ABSTRACT
A seroprevalence study was carried out on serum samples obtained from blood of cattle slaughtered at Sokoto metropolitan abattoir, Sokoto, Nigeria. Systematic random sampling was used to select the animals. Serum samples of 372 cattle of different sexes and breeds were analyzed using ID Screen® BVD p80 Antibody Competition ELISA Test kit for the presence of bovine viral diarrhea antibodies. Two hundred and forty seven (247), representing 66.39% of the samples, were found positive for the antibodies against bovine viral diarrhea virus (BVDV). More female animals appeared to have BVDV antibodies than the males (p<0.05). Sokoto Gudali breed of cattle recorded the highest number of positive cases of BVDV (p>0.05). The result of this research suggests that there is an existence of BVDV within the study area and that the spread of the BVDV antibodies seems to cut across sex and breed of cattle within the state. It is suggested that virological research on BVDV should be carried out in the state.
Key words: Bovine viral diarrhea virus, antibodies, cattle, Sokoto metropolitan abattoir
Economic livestock farming in Africa is faced with a lot of challenges. Apart from the environmental extremes, there are also a number of diseases which pose a significant health risk to humans and animals. With the eradication of rinderpest, it is essential to keep active surveillance on rinderpest-like diseases. Bovine viral diarrhea (BVD) is an endemic disease of cattle, inflicting substantial losses to both beef and dairy enterprises worldwide (Vanroose et al., 1999). Knowledge of the spread of BVDV within the target population is therefore crucial to the assessment of regional control options, the economic implications of infection and to the animal welfare issues.
The disease is caused by a virus BVDV of the genus pestivirus and family Flaviviridae (Donis, 1995). It is characterized by diarrhea which may contain blood or mucus, fever, anorexia, nasal and ocular discharges, and erosions in the oral cavity, muzzle, vaginal mucosa as well as, interdigital cleft. The disease is of economic importance because infections of the bovine fetus may result in abortions, stillbirths, teratogenic effects or persistent infection in the neonatal calf. Persistently viraemic animals may also be born weak, unthrifty or may appear as normal healthy calves and be unrecognized clinically. Some of these animals may later develop mucosal disease with anorexia, gastrointestinal erosions, and profuse diarrhea, leading invariably to death. Mucosal disease can arise only in persistently infected animals (Bolin, 1995).
The disease has a worldwide distribution and cattle of all ages are susceptible. The clinical picture is generally one of high morbidity and low mortality, though more severe disease is sometimes seen (Brownlie, 1990). Vertical transmission plays an important role in the epidemiology and pathogenesis of the disease (Bolin, 1995). Despite the various works carried out so far on the prevalence of this virus in various parts of Nigeria and Cameroons (Okeke et al., 1977; Baba et al., 1994; Handel et al., 2011) and the world at large (Paton et al., 1998; Ferreira et al., 2000; Falcone et al., 2001; Viltrop et al., 2002), there is still no research done to investigate and evaluate the prevalence of this virus among cattle in Sokoto, Nigeria.
The goal of this study therefore, was to investigate and estimate the BVDV seroprevalence in cattle slaughtered at Sokoto metropolitan abattoir and to determine the distribution of antibodies with regard to sex and breed of cattle in the study area.
Sokoto is the capital city of Sokoto State, located in the northwestern part of Nigeria. The state is located between longitudes 4° 8’E and 6° 54’E and between latitudes 12° N and 13° 58’N. The state shares common borders with the Republic of Niger to the North, Kebbi State to the West and Zamfara State to the East (Anonymous, 2001). The study involved cattle population slaughtered at Sokoto metropolitan abattoir where animals are brought from different parts of the state. Within the period of 3 to 4 months (July to October, 2012), 372 cattle were selected and sampled by systematic random sampling method. From each selected animal, approximately 10 ml of jugular blood sample were collected in a sterile test tube at the point of slaughter in the abattoir, then transferred to sterile plain sample bottles. The samples were labeled appropriately, and then transported in an ice packed kit to the Veterinary Microbiology Laboratory for analysis. Serum was extracted according to the methods described by Henry (1979). Whole blood was collected in a covered test tube that does not contain an anticoagulant. The blood was then allowed to clot after which the clot was then removed by centrifugation at 1500 rpm for 10 min. The serum was then immediately transferred to a clean polypropylene tube using a Pasteur pipette and then stored in a freezer (-20°C) until the time for analysis. The serum samples were screened for antibodies against bovine viral diarrhea virus using ID Screen® BVD p80 Antibody Competition Enzyme-Linked Immunosorbent Assay (ELISA) Test kit procured from ID.Vet, Garosud, France in Hematology Laboratory at Neurosurgical Department of Usmanu Danfodiyo University Teaching Hospital, Sokoto. The test was conducted according to the manufacturer’s instructions. For each of the serum samples, competition percentage was calculated using the formula:
Competition (%) = ODsample / ODNC × 100
Where ODsample is the optical density of the sample, ODNC is the mean value of the optical densities of the negative control. Samples presenting a competition percentage less than or equal to 40% were considered positive. Those presenting greater than 40% and less than or equal to 50% were considered doubtful whereas those presenting greater than 50% were considered negative. Chi square test (χ2-test) of independence was used to test for any significant association (using InStat statistical package, version 3.05 (2000) between the occurrences of BVDV antibodies with the sex and breed of the animals sampled. Z-test was also used to check for the differences between the proportions of the variables (Sergeant, 2014).
