ABSTRACT
An observational study on the effects of oxytetracycline treatment on contagious bovine pleuropneumonia in a naturally infected herd of 500 cattle was conducted. A total of 68 cattle that showed pneumonia-like clinical signs were treated. Treatment was effected the moment an animal showed signs of illness. A total of 429 cattle were slaughtered after diagnosis of contagious bovine pleuropneumonia and at slaughter, 40.8% (175) had lesions compared to 59.2% (254) that did not have lesions. Out of the total cattle that were treated with oxytetracycline, 57.4% (39) died from contagious bovine pleuropneumonia over a period of 9 weeks while 42.6% (29) survived. Of the treatment group that survived, 37.9% (11) had fibrous lesions indicative of healing, while 62.1% (18) had pathological lesions consistent of active contagious bovine pleuropneumonia (CBPP). Categorisation of carcases with pathological lesions within the treatment group showed 66.7% (12) and 33.3% (6) of acute and chronic lesions, respectively. The CBPP causative agent was isolated through culture and confirmed using polymerase chain reaction (PCR). The results obtained suggest that oxytetracycline did not stop the spread or death of cattle in this particular herd with the treatment of a proportion of the herd. However, large scale field trials are needed in order to validate these findings. It is therefore recommended that any antibiotic that will be developed and advocated for use in the treatment of contagious bovine pleuropneumonia should be effective to contain spread within the herd by treating only a proportion showing signs of the disease.
Key words: Contagious bovine pleuropneumonia, antibiotics, lung, lesions, oxytetracyline, treatmen, Zambia.
Contagious bovine pleuropneumonia (CBPP) is a highly infectious disease of cattle caused by a mollicute bacteria Mycoplasma mycoides subspecies mycoides (Mmm) and is characterised by severe fibrinous exudative pneumonia (OIE, 2010; Provost et al., 1987). The disease has been recognised as a major hindrance to increased livestock
production in sub-Saharan Africa causing great economic losses due to cattle mortality and morbidity leading to weakness, emaciation, reduced work ability and reduced fertility in affected herds (Amanfu, 2009). Tambi et al. (2006) described the economic importance due to the high financial and economic losses the disease causes to cattle owners and nations, the associated socio-economic implications of these losses and the economic wide impacts (resulting from reduced export earnings and a decline in economic activity in those industries that depend on cattle and their products).
Although CBPP has been successfully controlled in most developed countries (Scacchia et al., 2011), it has continued to spread and affect new areas in sub-Saharan Africa. The disease in Africa is complicated by the uncontrolled movement of cattle and the availability of chronic carriers that have been implicated in the perpetuation of the disease (Masiga et al., 1986).
The main control method that has been adopted by most countries in sub-Saharan Africa is vaccination using an attenuated live T1/44 vaccine strain. The vaccine confers a short immunity requiring booster vaccinations annually and can also revert to pathogenicity at the injection site that can cause death if not treated with antibiotics (Thiaucourt et al., 2007).
Alternative control of CBPP using antibiotics has officially been discouraged although it has been shown that it is widely practised in many African countries (Mariner and Catley, 2004). Mitchell et al. (2012) have demonstrated the effectiveness of oxytetracycline, danofloxacin and tulathromycin in biological matrices as well as artificial media in inhibiting the growth of Mmm, the causative agent of CBPP and suggested the possible use of the three in the treatment of CBPP. Provost et al. (1987) reported that the use of antibiotics may alleviate the clinical signs but would not inhibit the spread of the disease. Studies by Yaya et al. (2004) showed that in an experimental infection,oxytetracycline was able to reduce the losses due to CBPP but could not prevent the persistence of viable Mmm in the treated animals suggesting that treated animals could still spread the disease to susceptible animals. However, in similar studies of naturally infected cattle, Huebschle et al. (2006) reported success in reducing spread of the disease in a trial using danofloxacin, but failed to reduce clinical effects. In an investigation by Niang et al (2010), it was reported that oxytetracycline failed to induce sequestra formation under experimental conditions demonstrating the probability of cure and not the evolution of the disease to chronicity to the treated group. Nicholas et al. (2012) reported success in treatment and prevention of CBPP spread using danofloxacin in naturally infected herds in Caprivi Strip of Northern Namibia.
