ABSTRACT
Bovine herpesvirus-1 (BHV-1) causes Infectious bovine rhinotracheitis/Pustular vulvovaginitis in cattle. Glycoprotein D (gD) of BHV-1 represents a major component of the viral envelope and is a dominant immunogen. gD encoding gene was expressed in baculovirus-insect cell system. Viral genomic DNA extracted from BHV-1 grown on Madin-Darby Bovine Kidney (MDBK) cell monolayer was used as a template for PCR amplification of gD gene (1255 bp). Gel purified gD gene was used for directional cloning into pENTR/SD/D Directional TOPO vector to produce entry clone. Recombinant plasmids were screened by PCR and restriction enzyme (RE) digestion for gD gene insert. Endotoxin free purified plasmids were then subjected to LR recombination reaction with baculovirus linear DNA. LR recombination mix was transfected into Sf-9 cells and observed for appearance of cytopathic effects (CPE). Recombinant virus was serially passaged for 3 more generations and the 4th passage viral stock was used to infect fresh Sf-9 cells for gene expression study. Recombinant gD protein was immunoprecipitated and when subjected to SDS-PAGE and western blot analysis protein band of ~70 kDa was detected consistently. The recombinant gD protein was further weakly confirmed by dot-ELISA indicating its limited potential as a coating antigen in gD-based diagnostic ELISA.
Key words: Bovine herpesvirus-1 (BHV-1), Madin-Darby Bovine Kidney (MDBK) cells, Sf-9 cells, pENTR TOPO vector, Baculovirus mediated gene expression, glycoprotein D, eukaryotic expression,
Bovine herpesvirus type-1 (BHV-1) causes various diseases worldwide (Biswas et al., 2013; Levings and Roth, 2013). It is the most common viral agent found in the semen of bovines (Saminathan et al., 2016) and can be transmitted through natural or artificial insemination (Godhardt-Copper et al., 2009). Infectious bovine rhinotracheitis (IBR) and infectious pustular vulvovaginitis (IPV) (Davis et al., 2014; Majumder et al., 2014) are few of the most important diseases which cause huge economic loss to dairy industry due to abortion and drop in milk production (Saminathan et al., 2016). BHV-1, large double stranded DNA virus (Guo et al., 2015; Kirchhoff et al., 2014), is one of three viruses responsible for the bovine respiratory disease complex (BRDC) associated with reduced immunity (Kirchhoff et al., 2014). It causes a latent infection in sensory neurons but can reactivate following an increase in corticosteroid level (da Silva et al., 2013). Apart from IBR and IPV, BHV-1 is also responsible for many other clinical manifestations namely abortion, endometritis, infertility, mastitis, rhinotracheitis, encephalitis (Davis et al., 2014) conjunctivitis (Levings, 2012), balanoposthitis (Kaur et al., 2013; Muylkens et al., 2007; Saminathan et al., 2016) and is fatal to newborn calves (Engels and Ackermann, 1996). 12 envelope glycoproteins have been identified and seven among them, namely gB, gC, gD, gE, gH, gK and gL are known to be involved in viral attachment (Drummer et al., 2014; Biswas et al., 2013). gD gene is contiguous with gI gene with 141 bp region separating their two open reading frames (Chowdhury and Sharma, 2012). gB, gC, and gD are reported to be the major envelope glycoproteins recognized by both cellular and humoral immunity (Blanc et al., 2012) and consequently major BHV-1 immunogens (Collins et al., 1985).
All three glycoproteins are used in vaccine production and induce higher titer neutralizing antibodies with gD producing the highest titer (Dummer et al., 2014; Blanc et al., 2012). gD is reported to be potential candidate for production of subunit vaccines (Majumder et al., 2014) and its expression is limited to the alpha herpesvirus, the largest subfamily of herpesvirus (Connolly et al., 2001; Grauwet et al., 2014). Glycoproteins gB and gD participate in viral replication (Tikoo et al., 1995). gD abundance in the viral envelope and its role in initial stage of replication define it as a good target of the host immune response (Drummer et al., 2014) making it immunodominant and a good candidate for vaccine (Blanc et al., 2012; Majumder et al., 2014). These biological properties make the three glycoproteins powerful antigens in the study of BHV-1 immune response (Abdelmagid et al., 1998).
