ABSTRACT
Donkeys (Ass-Equus-assinus) and camels (Camelus dromedarius) in Uganda are mainly owned by low income earners and peasant farmers, mainly in the semi-arid North-eastern Uganda in Karamoja and Sebei sub-regions. The animals however, seem to receive little or relatively no veterinary care. This study was carried out in Moroto and Amudat districts, Karamoja sub-region, Uganda in March 2016. Faecal samples of 110 randomly selected camels and donkeys of all age and sex were collected directly from their rectum. The faecal samples were examined by flotation method, sedimentation technique, McMaster egg counting technique and faecal culture to identify and determine the burden of parasites in different age groups. Lungworms ovaculture revealed Dictyocaulus cameli (29.3%) of camels and Dictyocaulus arnfieldi (15.4%) of donkeys. Cestode eggs detection revealed family Anoplocephalidae which includes seven species of cestodes identifiable as eggs in faeces or as adults in the gastro-intestinal tract of camels (18.3%) and donkeys (15.4%). Coccidia species included Eimeria cameli (11%) and Eimeria Leuckarti (3.85%) in camels and donkeys, respectively. Trematodes were detected in 5 camels as Fasciola gigantica. Overall, EPG count was observed for strongyles at 58.5% in camels and 42.3% in donkeys. The results of this study will provide insights into the health of donkeys and camels in Uganda and provide a way forward to their veterinary care and management for improved production and productivity.
Key words: Gastrointestinal parasites, donkey, camels, Karamoja, Uganda.
Donkeys and camels are subject to severe work and in bondage, working in extremely strenuous conditions, over laden, underfed and ill-used especially for draught purposes. They are sore footed, constantly burdened by parasites (Atawalna et al., 2015; Tsegaye and Chala, 2015; Ismail et al., 2016; Anvari-Tafti et al., 2013), with little or no veterinary care. An attempt to study parasitism in these species has only been recent.
Parasitic helminthes are one of the most common factors that constrain the health and working performance of camels and donkeys worldwide. Parasites cause various degrees of damage depending on the species and number present, nutritional and the immune status of the animal (Sumbria et al., 2014; Sumbria and Singla,
2015). Infection by endo-parasites in camels and donkeys are responsible for problems including poor body condition, reduced power output, diarrhea, colic, emaciation, impaired growth, poor reproductive performance, short lifespan and predisposition to other infectious diseases (Ayele, 2006; Fikru et al., 2005; Getachew et al., 2010; Yoseph et al., 2005).
The prevalence, species composition and epidemiology of helminthes affecting donkeys and camels have not been previously investigated in Uganda. Donkeys and camels are used in arid and mountain villages, both as saddle animals and for carriage. In camels and donkeys, nematodes are seen to be most prevalent, while cestodes and trematodes are less occurring (Arslan and Umur, 1998; Bakirci et al., 2004; Demir et al., 1995). Eimeria species can also be encountered (Arslan and Umur, 1998; Bakirci et al., 2004; Ozer and Küçükerden, 1992).
Nematode infection was the most prevalent challenge in these species. A heavy internal parasite burden can adversely affect the health of donkeys and camels particularly when called upon to work and as it is often the case, is undernourished and stressed (Sonja et al., 2000).
The present study was therefore designed to generate baseline data on the prevalence and species composition of helminth parasites of donkeys and camels in Uganda.
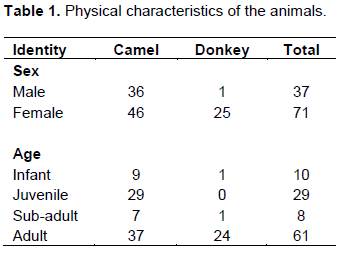
Faecal samples were collected from Karamoja sub-region in two districts namely: Moroto: N 2° 31' 41.604", E 34° 39' 28.794" and Amudat: N 1° 47' 29.841", E 34° 54' 23.583" districts, Uganda. The study was conducted in March 2016. The camels and donkeys were classified as: infant, juvenile, sub-adult and adult. Male and female animals were sampled (Table 1).Karamoja sub-region is a semi-arid region, the livelihoods of the people depend on pastoralism. Cattle, sheep and goat are kept in large numbers for meat, milk, blood, dowry, prestige. Donkeys and camels are kept for draught power, meat and milk. Faecal samples were collected directly from the rectum of the animal using a gloved hand, into polyethene bags, labeled using details such as source, age, sex, species and general condition of animal for easy identification, cold chain was maintained in the field and the samples were hurriedly taken to the parasitology laboratory for analysis to avoid larval development.