In FAO meeting of 2002 in Ghana, the effectiveness of antibiotic therapy was put to scrutiny and it was advocated that further research should be carried out to arrive at informed decisions concerning the use of antibiotics in the treatment of CBPP (FAO, 2003). This paper highlights the failure of long-acting oxytetracyclinein reducing the clinical lesions in individual cattle and in halting the spread of CBPP within a herd of naturally infected cattle that were in quarantine.
Study site
The study was limited to a commercial farm located in the Copper belt Province of Zambia. The farm is mainly a crop farming enterprise that was diversifying into cattle ranching. The farm imported a 500 herd of cattle from Tanzania as its start-up herd. The cattle under study were the only ones on the premises. The farm is triple fenced with a barbed wire, game fence and an electric fence all around its perimeter. It has additional fences within the farm to divide various areas for different activities.
Study cattle
The study was restricted to heifers that were imported from Tanzania and were in quarantine at the time of the study. The age range of the cattle was from 3 to 18 months.
History of illness and treatment
According to the available records, on the fourth day after arrival, 3 of the heifers died after showing difficulties in breathing. A post-mortem was conducted and lung samples showing pneumonic signs were collected, placed in the mobile car freezer with a temperature of -20ºC and submitted to the laboratory. They were not processed as the preservation in transit was not good for bacteriological assessment. However, a tentative diagnosis of Pasteurellosis was made in view of the long distance (over 2,800 km) that the heifers covered. Consequently, medication with oxytetracycline was instituted for those heifers showing respiratory discomfort and thereafter those that develop these signs. This led to a total of 13.6% (68) of the herd being treated. A dosage of 20 mg/kg per day (1 ml/10 kg body mass) was given for five days to each of the sick heifers. The sick cattle were not separated from the herd after institution of treatment. Over a 3 month period, 39 of the treated heifers died. The diagnosis of CBPP was only made 112 days after the initial deaths and by then, 92 were showing overt clinical disease characterised by coughing, dyspnoea, polypnoea, and nasal discharge. On running, these signs were accentuated.
Slaughter and collection of samples from the herd
After confirmation of CBPP on serology using CFT (OIE, 2010) and c-ELISA (Le Goff and Thiaucourt, 1998), the herd was ear marked for slaughter.
Pathology/post-mortem
All the cattle that had remained (n=429) were subjected to post-mortem upon slaughter. On the slaughter line, all the animals were identified according to the mark on the ear tagand thus treated and untreated individuals were recognised. Tissue samples that included lungs, lymph nodes and pleural fluid were collected from both groups and stored at -20°C until analysis.
Culture and PCR of isolates
A total of 20 tissue samples from both the treated (10) and untreated groups (10) were cultured in PPLO broth and agar medium (Himedia® India) containing 20% Horse serum as described by Razinand Freundt (1984) for 10 days.
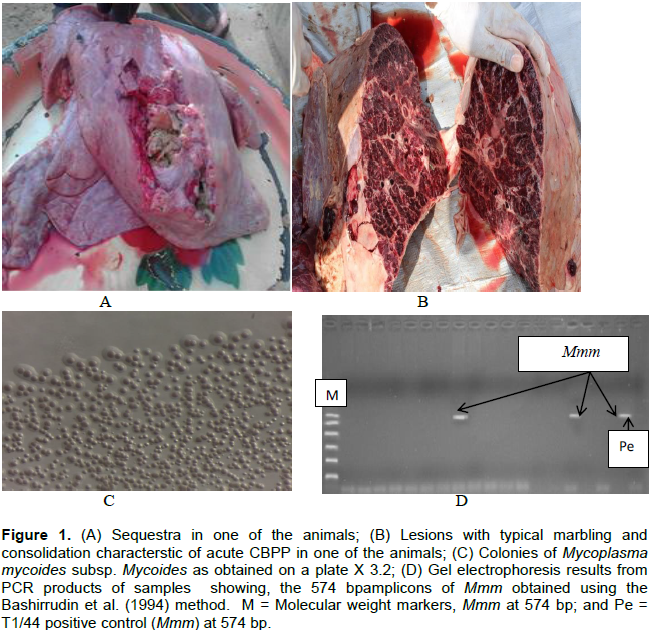
DNA was extracted from the broth cultures of Mmm and purified using the Maxwell ® DNA purification kit following the manufacturer’s instructions. They were subjected to PCR and then restriction enzyme digestion using AsnI as described by Bashiruddin et al. (1994), (Figure 1).