BHV-1 infection in India is endemic in nature and ELISA based diagnostic tests are being used commonly for seroprevalence studies in large population against several diseases including BHV-1. Most of these assays require viral antigen in bulk and recently diagnostic ELISAs based on recombinant protein are being developed as large scale production of recombinant viral protein is possible in safe and cost effective manner. With the recent development of marker viral vaccines, individual viral protein antigens will become a powerful tool for diagnostic evaluation of immune response as well as to differentiate infected animals from vaccinated ones (Kit and Kit, 1991; Drummer et al., 2014; Muylkens et al., 2007). Various expression system including insect cells have been developed for expression of different proteins of pathogens (Saminathan et al., 2016).
Keeping these points in view, this study was aimed at cloning for eukaryotic expression of BHV-1 gD and characterization of the expressed recombinant protein.
Cell, virus and antibodies
MDBK cells were propagated in Dulbecco’s minimum essentialmedium (DMEM) supplemented with 10% fetal bovine serum (FBS) and containing sodium bicarbonate, 2 mM L-glutamine, 10 mM HEPES. Sf-9 (Spodoptera frugiperda) cells were propagated in Grace’s Insect Medium supplemented with 10% FBS (Invitrogen). Sf-900TM II Serum Free Media (Invitrogen) also supplemented with 10% FBS was used in later stage for protein expression study. A solution having both antibiotic and antimycotic activity (100×, HIMEDIA) containing amphotericin B, penicillin and streptomycin was added to the medium.
25 cm2 cell culture flasks each containing (85-90%) monolayer of MDBK cells were used to propagate BHV-1 isolate IBR 216 II (available in the laboratory) at multiplicity of infection (m.o.i.) 0.1 and the virus was serially passaged 4 to 5 times.
Anti-sera against BHV-1 and Anti-gD mouse monoclonal antibody were used in this study; the first was available in animal biotechnology department and the second was procured from Biodot laboratory, New Delhi.
DNA extraction of BHV-1 and PCR
AuPrep GEN DNA Extraction kit (Life Technologies) was used to extract genomic DNA from Bovine herpesvirus-1 passaged in MDBK cell line. gD gene was amplified from 5 µl of template DNA by PCR. CACC overhang immediately preceding initiation codon sequence (ATG) and kozak sequence were two important features of the forward primer while the reverse primer was designed simply to amplify the gD gene from the codon immediately preceding the stop codon. The gD Forward primer was: 5’ CACCATGGAAGGGCCGACATTG 3’ and the reverse primer was 5’ CCCGGGCAGCGCGCTGTAGTTG 3’. The actual size of gD gene is 1251 bp but addition of 4 bp, CACC, overhang increased the size of amplified product to 1255 bp.
PCR was carried in 50 µl volume made of 150 ng of DNA, 1 µl of each of primers at 25 pmol concentration, 6 µl of deoxynucleoside triphosphates (dNTPs) mix at 2.5 mM, 1.5 µl of magnesium sulphate at 50 mM concentration, 3 µl of dimethyl sulfoxide, 5 µl of enhancer solution, Pfx platinum buffer 1X and Pfx platinum DNA polymerase at 1.5 U. The conditions of PCR were: 94°C for 2 min, then 35 cycles of 94°C for 30 s, 50°C for 40 s, 72°C for 2 min and lastly the final extension at 72°C for 10 min in thermal cycler (Eppendorf). PCR product was visualized using ethidium bromide post stained agarose gel (1.5%) and its molecular weight was determined by comparing it to the 100 bp ladder (Fermentas).
