ABSTRACT
The study was designed to assess the microbiological quality of Nile tilapia (Oreochromis niloticus) fillets and food safety practices of fish handlers in Arba Minch town, SNNPR, Ethiopia. A questionnaire survey was conducted to assess the food safety practices of fish handlers. The microbiological quality of frozen raw and undercooked (Asa leb-leb) tilapia fillets was assessed by determining the total plate count and isolating hygiene indicator bacteria that is, Escherichia coli and other major foodborne pathogens that is, Salmonella spp. and Staphylococcus aureus. Among interviewed fish handlers, 32.6% and 55.8% were not found to use overcoat and hair cover, respectively. Moreover, 30.2% of the fish handlers didn’t take training regarding sanitary handling of fish food. The highest mean bacterial count was observed in frozen raw tilapia fillet sample (4.63x106 cfu/g), which was significantly (p=0.037) different from undercooked tilapia fillet (4.92x103 cfu/g). From 40 frozen raw samples of tilapia fillet examined, 42.5% had E. coli, 7.5% had Salmonella spp. and 65% had S. aureus. In contrast, from 40 undercooked samples of tilapia fillet examined, 7.5% had E. coli and 17.5% had S. aureus whilst Salmonella spp. was not detected. A significant (p=0.000) difference in frequency of bacterial isolates were also observed between frozen raw and undercooked tilapia fillet. Generally, the study revealed that there was a gap on food safety practices and high microbial profile were observed. Hence, it could be wise to advice the government to improve the food safety practices and quality standards of fish sold in the town.
Keywords: Fish, food safety, microbiological quality, Nile tilapia, pathogens, undercooked.
Fish products are essential to food security, providing over 1 billion people with their main source of protein and more than 4.3 billion people with about 15% of their average per capita animal protein intake (FAO, 2012). In 2010, wild capture fisheries and aquaculture supplied the world with about 148 million tons of fish, worth US $ 217.5 billion. Approximately, 128 million tons or 86% were used for human consumption. Preliminary data for 2011 indicates that production has increased to 154 million tons, of which about 131 million tons, or 85%, were consumed as food (FAO, 2012; World Bank, 2010).
Ethiopia has only inland freshwater capture fisheries. Total annual fish production potential in the country is estimated to be 51,481 tons (FAO, 2012). In 2010/11 the total landing was about 18,058 tons, which is about 35% of the calculated potential (MoA, 2012). But the country overall per capita fish production is less than 240 g per person per year (FAO, 2006). However, in areas and communities where there is regular and sufficient supply, annual fish consumption can exceed 10 kg/person (FAO, 2012). This implies that fish consumption in the country is more highly influenced by supply factors (FAO, 2012). This supply factor is indirectly related to lack of preservation and processing techniques. In the country most (about 73%) of the total fish landed is marketed fresh in nearby markets. The rest reaches distant consumers chilled or frozen (26%), or as dried, smoked and canned (1%) forms (FAO, 2012).
Fish is one of the best protein sources available to the human body in quality and quantity. It is also a valuable source of vitamin A and B, iodine and oils containing polyunsaturated fatty acids (Adebayo et al., 2012). However, the increasing demand for fish and fish products all over the world is greatly challenged by microbial infection of fish and contamination of fish products (Pyatkin and Krivoshein, 1986).
Studies indicate that there are several bacteria that have been isolated from three main parts of fresh fish: slime layer, gills and the gastrointestinal tract. According to Cahill (1990) the microbiological diversity of the fresh fish muscle depends on the fishing grounds and environmental factors around it. Fish from warm waters mostly harbor mesophilic gram-negative bacteria while cold waters harbor mostly psychrophilic, gram-positive bacteria. These bacterial isolates are classified into two groups: indigenous and post-harvest bacteria. Previous studies have also demonstrated the presence of indicator microorganisms of fecal pollution, opportunistic and pathogenic bacteria to humans in fish (Tsai et al., 2002; Ferreira and Pinho, 2006; Tzikas et al., 2007).