Of the total that were treated, 57.4% (n=39) died with CBPP symptoms before the decision to slaughter the
whole herd was made while 42.6% (n=29) survived until the whole herd was slaughtered (Table 1).
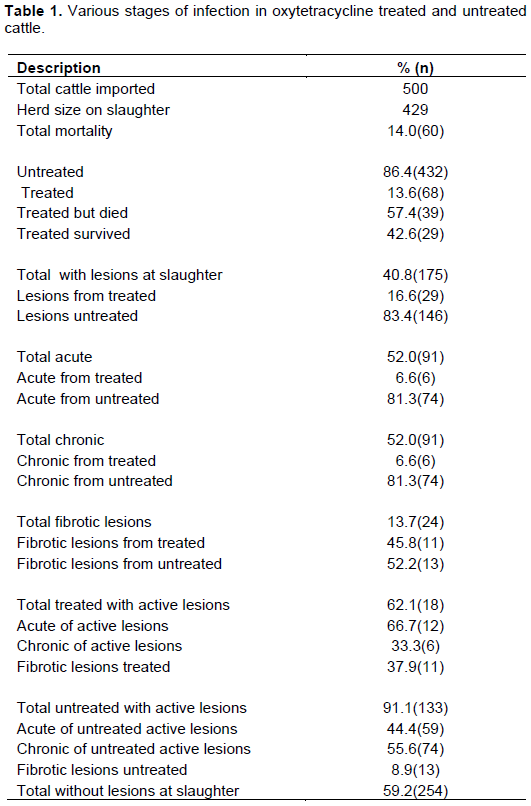
A total of 429 cattle were slaughtered and on post-mortem examination of all the carcasses, 40.8% (n=175) had lesions of various stages of disease progression while 59.2% (n=254) did not have any lesions. Of those with lesions, 16.6% (n=29) were those from the treated group while 83.4% (n=146) were of the untreated cattle. There was 13.7% (n=24) carcases with fibrotic lesions with indications of healing. Of these 45.8% (n=11) were from the treated group while 52.2% (n=13) were from the untreated group. When the carcasses with lesions and those showing signs of recovery were compared in the treated group, it was found that 37.9% (n=11) had lesions in the lungs showing signs of recovery, while 62.1% (n=18) had classical lesions of clinical CBPP. Those from the untreated group showed 8.9% (n=13) with signs of lesions of CBPP. When the carcasses with classical healing compared to 91.1% (n=133) that had classical lesions from the treated group were further examined, it was observed that 66.7% (n=12) had lesions in the acute phase of CBPP with typical marbled appearance of the lung, various degrees of adhesion to the pleural wall and copious pleural fluid, while 33.3% (n=6) had chronic lesions with sequestra of various sizes. Those from the untreated group showed 44.4% (n=59) in the acute phase of the disease while 55.6% (n=74) had lesions in the chronic phase of the disease.
Treatment of cattle infected with CBPP has long raised controversy due to the non-availability of adequate information on the repercussions of such interventions. This study has demonstrated the failure of oxytetracycline therapy in abetting clinical CBPP in a naturally infected herd where only cattle with clinical signs were treated. The proportion of cattle that was treated with the antibiotic was 13.6% (68) of the whole herd. Considering the population (500) at risk when clinical signs were noticed, this is too low for an infectious disease as CBPP. This augments the point by OIE (2012) which states that in a CBPP infection, the whole herd will need to be treated with antibiotics in order to achieve recovery. Indeed the cost implication of treating the whole herd in such a situation would be very costly and prohibitive to the peasant farmers who are usually the victims of CBPP in sub-Saharan Africa.