Cloning of BHV-1 gD gene
A QIA Quick gel extraction kit (Qiagen) was used to purify the PCR product followed by directional cloning of the PCR product in pENTRTM/SD/D-TOPO® vector (Invitrogen) as per the manufacturer’s guideline. 2 µl of the cloning reaction were used to transform chemically competent E. coli host strain TOP10 cells and LB agar plates with 100 µg/ml of kanamycin were used for selection of transformed colonies. Selected clones (5 to 6 numbers) were grown overnight in LB broth at 37°C in a shaker incubator in presence of kanamycin (100 µg/ml). Recombinant plasmids were extracted from the broth culture by alkaline lysis method (Sambrook et al, 1990). The presence of BHV-1 gD gene and its correct orientation in the expression vector was confirmed by Touchdown/Colony PCR followed by PCR from extracted plasmids and the recombinant plasmid digestion with Asc I and Not I restriction enzymes. Gen Elute Endotoxin-free Plasmid Midiprep kit (SIGMA-ALDRICH) was used to prepare the plasmid DNA (free from endotoxin) which was then used as entry clone for recombination with baculovirus genomic DNA before transfection of the insect cells.
Transfection in Sf-9 cell line
The transfer of the gene of interest (gD) into the BaculoDirectTM Linear DNA (Invitrogen) was mediated by LR recombination reaction. A transfection mixture was prepared using Cellfectin reagent and LR recombination mixture. Sf-9 cells in log phase with the viability greater than 95% were used for each transfection. Approximately 8×105 Sf-9 cells in 2 ml of complete growth medium were seeded per well in a six well plate followed by incubation at 27°C for one hour. Subsequently, in each well of Sf-9 cells, LR recombination mixture was added and again incubated. Selection of recombinant baculovirus encoding gD gene was done by adding 100 µM ganciclovir to the transfected Sf-9 cells. After 96 h of incubation at 27°C, cells were harvested upon observation of cytopathic effect. The P1 viral stock was used for 2 more passages up to P3. Sf-9 cells in 75 cm2 cell culture flasks were infected with this P3 viral stock for gene expression study.
Ni-NTA column chromatography and immunoprecipitation
Ni-NTA Column chromatography which uses Ni-NTA superflow columns (Qiagen) and immunoprecipitation which uses Protein G Immunoprecipitaion kit (SIGMA) as recommended by the manufacturer (cell lysate (60 µl), Monoclonal antibody (5 µl) and 1X immunoprecipitation buffer “IP buffer” (535µl) were added to one spin column and cell lysate (60µl), Polyclonal antibody (15µl) and 1X IP buffer (525 µl) were added to another spin column to make the final volume 600 µl in each column. Both spin columns were incubated for 1 h at 4°C after which 30 µl of Protein-G-Agarose beads washed twice with 1X IP buffer and finally mixed with 50 µl of fresh 1X IP buffer were transferred into each spin column. Spin columns were then centrifuged at 12,000 rpm for 20 s at 4°C and the effluent were discarded. Beads were again washed twice with 1X IP buffer and 0.5 M NaCl by centrifugation at 12,000 rpm for 20 s followed by 4 times washing with 1X IP buffer. The last washing was performer with 0.1X IP buffer) were used to purify the recombinant gD protein from the P3 virus infected Sf-9 cells. Purified protein samples thus obtained were subjected to SDS-PAGE and western blot analysis.
