all the antibiotics except Oxacillin. Isolate from Farms A and C were resistant to Tetracycline, Sulphadimethoxazole and Oxacillin.
Food-borne diseases are considered to be global public health concern. In this study, the overall prevalence of Salmonella spp. in beef samples from Jos and environs, Nigeria was found to be 11.0%. Samples within close proximity to the abattoir showed lower prevalence (1.0%) than those far from the abattoir (Rukuba Road 4.0%; Tudun Wada 4.0%). This might be attributed to the unhygienic post slaughter operations such as transportation, handling of the beef by the distributors and even the retailers/meat sellers in the study area. It is most likely that the tables where beef are kept and vehicles used in transporting the meat are not properly cleaned. More so, knives and other materials used during the sales may be sources of contamination. The beef sampled were also sold in open markets where flies and other dust particles can serve as sources of conta-mination. There is therefore the need to have minimum hygienic operation standards for retail meat premises in the area. The standard should include a range of requirements related to the receiving, storage, processing, display, packaging and transportation of meat. McDonough et al. (1999) reported that cattle are among the known reservoirs of Salmonella. This might be a potential contamination source in slaughter houses and in retail beef carcasses. Contamination arising from the butchers and beef retailers through poor toilet manners and poor hand hygiene is another possible explanation since their health status was not ascertain. Cross contamination of edible carcass tissue due to the presence of Salmonella in cattle at slaughter or contamination due to poor handling process present a significant food safety hazard. Because the presence of any Salmonella is potentially a health threat, consumption of raw eggs and or undercooked beef in the study area may serve as vehicle for the transmission of Salmonella to humans.
Prevalence of Salmonella contamination on the shells of quail eggs from farms and retail outlets in Jos, PlateauState, Nigeria was found to be 1.7%. In a similar study conducted in India, Harsha et al. (2011) reported a high (9.0%) prevalence of Salmonella contamination in shell of quail eggs and 0.0% in the egg content. Though the 0.8% prevalence of Salmonella revealed by the study may seem negligible, the public health implication of this in causing human salmonellosis can never be over emphasized. Since most of the positive samples in this study and those of other places were from egg shell, one may conclude that the contamination may be horizontal due to cross contamination from faeces or from the cage environment. Guard-petter, 2001 in a study reported that eggs can be contaminated with droppings from chickens excreting Salmonella. In such cases Salmonella in droppings can penetrate egg shell pores as egg cools and before the establishment of the proteinaceous cuticular barrier (St Louis, 1988). In view of this, S. enteritidis is able to persist on the surface of the egg shell and potentially cross contaminate the liquid portion of the egg when eggs are broken for preparation of food, which could pose a potential health risk to the society (Charles and Takayuki, 2010). This further suggests that prompt removal of the quails waste and disinfection can greatly reduce Salmonella contamination on the shell and the content. The study showed that Salmonella contamination in quail eggs (shell and content) was only found in samples obtained from Jos South Local Government Area.
No isolate was obtained in samples from Jos East and Jos North Local Government Areas. Agada et al. (2014) in a related study reported that prevalence of Salmonella isolates from human faeces/hand swabs, poultry droppings, swabs from shells of intact eggs and feeds was higher in Jos South Local Government Area. The distribution may not be unconnected with certain factors such as differences in the socio-demography and biosecurity practices in the areas. Salmonella entry into the farms can occur through environmental related factors such as litter, dust, mice, flies and even the poultry feed (Galis et al., 2013). Therefore, lack of proper biosecurity measures in poultry farms could lead to cross contamination from one poultry house to another.
The antibiotic sensitivity pattern of the Salmonella isolates from the study showed varying degrees of sensitivity with Ciprofloxacin, Chloramphenicol and Gentamycin recording the highest sensitivity. The isolates were moderately sensitive to Sulphademethoxazole and completely resistant to Tetracycline, Erythromycin, Neomycin and Oxacillin. This partially agrees with the findings of Agada et al. (2014) who in a similar study in Jos reported that all the Salmonella isolated were resistant to Oxacillin. This resistance may not be unconnected with indiscriminate use of this drug. The highest level of susceptibility was detected in Chloram-phenicol, Ciprofloxacin and Gentamycin. This is in agreement with Gordana et al. (2012) who reported similar susceptibility in chloramphenicol and ciprofloxacin.
High sensitivity to Ciprofloxacin, Chloramphenicol and Gentamycin adequately explains it increasingly and successfully used for treatment of scepticemic salmonellosis in humans (Agada et al., 2014).