Samples were kept in refrigerator at 4°C if immediate processing was not possible, but it had been processed within 48 h or as soon as possible. Direct faecal smear, sedimentation and floatation techniques were the utilized parasitological techniques to identify the eggs in faeces and examined microscopically (10 and 40×) for presence of parasite ova following their procedures (Forety, 2001). Identification of the eggs was made on the basis of their morphology (Soulsby, 1982). Quantitative faecal examination was performed by using McMaster technique (Gordon and Whitlock, 1939) to determine the number of egg per gram of faeces (EPG) and performed according to the procedure described by Urquhart et al. (1996). Faecal culture using Baermann technique to determine lungworm larvae, Dictyocaulus arnifieldi and Dictyocaulus cameli was also undertaken. Level of infection was extrapolated from infection severity index (Soulsby, 1986) where animals are said to have mild, moderate and severe nematode infestation if their faecal egg counts are less than 500, 500-1000 and more than 1000, respectively.
Data management and analysis
The data collected from the study area was entered into Microsoft Excel spread sheet and the data was coded appropriately and analyzed using SPSS version 16 statistical software. Analysis Of variance (ANOVA) test was applied to test the statistical association existing among the risk factors such as species, sex, age and body condition scoring with the presence of the infection.
The total number of donkeys was 26, while camels were 82 giving a total of 108 animal samples analyzed. Number of positive cases for Strongyle EPG was 48 for camels and 11 for donkeys giving a prevalence of 58.5% for camels and 42.3% for donkeys. The mean EPG count was 1056 for camels and 323 for donkeys. The number of positive cases for Anoplocephalidae family of cestodes was 15 for camels and 4 for donkeys, giving a mean count of 74.4, prevalence of 18.3% in camels and 27, prevalence of 15.4% in donkeys (Table 2). Lung worm larvae reported Dictyocaulus arnfieldi in donkeys with 4 cases at a mean count of 0.4 and a prevalence of 15.4%. While Dictyocaulus cameli recorded 24 cases with a mean count of 1 at a prevalence of 29.3%.
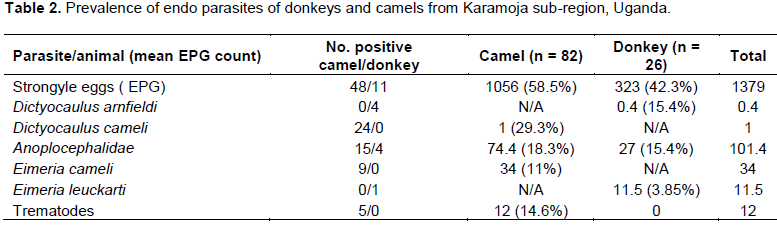
Coccidia reported Eimeria cameli in camels with 9 cases in camel at a mean count of 34 at a prevalence of 11%, while Eimeria leuckarti in donkeys reported 1 case with a mean count of 11.5 at a prevalence of 3.85%.Mixed infections were reported. Infections with one parasite species were 22 (26.8%) in camels and 8 (30.8%) in donkeys. Infections with 2 parasite species were 24 (29.3%) in camels and 6 (23%) in donkeys. Infections with 3 parasite species were 7 (8.5%) in camels and none in donkeys (Table 3).
Level of infection
Animals were said to have mild,mode rate and severe nematode infestation if their faecal egg counts are less than 500, 500-1000 and more than 1000, respectively (Table 4). Statistical analysis revealed a significant difference between the mean Strongyle EPG between the two districts, Moroto and Amudat (p=0.000), Eimeria EPG (p=0.018) and Trematode EPG (p=0.048), for the two districts; Mean Strongyle EPG Moroto (1281.8), Amudat (260); Mean Eimeria EPG Moroto (10.6), Amudat (60.0); Mean Trematode EPG Moroto (0.30), Amudat (0.25). The mean Strongyle EPG between species, camel and donkey was significant at p=0.020. Mean Eimeria EPG was statistically significant for location (p=0.018) and sex (p=0.017). Mean Trematode EPG was significant for location (p=0.048) and sex (p=0.006).
Lungworm infection characterized D. cameli of camels and D. arnfieldi as the parasite of horses and donkeys. D. arnfieldi has donkey as its normal host. While pathogenicity is limited in the donkey, the parasite may provoke severe clinical lungworm disease in the horse.
The family Anoplocephalidae comprises seven species of cestodes that inhabit the gastro-intestinal tract of camels and donkeys as adults or eggs found in the faeces. These include: Moniezia expanza, Moniezia benedeni, Stilesia globipunctata, Stilesia vittata, Avitellina centripunctata, Avitellina woodland and Thysaniezia ovilla.