SDS-PAGE and Western blot analysis
Sf-9 cells infected by recombinant baculovirus and non-infected ones, were harvested and prepared for SDS-PAGE by the method of sonication where centrifugation at 5000 rpm produced the pellet which was resuspended in 500 µl PBS and 5 µl protease inhibitor followed by sonication thrice at 5 microns for 10 s with a gap of 20 s each. Samples were then centrifuged again at 5000 rpm for 10 min and the supernatants were collected into new tubes for SDS-PAGE analysis. Samples along with the protein marker were run on 10% SDS-PAGE gel followed by staining with Coomassie Brilliant Blue (Sambrook et al., 1989). An unstained gel was blotted onto a PVDF membrane for Western blot and a SNAPid system (Millipore) was used for development. A solution of PBS with 0.1% Tween-20 and 1% BSA was used to block the membrane for 10 min at room temperature. The membrane was respectively incubated at room temperature with monoclonal antibody against BHV-1 gD and Goat anti-mouse HRP conjugate, the first at 1:300 dilution for 10 min followed by washing thrice with (PBS + 0.1% Tween 20) buffer but the second at 1:100 dilution for 10 min also followed by washing thrice with PBS. To develop the blot, DAB solution made of 1mg/ml diamino benzidine and 10 µl/ml hydrogen peroxide in PBS was used.
Dot ELISA
The following were used for Dot-ELISA: partially concentrated BHV-1, MDBK cell lysate, immunoprecipitated gD, Sf-9 infected cell lysate, non-infected Sf-9 cell lysate, PBS and blank. On dot-ELISA strip, 2 µl of each sample was spotted and it was kept for drying at 37°C for 1 h. 1% BSA was used for blocking for 30 min followed by 5 times washing with a solution of PBS containing 0.01% Tween-20, incubation with polyclonal antibody as primary antibody at 1:200 dilution and again washing once. The last incubation for 1 h at 37°C was done with secondary antibody, goat anti-rabbit HRP conjugate, diluted at 1:500 followed by washing once. DAB and hydrogen peroxide were used to develop the blot as described previously.
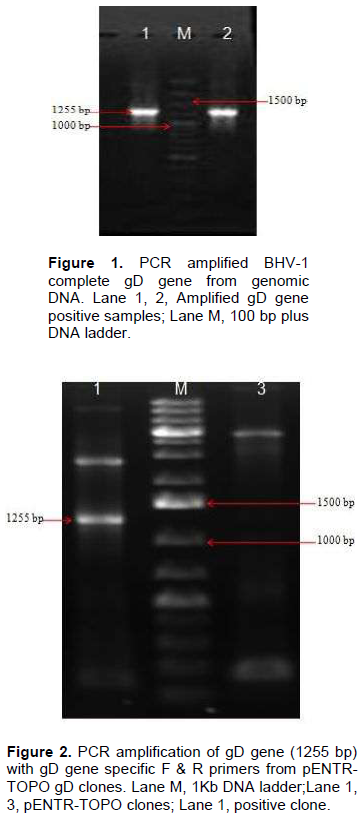
Cloning of gD gene and screening of the recombinant plasmids for presence of the insert
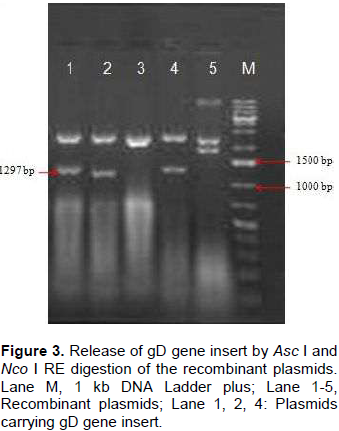
Genomic DNA of BHV-1 was isolated from partially purified virus and the complete gD gene (1255 bp) was amplified by PCR using specific primers to obtain gD gene fragments containing CACC base sequence at 5‘end just preceding the initiation codon (Figure 1). Blunt end PCR amplified gene products were gel purified and directly used for cloning into PENTR/SD/D-directional TOPO cloning vector. Screening clones for gD gene insert was done by touch down PCR for presence of gD gene insert where PCR product of 1255 bp could successfully be amplified from recombinant plasmids. Clones having gD gene insert were grown for plasmids isolation and again the presence of gD gene insert from the purified recombinant plasmids was confirmed by amplification of 1255 bp product (Figure 2). The confirmation of complete gD gene’s presence was also possible by digesting plasmids with Asc I and Nco I restriction enzymes which released 1297 bp fragment having additional 42 bp from multiple cloning site region of the plasmid (Figure 3).