The origin of most microbes in fish and fish products may not be precisely known, some workers have related microbial infections and/or contamination of fish and fish products to a number of factors including unfavorable conditions in the fish culture system (Okpokwasili and Ogbulie, 1993), pollution and seasonal changes (Obiajuru et al., 2010), fish handling and processing including personnel and processing equipment (Pelczer and Chan, 1986). Fish contains protein and nutrients favorable for microbial attack even after processing. This often leads to fish spoilage and nutritional deterioration. Recent studies in Owerri, Imo State of Nigeria, showed 20% prevalence of Salmonella in frozen fish (Ohalete et al., 2011). In developing countries like Ethiopia where there is a poor infrastructure for the sector, fish spoilage from microbial contamination is believed to be a critical problem of fisheries and public health (FAO, 1993). Hence, this study was designed to elucidate the microbiological quality of fish meat retailed at Arba Minch town in Southern Ethiopia.
Lake Abaya and Chamo, the two large rift valley lakes, are located about 10 km North and Southeast of Arba Minch town, respectively. The total annual average commercial catch from Abaya and Chamo Lake based on surface area was estimated at 10,100 and 5,500 tons, respectively (FAO, 1993). These two lakes supply fish for local and national markets, and Nile Tilapia (Oreochromis niloticus) is the common fish species harvested from them.
According to the data obtained from Arba Minch Zurya Woreda Marketing and Cooperative Office, authorized fishermen associations are the main supplier of fish to hotels and restaurants in Arba Minch town. Fish is transported to the town at the back of pick-up track. However, illegal fishermen have to walk daily to the town carrying fish in sacks on their heads in the sunny and hot weather conditions. Factors that could degrade the safety and quality of fish, such as lack of proper transportation facilities, poor handling and processing are common.
In Arba Minch, fish is processed and presented to the consumer in different types of dish. Among that, Asa leb-leb is the most famous and highly consumed fish dish in the town. The dish Asa leb-leb is lightly fried (undercooked) fish fillet, thus it may contain some pathogenic bacteria of great concern to public health (example, Escherichia coli) and their toxic metabolites (example, Staphylococcus toxins) which tolerate the temperature and time combination at which this dish is prepared. Moreover, several studies assured that cooking of sea food products would not destroy their toxic metabolites like thermostable enterotoxin of Staphylococcus aureus and thermostable enterotoxin of E. coli (Vieira et al., 2001; Ayulo et al., 1994). Even if this dish is prepared at the correct temperature and time combination which eliminate the above risks, there may be also a risk of recontamination from improper post-process handling. Therefore, consumers could be at risk of acquiring zoonotic pathogens and/or their toxic metabolites from consumption of Asa leb-leb dish.
Therefore, the general objective of this study was to assess the microbiological quality of Nile tilapia fillets and food safety practices of fish handlers in Arba Minch town, SNNPR, Ethiopia. The specific objectives of the study were:
1. To determine the microbial load of frozen raw and undercooked (Asa leb-leb) Nile tilapia fillets.
2. To isolate hygiene indicator bacteria that is, E. coli and other pathogenic bacteria of great concern to public health that is, Salmonella spp. and S. aureus from frozen raw and undercooked (Asa leb-leb) Nile tilapia fillets.
3. To assess food safety practices of fish handlers working in the kitchens of different hotels.
Description of the study area
The study was conducted from June to December, 2013 in Arba Minch town. Arba Minch is the capital town of Gamo Gofa Zone of Southern Nations, Nationalities and People’s Regional State (SNNPR). It is located 505 km far from the capital city of Ethiopia, Addis Ababa, in the coordination of 5°57' N latitude and 37°32' E. The area has an average annual temperature of 29.7°C and rain fall of 700 mm (CSA, 2007). Arba Minch is presently experiencing rapid population growth. As a result, the number of commercial food establishments in the town has visibly increased. According to the data obtained from Trade and Industry Office of the town, about 20 registered hotels are currently present in the town. The sources of fish for all these commercial food establishments are fish harvested from Abaya and Chamo Lakes.
Study design
The study involved a questionnaire survey and a laboratory assessment of the microbiological status of frozen raw and undercooked (Asa leb-leb) Nile tilapia fillets collected from the different hotels in Arba Minch town. A questionnaire survey was used to assess the current status of food safety practiced by fish handlers working in the kitchens of different hotels in the town.
Sample and sampling method
In the present study, frozen raw and undercooked (Asa leb-leb) Nile tilapia fillets were collected from selected registered hotels in Arba Minch town. The subjects for this study were fish handlers in the kitchens of hotels, registered under the Trade and Industry Office of the town. Due to limited resources, only representative hotels were selected by purposive sampling strategy.