Ugandan camels (Camelus dromedaries) were infected with E. cameli (11%) while 3.85% donkeys had Eimeria leuckarti. It is believed that the infection of coccidia is concern mainly with young horses and donkeys, especially foals. Although, it has been reported that E. leuckarti is more prevalent among young foals and only occasionally is it detected in adult animals (Souza et al., 2009). Watering devices should be protected from faecal contamination. Diagnosis is based on clinical signs and the demonstration of high numbers of oocysts in the diarrhoeic faeces. Cystic structures containing immature oocysts occur in the intestinal mucosa. In the present study, in all the cases, no clinical signs were documented in the animals excreting E. leuckarti oocysts, while there were some doubt about the pathogenicity of E. leuckarti, diarrhea of several days duration, and acute massive intestinal hemorrhage leading to rapid death have been described in foals (Radostits et al., 2006). However, the infection is rarely evidenced by clinical signs. Difficulties in the diagnosis of coccidia infection in camels, horses and donkeys cause the parasitosis not to be diagnosed in a routine coproscopical examination.
Trematodes were detected in 5 camels as Fasciola gigantica. Trematodes whose eggs may appear in the faeces including: Fasciola hepatica (liver flukes), Fasciola gigantica (giant liver fluke), Dicrocoelium spp. (liver flukes), Eurytrema pancreaticum (pancreas fluke), Schistosoma bovis and Schistosoma mattheei (blood flukes).
Strongyle eggs were quantified to determine egg per gram of faeces (EPG). Strongyles were taken as a group, speciation of the nematodes by ovaculture was not done. All the camels and donkeys were in good health and body condition except one sick donkey in Moroto which was in poor body condition. For this reason, autopsy was not done and hence adult worms were not studied. Therapy and prophylaxis for all the above mentioned helminth parasites is the same as for cattle.
Veterinary health care provision in Uganda does not cater for donkeys and camels at all. No veterinary intervention for these animals in Uganda is available at all. Yet these animals are kept in arid and mountainous areas of Karamoja and Sebei sub-region in North-eastern Uganda. They are kept for milk, meat, dowry, prestige and carriage.
Mixed infections were encountered comprising of double infections or triple infections of Strongyle, coccidian, cestode, trematode and lungworms. The challenge of mixed infections exacerbates the compromised health condition of the animal which could result in debilitation and death of the animal.
The number of eggs per gram can be calculated by counting the number of eggs within the grid of each of the 2 chambers, ignoring those outside the squares, then multipling the total by 50; this gives the eggs per gram of faeces (e.p.g.). Reading the count should not be delayed beyond the recommended time as the
flotation fluid may distort or destroy delicate eggs. Therefore, it is advisable to only process a few samples at a time.
When interpreting McMaster results, it must be remembered that a number of factors can influence the occurrence, recognition or numbers of helminth eggs found in a faecal sample. In particular, the number of eggs is not necessarily indicative of the number of worms present. Reasons for this include: Eggs are produced only by fertile adult female (or hermaphrodite) worms and will, therefore, be absent in immature or single sex infections. The daily output of eggs by fertile females is influenced by host-physiological factors such as stress or lactation (increased) or immunity (decreased). Chemotherapy can also affect egg-production e.g. corticosteroids (increased) or sub-lethal anthelmintic doses (decreased). Some food-stuffs may have a similar effect e.g. tannin-rich forages (decreased). The concentration of eggs (per gram of faeces) is influenced by the daily volume of faeces being produced by the host, the rate of passage by the ingesta through the intestine, and the distribution of eggs throughout the faecal mass. Some types of eggs are heavier than others and may not float well in solutions of lower specific gravity (e.g. Fasciola). Some eggs from different species are indistinguishable (particularly trichostrongylids and strongylids). This complicates clinical interpretation as some species (e.g. Haemonchus) produce many more eggs per day than others (e.g. Ostertagia).
The mean EPG was statistically significant for male and female animals for Eimeria (p=0.017) and Trematodes (p=0.006). Although in this study, mean EPG Eimeria was higher in males (68.6) than females (12.1), and Trematodes male (0.34) and females (0). Female animals are ideally more prone to helminth infections physiologically because of stress, pregnancy and lactation conditions which compromise their immunity. The mean Strongyle EPG was 1056.1 in camels and 323.1 in donkeys. The mean Strongyle EPG between species, camel and donkey was significant at (p=0.020). It could be explained that donkey is a hardier animal, closer to wildlife species. Hence, it has a stronger resistance to infections naturally, more tendency as a reservoir host. However, fewer donkeys than camels were sampled in this study.
Helminthes infection is endemic in camels and donkeys in Uganda. A wide range of species of helminthes that plague donkeys and camels was detected in Karamoja sub-region, Uganda. The observation of multiple infections with high prevalence and high overall EPG suggest the presence of favorable environmental conditions for survival, infection and perpetuation of helminthes of camels and donkeys in Uganda. There is complete lack of veterinary service provision to these animals and lack of awareness of animal welfare. Information on the different aspects of parasitology of these animals is also limited. Production and productivity of camels and donkeys in Uganda is compromised by the little or no veterinary care and welfare of these animals.