Analysis of recombinant protein
The optimum duration for maximum protein expression was 72 h post infection as revealed by Western blot analysis. Immunoprecipitated expressed protein (from 72 h post infected cell lysate) was analyzed by SDS-PAGE where two bands of ~70 kDa and ~50kDa were observed (Figure 4). On western blot analysis with polyclonal anti BHV-1 serum, strong reaction of protein was observed with band of ~70 and 50 kDa (Figure 5). On SDS-PAGE together with western blot analysis (Figure 6a), gD protein was detected from 24 to 144 h post infection from Sf-9 cells, however the maximum expression being observed at 72 h (Figure 6b).
When expressed protein purified by immunoprecipitation was run on SDS-PAGE, a band of ~70 kDa and an additional band of ~50 kDa were observed. On western blot analysis using polyclonal anti BHV-1 serum, strong reaction of protein was observed with these bands (Figure 5 and 7), however the reaction being intense with polyclonal anti-BHV-1 compared to monoclonal.
The recombinant gD protein which appeared to be of a molecular mass of ~70 kDa was absent from uninfected cells. On western blot analysis, authentic gD (~70 kDa) and a polypeptide with an apparent molecular mass of ~70kDa were recognized by polyclonal antibody respectively in MDBK cells infected with BHV-1 and Sf-9 cells infected with recombinant baculovirus. This suggests that the recombinant gD is equivalent to the authentic gD.
Dot ELISA
The reaction of both immunoprecipitated gD protein and gD protein recovered from infected Sf-9 cell lysate as shown in Figure 8, was also detected in Dot ELISA. As revealed by dark brown spots, the native protein gD recovered from both partially purified BHV-1 and BHV-1 infected cell lysate appeared to have fair reactivity with polyclonal antibody.
As Abdelmagid et al. (1998) reported earlier, 72 h post infection was the optimum period for protein expression. Two bands of 70 and 50 kDa for the recombinant gD proteins were observed as revealed by SDS-PAGE and western blot analysis. Parker et al. (1991) reported to have expressed BHV-1 gp IV (D) of an apparent molecular mass of 63 kDa by recombinant baculovirus and its lower apparent molecular mass compared to the native protein indicated the incomplete glycosylation. The expressed protein appeared to be antigenically intact in this study as its reaction with polyclonal and monoclonal antibodies specific to gD was comparable to that of gD from BHV-1. These findings are in accordance with the work of Parker et al. (1991) and supported by the fact that Sf-9 cell are higher eukaryote and therefore having proper posttranslational modification (Hu, 2005).
The reactivity of anti gD monoclonal antibody with recombinant gD protein was found to be lower as compared to polyclonal antibody suggesting that the expressed protein is not being recognized properly by monoclonal antibody used as revealed by both dot ELISA and Western blot. This may result from modification or loss of epitope conformation due to protein denaturation during sample preparation together with or improper glycosylation as baculovirus infected cell display inability to correctly process some proteins (Hu, 2005) due to the fundamental nature of insect glycoprotein processing pathways (Jarvis, 2003). The poor reactivity of monoclonal antibody with a recombinant protein due to loss of native conformation of baculovirus expressed protein was also reported by Abdelmagid et al (1998) in the case of glycoprotein B (gB).
With both monoclonal and polyclonal antibodies, protein bands of 70 and 50 KDa were consistently detected in SDS-PAGE and western blot analysis. Blanc et al (2012) reported to have expressed glycoprotein D (gD) of 71 kDa and according to Van Donkersgoed et al. (1994) glycoprotein D (gD) of 71 kDa containing both N-linked and O-linked Oligosaccharides is synthesized first as a partially glycosylated precursor of 63 KDa.