Sample collection, handling and transportation
A total of 80 (40 frozen raw and 40 undercooked (Asa leb-leb)) Nile tilapia fillets were collected in 10 trips from 10 (50% of total hotels) selected registered hotels of Arba Minch town. In each trip, two hotels were visited to take a total of eight samples of Nile tilapia fillets (two frozen raw and two undercooked from each hotels) to assess their microbiological quality. To make sure that samples were taken without being contaminated, inverted plastic bags were used for collection. The inner surface of the bag was used to touch nothing else but the sample. All samples were labeled with the type of the sample, the place, date of sampling and given an identification code and transported to Wolaita Sodo Regional Veterinary Laboratory in icebox containing ice packs for microbiological analysis. Upon arrival, the samples were immediately processed or stored at -20°C in a refrigerator until use and processed within 24 h of collection.
Microbiological analysis
Sample preparation
The frozen samples were thawed at room temperature for 5 to 6 h before processing (APHA, 2005). T10 g of each Nile tilapia fillet sample was weighed and placed into sterile stomacher bags, diluted with 90 ml of 0.1% sterile peptone water (HiMedia, India) and homogenized in a stomacher for 2 min per procedure describe by Fawole and Oso (2001).
Total plate count (TPC)
From the 10-fold dilutions of the homogenates, 0.1 ml of 10-2, 10-3, 10-4, 10-5 and 10-6 dilutions of the homogenates were pour-plated in duplicates on fresh standard plate count agar (HiMedia, India). The plates were then incubated at 37°C for 24 to 48 h. At the end of the incubation period, plates exhibiting 30 to 300 colonies were counted using illuminated colony counter. The counts for each plate were expressed as colony forming unit of the suspension (cfu/g) (Fawole and Oso, 2001).
Isolation and identification of bacteria
Isolation and identification of Salmonella
Salmonella was isolated from 10 g fish samples homogenized in 90 ml of 0.1% sterile peptone water. Aliquots of 1 ml were added to 10 ml of selenite broth (Difco, France). These were incubated at 37°C for 24 h. After gentle mixing, a loopful of culture from the enrichment broth was streaked on to xylose lysine desocholate (XLD) agar (HiMedia, India) and incubated at 37°C for 24 to 48 h. Typical Salmonella colonies which were pink with or without black centers were isolated. The colonies were purified on fresh nutrient agar (HiMedia, India), then streaked and stabbed into the butt of triple sugar iron (TSI) (HiMedia, India) slants. These were incubated at 37°C for 24 h. The test tubes that had alkaline (red) slants and acidic (yellow) butts, with or without the production of H2S (blackening) were presumed to be Salmonella isolates. Moreover, two or more colonies from pure isolate were inoculated on urea broth (SRL, India) and incubated at 37°C for 24 h. All test tubes that were urease negative were treated as suspects of Salmonella (FDA, 1992). In addition, isolates that were Gram-negative rod, oxidase negative, citrate positive, methyl red positive, indole negative, voges-proskauer negative and lactose and sucrose non-fermenter were accepted putatively as Salmonella (Fawole and Oso, 2001).
Isolation and identification of E. coli
For the isolation of E. coli, a loopful of homogenized sample was streaked on to eosin methylene blue (EMB) agar (HiMedia, India). The plates were incubated at 37°C for 24 to 48 h. Typical E. coli colonies which are blue-black with a green metallic sheen were isolated. The colonies were purified on fresh nutrient agar (HiMedia, India) and then streaked and stabbed into the butt of triple sugar iron (TSI) (HiMedia, India) slants. These were incubated at 37°C for 24 h. All test tubes that had acidic (yellow) slants and acidic (yellow) butts, without the production of H2S (blackening) were treated as suspects of E. coli (FDA, 1992). In addition, isolates that were gram-negative rod, oxidase negative, citrate negative, indole positive, urease negative, methyl red positive, voges-proskauer negative and lactose and sucrose fermenter were accepted putatively as E. coli (Fawole and Oso, 2001).