Antimicrobial resistant foodborne pathogens are considered to be acquired primarily through consumption of contaminated food of animal origin or water (Mead et al., 1999). Antimicrobial use and misuse has been considered to be the most vital selecting force to antimicrobial resistance of bacteria development and spread in both veterinary and human medicine (Okeke et al., 2005). Indiscriminate antibiotics usage in food animals especially poultry could predispose consumers to risks of antibiotic resistant bacterial infections. This situation is further complicated by the potential of resistant bacteria to transfer their resistance determinants to resident constituents of the human microflora and other pathogenic bacteria. The antimicrobial susceptibility test of the Salmonella isolates encountered in quail eggs in the present study revealed that the isolate from farm A had acquired resistance to more than 5 antibiotics. This study has revealed a multi-drug resistance of isolate of Salmonella in farm A and a lower resistance in isolates from farm B and C. More so, the isolate from farm A was resistant to Ciprofloxacin while isolates from farms B and C were not. However, the isolates from farms A and C were resistant to Tetracycline and the isolates from the three farms were resistant to Oxacillin and Sulphadimethoxazole. These may not be unconnected to the frequent and indiscriminate use of these antibiotics in quail birds at sub-therapeutic level or prophylactic doses. This promotes on-farm selection of antimicrobial resistant strains and also increases the human health risks associated with consumption of contaminated quail eggs.
Despite the seeming poor and unhygienic practices involved in the handing of beef in the study area, prevalence of Salmonella spp. was relatively low. Samples within close proximity to the abattoir showed lower prevalence (1.0%) than those far from the abattoir. The antibiotic sensitivity pattern of the Salmonella isolates from the study showed varying degree of sensitivity with Ciprofloxacin, Chloramphenicol and Gentamycin recording the highest sensitivity. The isolates were moderately sensitive to Sulphademethoxazole, Tetracyline and completely resistant to Oxacillin.
The study has also revealed a low prevalence Salmonella species in commercial quail eggs from different retail outlets of the three local government areas in Jos, Plateau State. More so, multi-drug resistance was reported in isolate from farm A. All the isolates from the three farms were resistant to Sulphadimethoxazole while two (from farms A and C) were resistant to Tetracycline and Oxacillin.
Improving the conditions in which quails are raised by periodic disposal of waste and disinfection may help in maintaining a low level of Salmonella contamination in quail eggs. Strict hygiene during post slaughter operations such as transportation, handling of the beef by the distributors and the retailers/meat sellers may reduce Salmonella contamination in beef. We also, recommend that further studies be carried out to determine the serovars of the Salmonella isolates from beef and quail eggs in the area.
The authors have not declared any conflict of interests.
REFERENCES
|
Agada GOA, Abdullahi IO, Aminu M, Odugbo M, Chollom SC, Kumbish, PR, Okwori AEJ (2014). Prevalence and Antibiotic Resistance Profile of Salmoella Isolates from commercial poultry and poultry farm-handlers in Jos, Plateau State Nigeria. Br. Microbiol. Res. J. 4(4):462-479
Crossref
|
|
|
|
APHIS (2001). Animal and plant health inspection service; prevalence and antimicrobial susceptibility patterns of salmonella from beef cows.
View
|
|
|
|
|
Barton MD (2000). A paper presented on Public Health Risk: Antibiotic Resistance. Apec China Seminar on Public Health Issues in Animal Production/Animal products; Retrieved November, 2014, Friendship Hotel Beijing P.R. China. pp. 101-102.
|
|
|
|
|
Bhandare SG, Sherikarv AT, Paturkar AM, Waskar VS, Zende RJ (2007). A comparison of microbial contamination of sheep/goat carcasses in a modern Indian abattoir and traditional meat shops. Food Control. 18:854-868
Crossref
|
|
|
|
|
Centre for disease control and prevention (CDC) (1996). Salmonella Enteritidis infection and shell eggs–United States. Morb. Mortal. Wkly. Rep. 39:900-912.
|
|
|
|
|
Charles OAO, Takayuki K (2010). Salmonella enterica serovar Enteritidis: a mini-review of contamination routes and limitations to effective control. JARQ 44(1):7-16
Crossref
|
|
|
|
|
CLSI (2008). Clinical and Laboratory Standard Institute. Performance standard for antimicrobial desk and dilation susceptibility test for bacteria isolated from animals: Approved standard CLSI document M31-A3, 3rd edition.
|
|
|
|
|
Dipeolu MA, Alonge DO (2002). Residues of Streptomycin Antibiotic in Meat Sold for Human consumption in Some States of SW Nigeria. Arch. Zootecnia. 51:477-480.
|
|
|
|
|
Doyle ME (2006). Veterinary Drug Residues in Processed Meat-Potential Health Risk. Food Research Institute Briefings. University of Wisconsin-Madison. Retrieved November, 2014.