BHV-1 gD could be successfully expressed using baculovirus based vector and insect cell system which was visualized as protein bands of 70 and 50 KDA in SDS-PAGE and Western blot assays. The expressed gD is equivalent to the authentic gD and has fair potential to be used as a diagnostic antigen for detection of BHV-1 specific antibodies.
The authors have not declared any conflict of interest.
REFERENCES
|
Abdelmagid OY, Mansour MM, Minocha HC, van Drunen Littel-van den Hurk S (1998). Evaluation of baculovirus-expressed bovine herpesvirus-1 (BHV-1) glycoproteins for detection and analysis of BHV-1-specific antibody responses. Vet. Microbiol. 61:249-259.
Crossref
|
|
|
|
Biswas S, Bandyopadhyay S, Dimri U, Patra HP (2013). Bovine herpesvirus-1 (BHV-1)–a re-emerging concern in livestock: a revisit to its biology, epidemiology, diagnosis, and prophylaxis. Vet. Q. 33(2):68-81.
Crossref
|
|
|
|
|
Blanc AM, Berois MB, Tomé LM, Alberto L, Epstein AL, Arbiza JR (2012). Induction of humoral responses to BHV-1 glycoprotein D expressed by HSV-1 amplicon vectors. J. Vet. Sci. 13(1):59-65.
Crossref
|
|
|
|
|
Connolly SA, Whitbeck JC, Rux AH, Krummenacher C, Cohen GH, Eisenberg RJ (2001). Glycoprotein D homologs in herpes simplex virus type 1, pseudorabies virus, and bovine herpes virus type 1 bind directly to human HveC (nectin-1) with different affinities. Virol. 280(1):7-18.
Crossref
|
|
|
|
|
Chowdhury S, Sharma B (2012). Transcription of gD and gI genes in BHV1-infected cells. J. Biosci. 37:971-977.
Crossref
|
|
|
|
|
Collins JK, Butcher AC, Riegel CA (1985). Immune response to Bovine herpesvirus type 1 infections: Virus-specific antibodies in sera from infected animals. J. Clin. Microbiol .21:456-552.
|
|
|
|
|
da Silva LF, Kook I, Doster A, Jones C (2013). Bovine herpesvirus 1 regulatory proteins bICP0 and VP16 are readily detected in trigeminal ganglionic neurons expressing the glucocorticoid receptor during the early stages of reactivation from latency. J. Virol. 11214-11222.
Crossref
|
|
|
|
|
Davis KJ, Syam RS, Chauhan RS (2014). Epidemiological studies on infectious bovine rhinotracheitis (IBR) in different parts of India. Int. J. Live. Res. 4(5).
|
|
|
|
|
Dummer LA, Leite FBL, van Drunen Littel-van den Hurk S (2014). Bovine herpesvirus glycoprotein D: a review of its structural characteristics and applications in vaccinology. Vet. Res. pp. 45:111.
|
|
|
|
|
Engels M, Ackermann M, (1996). Pathogenesis of ruminant herpesvirus infections. Vet. Microbiol. 53:3-15.
Crossref
|
|
|
|
|
Godhardt-Cooper JA, Zoromski J, Toohey-Kurth K (2009). Evaluation of a blocking enzyme-linked immunosorbent assay for serological diagnosis of Bovine herpesvirus1. J. Vet. Diagn Invest.21:523-526.
Crossref
|
|
|
|
|
Grauwet K, Cantoni C, Parodi M, Andrea de Maria, Devriendt B, Pende D Moretta L, Vitale M, Favoreel HW (2014). Modulation of CD112 by the Alphaherpesvirus gD protein suppresses DNAM-1–dependent NK cell-mediated lysis of infected cells. Proc. Natl. Sci. Acad.111(45):16118-16123.