Isolation and identification of S. aureus
For the isolation of S. aureus, a loopful of homogenized sample was streaked on to mannitol salt agar (HiMedia, India). The plates were incubated at 37°C for 24 to 48 h. Typical S. aureus colonies which are yellow with a yellow halo around the colony were isolated. The colonies were purified on fresh nutrient agar (HiMedia, India) and then transferred into small tubes with 0.5 mL of rabbit plasma (NVI, Ethiopia). The tubes were rotated gently to mix the content and incubated at 37°C for 2 to 24 h, after which tubes that had clotted plasma were treated as suspects of S. aureus (APHA, 2005). Moreover, the smear was prepared from the isolated culture on clean grease free microscopic glass slide and stained with Gram’s Method of staining. The stained smears were observed under microscope. Smear that revealed gram positive, spherical cells arranged in irregular clusters resembling to bunch of grapes were considered for Staphylococci presumptive. The isolates were further characterized by catalase, voges-proskauer and sugar (mannitol and maltose) fermentation test (Fawole and Oso, 2001).
Questionnaire survey and observation
Hygiene and sanitation conditions were determined by the use of semi-structured questionnaire. Moreover, direct observation was employed to assess the hygienic status and practices by the fish handlers working in the kitchens of different hotels found in Arba Minch town. All fish handlers observed in all 10 purposively selected hotels for this study were interviewed by the use of detailed and semi-structured questionnaire in order to obtain relevant information on food safety practices and fish food handling. Personal information, like educational status and training of fish handlers on sanitary handling of fish food and practices were collected through questionnaire and observation. Informed consent was obtained from owners/managers of the hotels and fish handlers after a brief explanation about the benefits of the study. Confidentiality of the respondent was maintained by assuring that his/her answers would not be released to anyone and would remain anonymous and his/her name would not be written on the questionnaire or be kept in any other records.
Data quality control
The quality of the data was kept by preparing and using standard operational procedures for laboratory investigation and media preparation. Semi-structured questionnaire was tested using pretest before conducting the study. Sample collection and processing were carried out using aseptic techniques. The samples were labeled properly. Culture and bacterial colony count were carried out under supervision of experienced laboratory personnel. All media were prepared according to the manufacturer’s specification and sterilized at 121°C 15 psi pressure unit for 15 min. The sterility test of prepared media was checked by incubating at 37°C (APHA, 2005).
Statistical analysis
Basic data entry and handling was done by Microsoft Excel database system. The data were then analyzed using statistical package for social sciences (SPSS) (2012). Descriptive statistics such as, means and frequencies were used to present the findings of both questionnaire survey and laboratory assessment. Also, a t-test was used to check whether the mean of two different groups under comparison were significantly different in the normally distributed population from which the samples were drawn.
Practices of fish handlers
Since food handlers may be the source of food contamination either as carriers of pathogen or through poor hygienic practices. Thus all food handlers have basic responsibility to maintain a high degree of personal cleanliness and implement hygienic and safe food handling practices. Among the precautions that a food handler must maintain the major ones are; keeping hands clean and fingernails short, wearing clean working garment and hair cover (hair net and cap) (Kaferstein, 2003). However, the result of this study showed that 32.6% of the fish handlers did not wear appropriate overcoat (Table 1). This finding is higher than the result of similar study done on food handlers in Hawassa (14%) (Teklemariam et al., 2000) but lower than the finding of Fisseha et al. (1999) in Addis Ababa (45.8%). Moreover, 44.2% of fish handlers were also found with covered hair, which is consistent with that of Kumie et al. (2000) done in Zeway (40.1%) but better than the result of Teklemariam et al. (2000) assessed in Hawassa (11.8%).
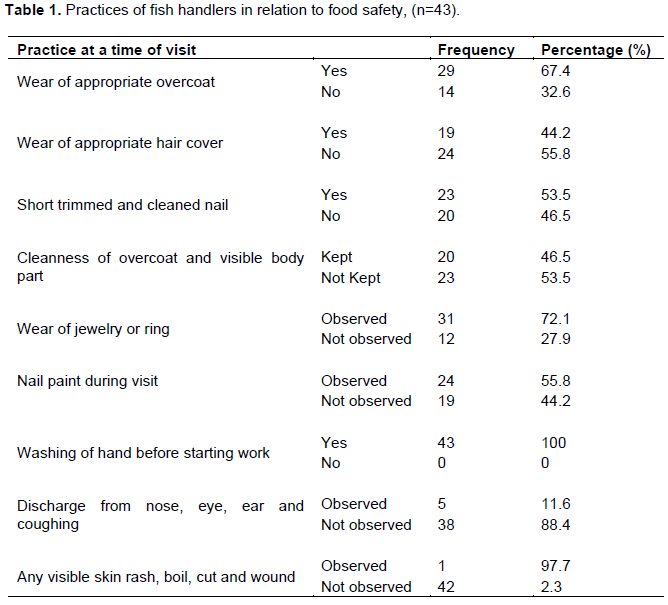
In addition, nail of 46.5% of the fish handlers in this study were not shortly trimmed and clean (Table 1). This finding is higher than the result of Teklemariam et al. (2000) who found that 21.2% of food handlers in Hawassa city hotels did not trim their nail shortly. It was also observed that 72.1% of fish handlers in this study wore rings on their finger during food preparations, which was markedly higher than the report of Kumie et al. (2000) done in Zeway (28.7%). As food handlers can be the probable sources of contamination for micro-organisms, it is important to take all possible measures so that such contaminations would be reduced or eliminated (Muinde and Kuria, 2005).