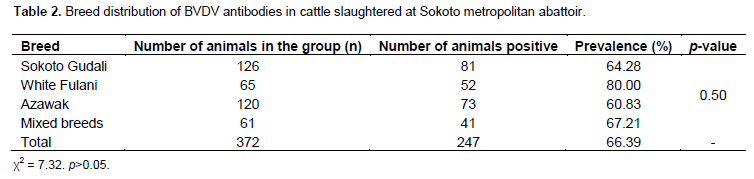
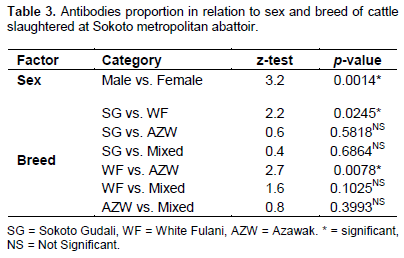
Within the period of three months, 372 serum samples from cattle were analyzed in this study. Of the total number of samples tested, 247 (66.40%) samples were found positive for antibodies against BVDV (Table 1). BVDV antibodies were found to be more prevalent in female cattle (75.00%) than in male cattle (59.31%) as shown in Table 1 and the difference being statistically significant at p<0.05. In terms of breed distribution, the highest prevalence was recorded in White Fulani (80%) while the least prevalence was recorded in Azawak (60.83%) as shown in Table 2. However, no statistical significant association was found to exist between the occurrences of BVDV antibodies with the breed of the animals sampled (Table 3). This is the first report on the seroprevalence of bovine viral diarrhea virus in Sokoto State, even though numerous investigations had been carried out previously in other parts of Nigeria (Plateau and Borno States), in Africa, as well as European countries where several prevalence had been estimated.

The prevalence observed in the study was higher than the 13.4% seroprevalence reported by Okeke et al. (1977) in Plateau; 27% seroprevalence reported by Baba et al. (1994) in Borno States; and 36.8% prevalence reported by Ferreira et al. (2000) in South African dairy herds. These differences could however be attributed to the variations in the techniques employed by former researchers in the analysis of sera. The result of this research on the other hand was lower than the 92% seroprevalence reported by Handel et al. (2011) in the Adamawa region of Cameroon.
This could also be attributed to the management system practiced by most farmers within those regions. Herds managed semi intensively was more likely to have been in contact with persistently infected animals and as such were more likely to belong to herds with recent and ongoing active BVDV infection. The result of the research is in line with the work of Paton et al. (1998) who reported that 65% of 1,070 dairy herds in England and Wales were likely to have undergone recent BVDV infection. The results are also similar with the findings of Falcone et al. (2001) who reported a seroprevalence rate of 62% in parts of Italy (Lombardia and Emilia Romagna). The research findings, on the other hand, were slightly different from the results of the study in Estonian cattle by Viltrop et al. (2002).
They observed a prevalence of herds potentially having PI animals of 46, 16 and 18% for three consecutive time periods. The substantial difference in prevalence observed in the study leads us to stress that results of the former studies describe only a snapshot of the spread of an endemic disease which is likely to fluctuate over time. The results of this research also indicate that female cattle had a higher seroprevalence (75%) than the male cattle (59.31%). This could be due to the fact that most of the female animals presented to the abattoir for slaughter were aged animals and as such their immunity against diseases was weak, more especially during pregnancy or lactation. It could also be due to fact that female animals stay more in the herd for reproduction than the male animals (Sonfada and Garba, 2000). In terms of breed distribution, white Fulani breed of cattle had the highest seroprevalence rate of bovine viral diarrhea virus antibodies, followed by Sokoto Gudali, while the Azawak had the least seroprevalence rate. White Fulani and Sokoto Gudali were the most predominant breeds of cattle found in Sokoto (often referred to as indigenous breeds) commonly reared for beef or milk production. This could be the reason why they were mostly seen in the abattoir for slaughter and could also explain why they had the higher seroprevalence rate than the Azawak breed of cattle that enters Nigeria from Niger and mostly kept for nomadism.