The animals were in quarantine from the time they arrived in Zambia to the time of slaughter. They were not in contact with any other animals and as such, the disease was contracted at source in Tanzania. Thus the exact stages of the disease in these animals at commencement of treatment are not known and the time between exposure and transportation was not determined. However, this is the kind of situation faced in the field where treatment is initiated only after exhibition of clinical signs and the time of acquiring the disease is not known. Infected and non-infected cattle in herds usually share pasture and watering points. In an experiment by Niang et al. (2010), it was noted that all the cattle that were subjected to oxytetracycline therapy recovered with the cicatrical lesions found on slaughter. In their study, all the cattle were treated early with the period of infection to therapy clearly outlined. In the current study however, the period between infection and therapy was not known.
It was observed that 57.4% (n=39) of the treated cattle succumbed to CBPP prior to mandatory slaughter of the herd. This is within the mortality rates expected in a CBPP outbreak where it has been shown that an outbreak usually causes mortality rates of 50 to 80% in a herd (Thomson, 2005). These results are also in agreement with studies of Huebschle et al. (2006) who showed that there was no difference between danofloxacin treated and untreated groups in terms of death due to CBPP. However, this is in contrast to reports made by Nicholas et al. (2012) where all the cattle that were treated using danofloxacin survived except for three that died in the first three months.
The lesions seen in the treated group at slaughter showed that 65.5% (n=19) were exhibiting signs of recovery indicated by fibrotic scar lesions while 34.5% (n=10) had lesions typical of active disease at various stages. This study also showed that 34.5% of the treated cattle had lesions of active CBPP. Of these 70% (n=7) had acute CBPP and 30% (n=3) had chronic lesions with sequestra of various sizes. The presence of sequestra indicates the transition of the disease into chronic phase which is known to be the probable cause of perpetuation of the disease where it exists. This finding is in contrast with the findings of Niang et al. (2010) who showed that none of the cattle treated with oxytetracyclinedeveloped sequestra.
The demonstration of Mmm colonies in pathological lesions from the treated group and the eventual confirmation using PCR indicate the presence of viable pathogen. This shows that these animals could still transmit the disease to susceptible individuals in the herd. This is in agreement with Yaya et al. (2004) who stated that the presence of Mmm in oxytetracycline treated individuals could still pose a risk of disease spread in the herds.
The observation of effective minimum inhibitory concentrations (MIC) by Mitchell et al. (2012) of some antibiotics including oxytetracycline on the growth of Mmm in biological matrices demonstrates their chemical effect in vivo. In vitro however, the concentration of Mmm is in many body fluids and tissues and the mycoplasmastatic effect of oxytetracyclinemay possibly not affect all the available pathogens. This could explain the observations made in this particular study.
This observation study has shown that the effects of oxytetracycline treatment of naturally infected CBPP cattle in a herdis inconclusive and still requires further study. It has however shown that healing in some animals is possible.
The authors have not declared any conflict of interests.
REFERENCES
|
Amanfu W (2009). Contagious Bovine Pleuropneumonia (Lungsickness) in Africa. Onderstepoort J. Vet. Res. 76:14-17.
Crossref
|
|
|
|
Bashiruddin BJ, Taylor KT, Gould RA (1994). A PCR based test for the specific identification of Mycoplasma mycoides subspecies mycoides SC. J.Vet. Diagn. Invest. 6:428-434.
hCrossref
|
|
|
|
Food and Agriculture Organization FAO (2003). towards sustainable CBPP control programmes in Africa. FAO-OIE-AU/IBAR-IAEA consultative Group on Contagious Bovine Pleuropneumonia Third Meeting, Rome. pp.12-14.
|
|
|
|
Huebschle BJO, Ayling DR, Godinho K, Lukhele O, Tjipura-Zaire G, Rowan GT, Nicholas RAJR (2006). Danofloxacin (AdvocinTM) reduces the spread of contagious bovine pleuropneumonia to healthy in-contact cattle. Res. Vet. Sci. 81:304-309.