Training of fish handlers on sanitary handling of food
According to Adams and Moss (1997), training of food handlers regarding the basic concepts and requirements of personal hygiene and sanitary handling of food play an integral part in ensuring a safe product to the consumer. But, the result of this study showed that 30.2% of the fish handlers have taken no training concerning sanitary handling of food. This result is lower than the previous result of Mekonnen et al. (2012) who obtained 61.5% of meat handlers not to take training on sanitary and food hygiene.
Total plate count (TPC)
Bacterial growth is the main cause of fish spoilage and public health concern therefore total bacterial count is used as a general index of fish quality. In this study the mean bacterial count (cfu/g) was found to be 4.63x106 in frozen raw Nile tilapia fillet samples (Table 2). This value was found to be higher than the result of Dhanapal et al. (2012) who found 4.9x104 in frozen raw Nile tilapia fillet samples. This high load is mainly the result of poor handling during the transportation, and/or poor personal hygiene during filleting.
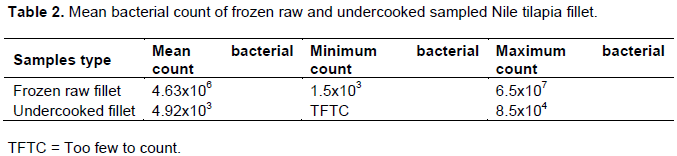
In the current study, the mean bacterial count (cfu/g) was found to be 4.92x103 in undercooked Nile tilapia fillet samples. This result indicated that low mean value of bacterial load was found in the undercooked Nile tilapia fillet samples, which was significantly different from frozen raw Nile tilapia fillet samples (p=0.037). This is due to the exposure to heat during processing for the undercooked Nile tilapia fillet samples. Almost similar result has been` reported by Salaudeen et al. (2010) and Dhanapal et al. (2012). For ready to eat seafood, the microbiological limit for human consumption proposed by the International Commission on Microbiological Specifications for Foods (ICMSF) (1986) is 5x105 to 107 cfu/g in aerobic plate count analysis, which is also in line with this study result.
Bacterial isolation
Table 3 summarizes the frequency of bacterial isolate from frozen raw and undercooked Nile tilapia fillet samples. As it is shown, the lowest frequency of bacterial isolates were observed in undercooked Nile tilapia fillet samples which was significantly different from frozen raw Nile tilapia fillet samples (p=0.000). This could be due to the negative effect of heat on the growth of bacterial contaminants. Regarding the frequency of bacterial
isolate from heat treated fish and other ready-to-eat food stuffs similar observations were reported by other researchers (Mezgebu and Mogessie, 1998; Thailambal, 2006; Omenwa et al., 2012).
Finding of this study indicated that 42.5% of frozen raw Nile tilapia fillet samples carried E. coli. This result is higher than the result of Vieira et al. (2001) who reported that 12.5% of all samples from Brazilian markets had E. coli. The detectable presence of E. coli in the present study is a sign of faecal contamination of fish. This is in accordance with results obtained by Monzur-Hassan et al. (1994) and Yousuf et al. (2008) those associated with the detectable presence of E. coli in fish to the faecal contamination of fish sourced from the warm blooded animal.
Moreover, this study indicated that 7.5% of undercooked Nile tilapia fillet samples had E. coli. This result complies with previous report by El-Gohany (1994) that foods of animal origin either cooked or uncooked were predominantly contaminated with E. coli. Besides, Ohalete et al. (2013), reported 58.3% of E. coli contamination in fried fish samples from different bars and eating houses in Owerri, Nigeria. This is also in accordance to the assertion of Okonko et al. (2008, 2009a,b) that improper handling and improper hygiene might lead to the contamination of ready-to-eat foods and this might eventually affects the health of the consumers.