|
|
|
|
|
Ejeta G, Molla B, Alemayehu D, Muckle A (2004). Salmonells Serotypes Isolated from minced beef meat, mutton and pork in Addis Ababa, Ethiopia. Rev. Med. Vet. 155:547-551.
|
|
|
|
|
Galis AM, March C, Marlier D, Portelle D, Van Illie, Beckers Y, Thewis A. (2013). Control of Salmonella contamination of shell egg –preharvest and postharvest methods - A review. Compr. Rev. Food Sci. Food Saf. 12:1-182
Crossref
|
|
|
|
|
Gordana M, Bogdanka A, Dragica T, Milena L, Brankica D (2012). Antibiotic Susceptibility of Salmonella spp: A comparison of two surveys with a 5 years interval. J. Imab-annual Proceeding (scientific papers). 18(1):216-219.
|
|
|
|
|
Guard-petter J (2001). The chicken, the egg and Salmonella Enteritidis. Environ. Microbiol. 3:421-430.
Crossref
|
|
|
|
|
Habtamu MT, Rathore R, Dhama K, Rajesh K A (2011). Isolation, Identification and Polymerase Chain Reaction (PCR). Detection of Salmonella species from materials of Poultry origin. Int. J. Microbiol. Res. (2):135-142.
|
|
|
|
|
Harsha HT, Reshmi R, Rinoy V, Divya PS, Mujeeb RKM, Mohamed H AA (2011). Prevalence and antibiotic resistance of Salmonella from the eggs of commercial samples. J. Microbiol. Infect. Dis. 1 (3):93-100.
Crossref
|
|
|
|
|
Hogue A, White P, Guard-petter J, Schlosser W, Gast R, Ebel E, Farrar, J, Gmez T, Madden J, Madison M, McNamara AM, Morales R, Parham D, Sparling P, Sutherlin W, Swerdlow D (1997). Epidemiology and control of eggs associated Salmonella enteritidis in United State of America. Rev. Sci. Tech. 16:542-553.
Crossref
|
|
|
|
|
International Organization of Standardization (ISO) 6579. (2002). Microbiology general guidelines on methods for the detection of Salmonella. International organization of standardization, Geneva, Switzerland.
|
|
|
|
|
Mead P, Slutsker L, Dietz V, McCaign L, Bresee J, Shapiro C (1999). Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625.
Crossref
|
|
|
|
|
McDonough PL, Fogelman D, Shin SJ, Brunner MA, Lein DH (1999) Salmonella enteric serotype Dublin infection: an emerging infectious disease for the north-eastern United States. J. Clin. Microbiol. 37:2418-2427.
|
|
|
|
|
Muragkar HV, Rahman H, Ashok K, Bhattacharyya D (2004). Isolation, phage typing and antibiogram of Salmonella from man and animals in northeastern India. Indian J. Med. Res. 122:237-242
|
|
|
|
|
Office International des Epizooties (OIE). (2012). Fowl typhoid and pullorum disease. In: Terrestrial manual. Office International des Epizooties, Paris, France, pp. 3-5.
|
|
|
|
|
Okeke IN, Laxminarayan R, Butta ZA, Duse AG, Jenkins P, O'Brien TF, Pabloso-Mendez A, Klugman KP (2005). Antimicrobial resistance in developing countries. Part i Recent Trends and current status. Lancet Infect. Dis. pp. 481-493.
Crossref
|
|
|
|
|
Podpecan, B., Pengov, A. and Vadnjal, S. (2007). The source of contamination of ground meat for production of meat products with bacteria Staphylococccus aureus. Slov. Vet. Res. 44:24-30
|
|
|
|
|
Saba CK, Escudero JA, Herrera-Leon S, Porrero MC, Suarez M, Dominguez L, Demuyakor B, Gonzalez-Zorn B (2013). First identification of Salmonella Urbana and Salmonella Ouakam in humans in Africa. J. Infect. Dev. Ctries. 7(10):691-695.
Crossref
|
|
|
|
|
St Louis ME (1988). The emergence of grade A eggs as a major source of Salmonella Enteritidis infections. New implications for the control of salmonellosis. JAMA 259:2103-2107.
Crossref
|
|
|
|
|
State Creation for Rapid Development (SCRD). (2011). History of Plateau State.
|
|
|
|
|
Thrusfield M (1997). Veterinary epidemiology, second edition. Blackwell publications, Oxford pp. 182-183.
|
|
|
|
|
Wallace HA, Thomas SH (2003). Food sampling/preparation of sample homogenate. Bacteriological Analytical Manual by Food and Drug Administration USA, Revised section A.
|
|