CONCLUSION AND RECOMMENDATIONS
The results of the research suggest an existence of BVDV antibodies and spread of the BVDV antibodies seems to cut across sex and breed of cattle in the studied area. Thus there is need to establish the actual prevalence of the genotypes and biotypes of BVDV in Nigeria as a whole in order to provide broad based data for further researches and policy making.
The authors have not declared any conflict of interests.
REFERENCES
|
Anonymous (2001). Sokoto State-The Seat of the Caliphate. In: Sokoto State Government Diary. pp. 2-8.
|
|
|
|
Baba SS, Bobbo AG, Akoma MB, Osiyemi TI (1994). Slaughter house survey for antibodies against selected viruses in ruminant sera in Maiduguri, Borno State, Nigeria. Dev. Trop. Zeits. Land. Tropen. Subtrop. 95:55-62.
|
|
|
|
|
Bolin SR (1995). The pathogenesis of mucosal disease. Vet. Clin. North. Am. Food. Anim. Pract. 11:489-500.
Crossref
|
|
|
|
|
Brownlie J (1990). The pathogenesis of bovine virus diarrhoea virus infections. Rev. Sci. Tech. Off. Int. Epiz. 9:43-59.
|
|
|
|
|
Donis RO (1995). Molecular biology of bovine viral diarrhea virus and its interactions with the host. Vet. Clin. North Am. Food Anim. Pract. 11(3):393-423.
Crossref
|
|
|
|
|
Falcone E, Cordioli P, Sala G, Tarantino M, Tollis M (2001). Genotyping of bovine viral diarrhoea viruses isolated from cattle in northern Italy. Vet. Res. Commun. 25(2):161-167.
Crossref
|
|
|
|
|
Ferreira GM, Lourens DC, Van Vuuren M (2000). The prevalence of bovine viral diarrhea antibodies in selected South African dairy herds, and control of the disease. J. South Afr. Vet. Assoc. 71(1):10-13.
Crossref
|
|
|
|
|
Handel IG, Willonghby K, Land F, Koterwas B, Morgan KL, Vincent NT, Bronsvoort BM (2011). Seroepidemiology of bovine viral diarrhea virus (BVDV) in the Adamawa region of Cameroon and use of SPOT test to identify herds with PI calves. J. Pone. 6(7):1371
|
|
|
|
|
Henry JB (1979). Clinical diagnosis and management by laboratory methods. Vol. 1, W.B Saunders Company, Philadelphia, P.A. P 60.
|
|
|
|
|
Okeke AN, Taylor WP, Shidali NN (1977). Experimental infection of Nigerian sheep and goats with bovine viral diarrhea virus. Trop. Anim. Health Prod. 9:249-251.
Crossref
|
|
|
|
|
Paton DJ, Christiansen KH, Alenius S, Cranwell MP, Pritchard GC, Drew TW (1998). Prevalence of antibod ies to bovine virus diarrhoea virus and other viruses in bulk tank milk in England and Wales. Vet. Rec. 142(15):385-391.
Crossref
|
|
|
|
|
Sergeant ESG (2014). Epitools epidemiological calculators. AusVet Animal Health Services and Australian Biosecurity Cooperative Research Centre for Emerging Infectious Disease. Available at: http://epitools.ausvet.com.au.
|
|
|
|
|
Sonfada ML, Garba HS (2000). A retrospective study of bovine tuberculosis in cattle slaughtered in Sokoto abattoirs. Sokoto J. Vet. Sci. pp. 36-39.
|
|
|
|
|
Vanroose G, Nauwynck H, Van Soom A, Vanopdenbosch E, De Kruif A (1999). Effects of bovine herpes virus-1 or bovine viral diarrhea virus on development of in vitro-produced bovine embryos. Mol. Reprod. Dev. 54(3):255-63.
Crossref
|
|
|
|
|
Viltrop A, Alaots J, Pam M, Must K (2002). Natural changes in the spread of bovine viral diarrhea virus (BVDV) among Estonian cattle. J. Vet. Med. Ser. B-Infect. Dis. Vet. Pub Health 49:263-269.
Crossref
|
|