The result of this study further depicted that 65% of frozen raw and 17.5% of undercooked Nile tilapia fillet samples to harbor S. aureus. The occurrence of S. aureus in frozen raw Nile tilapia samples in this study was higher than the result of Mhango et al. (2010) who isolated S. aureus in 41% of the frozen gutted Nile tilapia samples studied and Ayulo et al. (1994) who isolated S. aureus in 20% of fresh fish and fish fillets (Cynoscion leiarchus). However, the presence of S. aureus in undercooked (Asa leb-leb) Nile tilapia samples in this study indicated that this type of heat treatment was not sufficient to eliminate the pathogen and/or the risk of recontamination from improper post-process handling. Generally, the high prevalence of Staphylococcus in the fish samples indicated the unhygienic handling of the fish since this species is found on human skin.
The frequency of isolation of Salmonella spp. from frozen raw Nile tilapia fillet samples in this study was 7.5%. This result is lower than the result of Ohalete et al. (2011) who isolated 20% of Salmonella spp. from frozen fish samples. Moreover, while in this study Salmonella spp. was detected, Rokibul and his team didn’t isolate Salmonella spp. from frozen fish samples (Rokibul et al., 2012).
In contrast, no Salmonella spp. was isolated from undercooked Nile tilapia fillet samples in this study. The absence of Salmonella spp. in undercooked Nile tilapia samples in this study agrees with the result of Odu and Ameweiye (2013) that isolated no Salmonella spp. from ready-to-eat bole fishes.
But the presence of E. coli in undercooked Nile tilapia samples in this study indicated that Salmonella could also survive the heat treatment if it was there.
CONCLUSION AND RECOMMENDATIONS
The findings of this study revealed the presence of gap on food safety practices by fish handlers who work in different hotels of the Arba Minch town. The microbiological examination of frozen raw and undercooked (Asa leb-leb) Nile tilapia fillet samples revealed that the frozen raw Nile tilapia fillets are more contaminated than the undercooked Nile tilapia fillets. However, the detection of some zoonotic pathogens in undercooked (Asa leb-leb) Nile tilapia samples in this study indicated that this type of heat treatment was not sufficient to eliminate all zoonotic pathogens and/or the risk of recontamination from improper post-process handling.
In the light of these major findings, the following recommendations are made to safeguard the public against the risks of foodborne infections and intoxication:
1. Fish handlers should be trained on the adverse effect of lack of proper personal and environmental hygiene and sanitation.
2. Proper attention should be paid to the safety of both frozen raw and undercooked (Asa leb-leb) fish through proper handling and use of adequate processing procedures such as proper heating method.
3. Public health authorities in the state should ensure adequate supervision and monitoring of fish handling and sales especially ready-to-eat products like Asa leb-leb dish.
4. Further investigations should be conducted to assess the issues that are not addressed through this study.
The authors have none to declare.
REFERENCES
|
Adams MR, Moss MO (1997). Food Microbiology. Royal Society of Chemistry. Science Park, Cambridge.
|
|
|
|
Adebayo BC, Odu NN, Igiwiloh NJ, Okonko IO (2012). Microbiological and Physicochemical Level of Fresh Catfish (Arius hendelotic) from Different Markets in Akwa Ibom State, Nigeria. New York Sci. J. 5(4):46-52. Agric. Nutr. Dev.10:4202-4218.
|
|
|
|
|
American Public Health Association (APHA) (2005). Compendium of methods for microbiological examination of foods, 2nded. American Public Health Association, Washington, DC, USA.
|
|
|
|
|
Ayulo AM, Machado RA, Scussel VM (1994). Enterotoxigenic Escherichia coli and Staphylococcus aureus in fish and seafood from the southern region of Brazil. Int. J. Food Microbiol. 24:171-178.
Crossref
|
|
|
|
|
Babalola ET, Ogun AA (2008). Microbial studies on frozen shrimps processed in Ibadan and Lagos, Nigeria. Sci. Res. Ess. 3(11):537- 546.
|
|
|
|
|
Cahill MM (1990). Bacterial flora of fishes: a review. J. Microbial Ecol. 19(1):21-41.