Crossref
|
|
|
|
|
Guo L, Yanling Yang Y, Liu L, Liao P, Wen Y, Wu H, Cheng S (2015). A proteomic study of the differential protein expression in MDBK cells after bovine herpesvirus type 1 infection (BHV-1) strain treatment. Int. J. Clin. Exp. Med. 8(3):4204-4211.
|
|
|
|
|
HU Y (2005). Baculovirus as a highly efficient expression vector in insect and mammalian cells. Acta Pharmacol. Sin. 26 (4):405-416.
Crossref
|
|
|
|
|
Jarvis DL (2003). Developing baculovirus-insect cell expression systems for humanized recombinant glycoprotein production.Virology 310:1-7.
Crossref
|
|
|
|
|
Kaur G, Dwivedi P N, Verma R, Sharma NS (2013). Prevalence of Bovine herpesvirus-1 in cattle and buffaloes in Punjab. Vet. World 6(6):343-345.
Crossref
|
|
|
|
|
Kirchhoff J, Uhlenbruck S, Goris K, Keil GM, Herrler G (2014). Three viruses of the bovine respiratory disease complex apply different strategies to initiate infection. Vet. Res. pp.45:20.
|
|
|
|
|
Kit M, Kit S (1991). Sensitive glycoprotein gIII blocking ELISA to distinguish between pseudorabies (Aujeszky's disease)-infected and vaccinated pigs. Vet. Microbiol. 28:141-155.
Crossref
|
|
|
|
|
Levings RL (2012). "Generation and characterization of virus-neutralizing bovine monoclonal antibodies to bovine herpesvirus 1 glycoproteins gB, gC, and gD" Graduate theses and dissertations. Paper 12862.
|
|
|
|
|
Levings RL, Roth JA (2013). Immunity to Bovine herpesvirus 1: II. Adaptive immunity and vaccinology. Anim. Health Res. Rev. 14(1):103-123.
Crossref
|
|
|
|
|
Majumder S, Pandey AB, Ramakrishnan MA (2014). Cloning and sequence analysis of glycoprotein D gene of bovine herpesvirus 1. Adv. Anim. Vet. Sci. 2(2S):19-22.
Crossref
|
|
|
|
|
Muylkens B, Thiry J, Kirten P. Schynts F,Thiry E (2007). Bovine herpesvirus 1 infection and infectious bovine rhinotracheitis Vet. Res. 38:181-209.
Crossref
|
|
|
|
|
Sambrook J, Fritsch E, Maniatis T (1989). Molecular cloning (Vol. 2, pp. 14-9). New York: Cold spring harbor laboratory press.
|
|
|
|
|
Sambrook J, Fritsch EF, Maniatis T (1990). Molecular cloning: a laboratory manual, Ed 2nd.
|
|
|
|
|
Saminathan M, Rana R, Ramakrishnan MA, Karthik K, Malik YS, Dhama K (2016). Prevalence, diagnosis, management and control of important diseases of ruminants with special reference to Indian scenario. J. Exp. Bio. Agri. Sci. 4(3S).
|
|
|
|
|
Tikoo S, Campos M, Babiuk L (1995). Bovine herpesvirus 1 (BHV-1): Biology, pathogenesis, and control. Adv. Virus Res. 45:191-232.
Crossref
|
|
|
|
|
Parker MD, Fitzpatrick DR, Zamb TJ, van den Hurk JV, Campos M, Harland R, Babiuk LA (1991). Expression of Bovine herpesvirus 1 glycoprotein IV by recombinant Baculovirus and analysis of its immunogenic propeerties. J. Virol. 65:263-71.
|
|
|
|
|
Van Donkersgoed J, Kowalski J, Van den Hurk JV, Harland R, Babiuk LA, Zamb TJ (1994). A subunit gIV vaccine, produced by transfected mammalian cells in culture induces mucosal immunity against bovine herpesvirus-1 in cattle. Vaccine 12:1295-302
Crossref
|
|