Crossref
|
|
|
|
Le Goff C, Thiaucourt F (1998). A competitive ELISA for the specific diagnosis of contagious bovine pleuropneumonia (CBPP). Vet. Microbiol. 60:179-191.
Crossref
|
|
|
|
Mariner J, Catley A, (2004).The dynamics of CBPP endemism and development of effective control strategies.Proceedings of the third FAO-AU/IBAR-IAEA consultative Group meeting on CBPP in Africa. Rome. pp.76-80.
|
|
|
|
Masiga WN, Domenech J, Windsor RS (1986).Manifestation and epidemiology of contagious bovine pleuropneumonia in Africa. Rev. Sci. Tech.15(4):1283-1308.
Crossref
|
|
|
|
Mitchell DJ, McKellar AQ, McKeever JD (2012). Pharmacodynamics of Antimicrobial against Mycoplasma mycoidesmycoides Small Colony, the causative agent of Contagious Bovine Pleuropneumonia. PLoS ONE 7(8):e44158.
Crossref
|
|
|
|
Niang M, Sery A, Doucoure M, Kone M, N'Diaye M, Amanfu W, Thiaucourt F (2010). Experimental studies on the effect of long-acting oxytetracycline treatment in the development of sequestra in contagious bovine pleuropneumonia-infected cattle. J. Vet. Med. Anim. Health 2(4):35-45.
|
|
|
|
Nicholas JAR, Ayling DR, Tjipura-Zaire G, Rowan T (2012). Treatment of Contagious Bovine Pleuropneumonia. Vet. Rec. 171:510-511.
Crossref
|
|
|
|
Office International des Epizooties (OIE) (2012). Manual of Diagnostic tests and vaccines for Terrestial animals (Mammals, birds and bees). 7:701-716.
|
|
|
|
Office International des Epizooties (OIE) (2010). Manual of Diagnostic tests and vaccines for Terrestial animals (Mammals, birds and bees). 6:712-724.
|
|
|
|
Provost A, Perreau P, Breard A, le Goff C, Martel JL,Cottew GS (1987). Contagious Bovine Pleuropneumonia. Rev. Sci. Tech 6:625-679.
Crossref
|
|
|
|
Razin S, Freundt EA (1984).The Mycoplasmas. In: Krieg NR, Holt JG (eds) 67th Bergey's manual of systematic Bacteriology. 1:740-770.
|
|
|
|
Scacchia M, Tjipura-Zaire G, Rossella L, Sacchini F,Pini A (2011). Contagious Bovine Pleuropneumonia: humoral and pathological events in cattle infected by endotracheal intubation or by exposure to infected animals. Vet. Ital 47(4):407-413.
|
|
|
|
Tambi NE, Maina WO,Ndi C (2006). An estimation of economic impact of Contagious Bovine Pleuropneumonia (CBPP) in Africa. Sci. Tech. Rev. 25:999-1011.
Crossref
|
|
|
|
Thiaucourt F, Vander Lugt JJ, Provost A (2007).Contagious Bovine Pleuropneumonia. In Coetzer WAJ, Thomson RG, Tustin CR (eds), Infectious Diseases of Livestock with Reference to Southern Africa, 3rdEdition, Oxford University, Press.
|
|
|
|
Thomson G (2005). Contagious Bovine Pleuropneumonia and poverty: A strategy for addressing the effects of the disease in sub-Saharan Africa. Research Report DFID Animal Health Programme, Trop. Vet. Med. Uni. Edin. UK.
|
|
|
|
Yaya A, Wesonga H, Thiaucourt F (2004). Use of long acting tetracyclines for CBPP-preliminary results.Report of the third meeting of the FAO/OIE/OAU-IBAR consultative group on CBPP, FAO, Rome. pp. 112-113.
|