Crossref
|
|
|
|
|
Central Statistics Agency (CSA) (2007). Central Statistics Agency of Federal Democratic Republic of Ethiopia; Summary and statistical report of population and housing.
|
|
|
|
|
Dhanapal KG, Vidya SL, Binay BN, Venkateswarlu G, Devivaraprasad AR, Basu S (2012). Effect of cooking on physical, biochemical, bacteriological characteristics and fatty acid profile of Tilapia (Oreochromis mossambicus) fish steaks. Arch. Appl. Sci. Res. 4(2):1142-1149.
|
|
|
|
|
El-Gohany AH (1994). Sausage and minced meat as a source of food poisoning microorganisms to man. Assiut. Vet. Med. J. 30:146-215.
|
|
|
|
|
Fawole MO, Oso BA (2001). Laboratory manual of microbiology: Revised edition. Ibadan: Spectrum books Ltd, p. 127.
|
|
|
|
|
Ferreira IM, Pinho O (2006). Biogenic amines in Portuguese traditional foods and wines. J. Food Prot. 69(11):2293-2303.
Crossref
|
|
|
|
|
Fisseha G, Berhane Y, Teka GE (1999). Food Handling Practice in Public Caterings in Addis Ababa, Ethiopia. Ethiop. Med. J. 37(1):1-10.
|
|
|
|
|
Food and Agricultural Organization (FAO) (1993). Contribution to the formulation of a short-medium term plan for the fisheries sector in Ethiopia. Technical report, part 1: Sector review, Rome.
|
|
|
|
|
Food and Agricultural Organization (FAO) (2006). Fishery Information, Data and Statistics Unit. Capture production: quantities 1950-2004. Rome. Retrieved from
View.
|
|
|
|
|
Food and Agricultural Organization (FAO) (2012). The State of World Fisheries and Aquaculture 2012. Food and Agricultural Organization of the United Nations.
|
|
|
|
|
Food and Drug Administration (FDA) (1992). Bacteriological Analytical Manual. 7th Edition. AOAC international 2200 Wilson Blvd, Suite 400, Arlington, VA.
|
|
|
|
|
International Commission on Microbiological Specifications for Foods (ICMSF) (1986). Sampling plan and recommended microbiological limits for Seafood.
|
|
|
|
|
Kaferstein FK (2003). Food Safety: The fourth pillar in the Strategy to prevent infant Diarrhea. Bulletin of the WHO 81(11):842-843.
|
|
|
|
|
Kumie A, Genete K, Worku H, Kebede E, Ayele F, Mulugeta H (2000). The Sanitary Conditions of Public Food and Drink Establishments in the District town of Zeway, Southern Ethiopia. Ethiop. J. Health Dev.16(1):95-104.
|
|
|
|
|
Mekonnen H, Habtamu T, Kelali A, Shewit K (2012). Food safety knowledge and practices of abattoir and butchery shops and the microbial profile of meat in Mekelle City, Ethiopia. Asian Pac. J. Trop. Biomed. 3(5):952-957.
|
|
|
|
|
Mezgebu T, Mogessie A (1998). Microbial load and incidence of Salmonella spp. in 'kitfo', a traditional Ethiopian spiced, minced meat dish. Ethiop. J. Health Dev.12(2):135-140.
|
|
|
|
|
Mhango M, Mpuchane SF, Gashe BA (2010). Incidence of indicator organisms, opportunistic and pathogenic bacteria in fish. Afr. J. Food
Crossref
|
|
|
|
|
Ministry of Agriculture (MoA) (2012). Ethiopian Ministry of Agriculture (MoA). National summary of agricultural production in Ethiopia. Annual report. pp. 56-72.
|
|
|
|
|
Monzur-Hassan MM, Rahman KM, Nahar A (1994). Studies on the bacterial flora of fish which are potential pathogens for human. Bangladesh Med. Res. Council Bull. 20(2):43-51.
|
|
|
|
|
Muinde OK, Kuria E (2005). Hygienic and sanitary practices of vendors of street foods in Nairobi, Kenya. Afr. J. Food Agric. Nutr. Dev. 5:1. Obiajuru IOC, Ozumba UC (2009). Laboratory Methods for Medical Microbiology and Parasitology. Lifeway Amalgamations, Owerri. P 183.
|
|
|
|
|
Odu NN, Ameweiye NB (2013). Microbiological Quality of Street- Vended-Ready-to-Eat "Bole" Fish In Port Harcourt Metropoplis. New York Sci. J. 6(2):92-101.