The authors have not declared any conflict of interest
REFERENCES
|
Anvari-Tafti M, Sazmand A, Hekmatimoghaddam S, Moobedi I (2013). Gastrointestinal helminths of camels (Camelus dromedarius) in center of Iran. Trop. Biomed. 30(1):56-61.
|
|
|
|
Arslan MO, Umur S (1998). The helminth and Eimeria (Protozoa) species in horse and donkey in Kars. Acta. Parasitol. Turcica. 22:180-184.
|
|
|
|
|
Atawalna J, Emikpe BO, Sallah EK, Shaibu W, Folitse RD (2015). The Health Problems, Gastrointestinal and Blood Parasites Commonly Associated With Donkeys in the Upper East Region of Ghana. Afr. J. Biomed. Res. 18:37-41.
|
|
|
|
|
Ayele CF (2006). Principal health problems of donkeys in Dugda Bora district of Ethiopia. In: Proceedings of the 5th International Colloquium on Working Equines Thethe Future for Working Equines. Eds: Pearson AA, Muir CC, Farrow MM. The Donkey Sanctuary, Sidmouth. pp. 162-168.
|
|
|
|
|
Bakirci S, Çirak VY, Gülegen E, Karabacak A (2004). Parasites found by faecal examinations in horses in the Gemlik Military Stud Farm. Acta. Parasitol. Turcica. 28:35-37.
|
|
|
|
|
Demir S, Tinar R, Kaplan A (1995). Helminths obtained from a donkey. Acta. Parasitol. Turcica. 19:119-123.
|
|
|
|
|
Fikru R, Reta D, Teshale S, Bizunesh M (2005). Prevalence of equine gastrointestinal parasites in western highlands of Oromia, Ethiopia. Bull. Anim. Health Prod. Afr. 53:161-166.
Crossref
|
|
|
|
|
Forety WJ (2001). Veterinary Parasitology. Reference Manual, Fifth Edition, Iowa State University Press.
|
|
|
|
|
Getachew AM, Trawford AF, Feseha G, Reid SWJ (2010). Gastrointestinal parasites of working donkeys in Ethiopia, Trop. Anim. Health Prod. 42:27-33.
Crossref
|
|
|
|
|
Gordon HM, Whitlock HV (1939). A new technique for counting nematode eggs in sheep faeces. J. Counc. Sci. Ind. Res. 12:50-52.
|
|
|
|
|
Ismail AA, Seri HI, Ahmed NK, El Tigani-Asil El TA, Bashar AE, Abakar AD (2016). A Survey of Seasonal Gastrointestinal Parasitic Infections in Donkeys from a Semiarid Sub-Saharan Region, Sudan. Hindawi. J. Pathog. P 8.
Crossref
|
|
|
|
|
Ozer E, Küçükerden N (1992). Helminth and Eimeria sp. found in equids in Elazig and region. Doga Tr. J. Vet. Anim. Sci. 17:217-221.
|
|
|
|
|
Radostits OM, Gay CC, Hinchcliff KW, Constable PD (2006). A text-book of the diseases of cattle, horses, sheep, pigs and goats. Veterinary Medicine E-Book, 10th Edition. Vet. med. Saunders.
|
|
|
|
|
Sonja M, Rosina CK, Susan AM (2000). Prevalence and Biodiversity of helminth parasites in donkey from South Africa. J. Parasitol. 86:756-762.
Crossref
|
|
|
|
|
Soulsby E (1982). "Helminths, Arthropods and Protozoa of Domestic Animals," Bailliere TinDall, Toronto.
|
|
|
|
|
Soulsby EJL (1986). Helminthes, Arthropods and Protozoa of Domesticated Animals (7thedn) Bailliere Tindall London. 167-174
|
|
|
|
|
Souza PNBD, Bomfimb TCB, Huber F, Abboud LCS, Gomes RS (2009). Natural infection by Cryptosporidiumsp. Giardia sp. and Eimeria leuckarti in three groups of equines with different handlings in Rio de Janeiro. Brazil. Vet. Parasitol. 160:327-333.
Crossref
|
|
|
|
|
Sumbria D, Moudgil AD, Singla LD (2014). Equine Piroplasmosis: Current status. Veterinaria 1(1):9-14.
|
|
|
|
|
Sumbria D, Singla LD (2015). Recent Diagnostic and control approaches in Equine piroplasmosis. Veterinaria 2(1):1-6
|
|
|
|
|
Tsegaye B, Chala A (2015). Prevalence of endoparasitic helminths of donkeys in and around Haramaya district, Eastern Ethiopia. JVMAH 7:221-224.
|
|
|
|
|
Yoseph S, Smith DG, Mengistu A, Teklu F, Firew T, Betere Y (2005). Seasonal variation in the parasite burden and body condition of working donkeys in East Shewa and West Shewa regions of Ethiopia. Trop. Anim. Health Prod. 37:35-45.
Crossref
|
|