|
|
|
|
|
Ohalete CN, Dozie INS, Obiajuru IOC, Ekeh IH (2011). Studies on the ecology of Salmonella bacilli in Owerri metropolis Imo State, Nigeria. GRJS 1:109-116.
|
|
|
|
|
Ohalete CN, Obiajuru IOC, Obiukwu CE, Uwaezuoke JC, Nwaehiri UL, Daniel UN (2013). Microbiological quality of fried and smoked fish in Owerri, Imo State Nigeria. WJPPS. 2:1-19.
|
|
|
|
|
Okonko IO, Donbraye E, Babatunde SOI (2009b). Microbiological Quality of Seafood processors and water used in two different sea processing plants in Nigeria. EJEAFche 8(8):621-629.
|
|
|
|
|
Okonko IO, Ogunnusi TA, Ogunjobi AA, Adedeji AO, Adejoye OD,
|
|
|
|
|
Okpokwasili GC, Ogbulie JN (1993). Bacterial and Metal quality of Tilapia (Oreochromis niloticus) aquaculture systems. Int. J. Environ. Health Res. 13:190-202.
Crossref
|
|
|
|
|
Omenwa VC, Ansa EJ, Agokei OE, Uka A, George OS (2012). Microbiological quality of raw and processed farm-reared periwinkles from brackish water earthen pond Buguma, Nigeria. Afr. J. Food Agric. Nutr. Dev.11:4623-4631.
|
|
|
|
|
Pelczer MJ, Chan ECS (1986). Elements of Microbiology. McGraw – Hill Book co. New York.
|
|
|
|
|
Pyatkin KD, Krivoshein YuS (1986). Microbiology. MIR Publishers, Moscow. P 167.
|
|
|
|
|
Rokibul H, Mrityunjoy A, Eshita D, Kamal KD, Tasnia A, Muhammad AA, Kazi KF, Rashed N (2012). Microbiological study of sea fish samples collected from local markets in Dhaka city. Int. Food Res. J. 20(3):1491-1495.
|
|
|
|
|
Salaudeen M, Akande G, Oguntade O, Afolabi O, Olusola A, Ezekiel M (2010). Effect of preservatives on microbial safety and quality of smoked catfish (Clarias gariepinus) during ambient storage. Acta SATECH 3(2):81-86.
|
|
|
|
|
Statistical Package for Social Science (SPSS) (2012). SPSS for Window (Version 19) SPSS, Chicago, IL, USA.
|
|
|
|
|
Teklemariam S, Roma B, Sorsa S, Worku S, Erosie L (2000). Assessment of Sanitary and Hygiene Status of Catering Establishments of Awassa Town. Ethiop. J. Health Dev. 14(1):91-98.
|
|
|
|
|
Thailambal AS (2006). Effect of processing on bacterial population of cuttle fish and crab and determination of bacterial spoilage and rancidity developing on frozen storage. J. Food Process Preserv. 31(1):13-31.
|
|
|
|
|
Tsai YH, Chang SC, Kung HF, Wei C, Hwang DF (2002). Histamine Production by E. aeregenes in Salifish and Milkfish at Various Storage Temperatures. J. Food Prot. 68(8):1690-1695.
Crossref
|
|
|
|
|
Tzikas Z, Amvrosiadis I, Soultos N, Georgakis SP (2007). Seasonal size distribution, condition status and muscle yield of of Mediterranean Horse Mackerel (Trachurus mediterraneus) from the North Aegean Sea Greece. J. Fish. Sci. 73(2):453-462.
Crossref
|
|
|
|
|
Vieira RHSF, Rodrigues DP, Gocalves FA, Menezes FGR, Aragao JS, Sousa OV (2001). Microbicidal effect of medicinal plant extracts (Psidium guajava Linn. and Carica papaya Linn.) upon bacteria isolated from fish muscle and known to induce diarrhea in children. Rev. Inst. Med. Trop. S. Paulo 43:145-148.
Crossref
|
|
|
|
|
World Bank (2010). The hidden harvests: the global contribution of capture fisheries. World Bank, Washington.
|
|
|
|
|
Yousuf AHM, Ahmed MK, Yeasmin S, Ahsa N, Rahman MM, Islam MM (2008). Prevalence of Microbial Load in Shrimp, Penaeus monodon and Prawn, Macrobrachium rosenbergii from Bangladesh. World J. Agric. Sci. 4(5):852-855
|
|