ABSTRACT
Cellulases and xylanases are enzymes of industrial significance, particularly in the pulp, paper, textile, and animal feed industries. Moreover, their utilization in the food industry, among them, bakery, brewery, winery and fruit and vegetable juice production, cannot be underestimated. One of the potential sources of enzymes is the filamentous fungi, and hence bio-prospecting of this specific group of microorganisms with the highest levels of cellulase and xylanase secretions is being continuously undertaken. The specific aim of this study was to isolate and characterize cellulase- and xylanase-producing filamentous fungi from termite mounds. Termite mounds have long been established as very good sources of filamentous fungi with the ability to secrete high levels of lignocellulolytic enzymes, and hence an ideal target for the bio-prospecting of cellulases and xylanases. In this study, various groups of filamentous fungi were isolated through enrichment and repeated sub-culturing. This was followed by screening using the Congo red plate-based assay. Cellulase and xylanase activities during the solid-state fermentation of wheat bran were detected and analyzed through spectrophotometry via the 3,5-dinitrosalicylic acid detection system for reducing sugars. The obtained fungal isolates were then finally characterized through zymography, reaction kinetics and morphological studies. Overall, a total of eight different groups of fungi, capable of decomposing cellulose and hemicellulose, were isolated, and their tentative identities established as Fusarium, Didymostible, Penicillium, Phytophthora, Oedocephalum, Aspergillus, Monosporascus and Acremonium. Taken together, findings of this study conceivably showed that termite mounds are a good source of filamentous fungi that in turn are also a good source of cellulases and xylanases that arguably, can be recommended for use in industrial and commercial settings.
Key words: Filamentous fungi, lignocellulosic substrates, lignocellulolytic enzymes, cellulases, xylanases, termite mounds, termite nests, fungal combs.
Termites are soil insects that efficiently decompose lignocellulosic material with the aid of their associated microbial symbionts (Ohkuma, 2003). Termites live in termite mounds, which they build themselves, and these
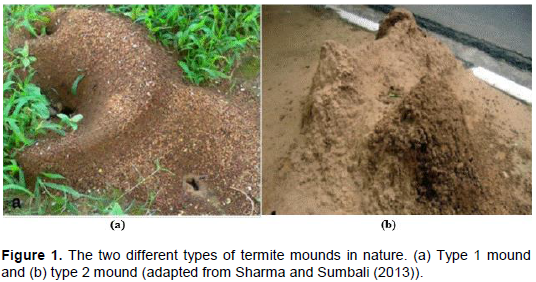
mounds are very predominant in Africa and Asia due to the warmness nature of these two continental regions (Makonde et al., 2015). Naturally, termite mounds are of two kinds; type 1 and type 2. Type 1 mound is generally small and susceptible to erosion while type 2 mound is large, epigeic and cemented (Sharma and Sumbali, 2013)as shown in Figure 1. Within the mound, termites formulate nests of diverse forms that are essentially covered with various plant and termite material, termed combs. In these combs, various microorganisms such as fungi, bacteria and yeasts are also found due to the ubiquitous nature of microorganisms and availability of food, and of which most live synergistically with the resident termites. If a comb is predominantly made up of fungi, it is termed a fungal comb whilst those of bacteria and yeast are also termed bacterial and yeast combs respectively.
Termites of the sub-family Macrotermitinae, also known as fungus-growing termites, cultivate symbiotic fungi of the genus Termitomyces on fungal combs, which are synergistically maintained in nests of the termite mounds (Hyodo et al., 2003; Taprab et al., 2002). In nature, termites solely depend on plant litter as their main source of food, however, these insects naturally lack the lignocellulolytic enzymes responsible for the breakdown of lignin and cellulose (Ni and Tokuda, 2013). Hence, to efficiently utilize lignocellulosic material, termites co-operate with symbiotic fungi in a sophisticated but well-coordinated manner. In this relationship, the symbiotic fungi have four major functional significances to the termites. Firstly, to provide ligninolytic enzymes that avail cellulose; secondly, to provide cellulolytic enzymes that avail hemicellulose; thirdly, to provide hemicellulases, which synergistically work with the endogenous termite enzymes to breakdown hemicellulose into various simple and soluble sugars; and lastly, to act as sole source of nitrogen for the termites (Hyodo et al., 2003; Ohkuma, 2003).
Symbiotic fungi avail cellulose and hemicellulose to both termites and other associated soil microbes. Due to the ubiquitous nature of microbes, there is a high likelihood of also finding other lignocellulolytic enzyme-producing microorganisms (bacteria, yeasts and fungi) in termite nests, since substrates are readily available. Among the termite nest microorganisms, fungi are industrially recognized as important producers of lignocellulolytic enzymes because of their ability to excrete large quantities of enzymes extracellularly (Adesina and Onilude, 2013; Polizeli et al., 2005), thus making the general extraction and purification process of the enzymes much easier and cheaper.
Lignocellulolytic enzymes constitute a very large group of extracellular proteins mainly, cellulases, hemicellulases, pectinases and ligninases (Mtui, 2012). Of these, cellulases and xylanases are of significant industrial value and relevance. These two groups of enzymes have numerous applications and massive biotechnological potentials for the various industrial sectors, including chemical, food, brewery and wine, animal feed, textile and laundry, pulp and paper and agriculture (Bhat, 2000; Kuhad et al., 2011; Polizeli et al., 2005; Subramaniyan and Prema, 2002). In addition to these applications, cellulases and xylanases are key enzymes that can be effectively used to solve challenges associated with energy inadequacy and environmental pollution (Favaro et al., 2013). These two groups of enzymes are currently being used significantly in the commercial bio-conversion of lignocellulosic biomass to bio-ethanol (Limayem and Ricke, 2012). It is therefore on the basis of this industrial demand and significance that the bio-prospecting of diverse cellulases and xylanases, particularly in termite nests, has become a necessity. In this study were report the isolation, screening and characterization of cellulase- and xylanase-producing filamentous fungi from termite mounds for possible recommendation in industrial and commercial systems.
Isolation of the lignocellulolytic enzyme-producing filamentous fungi from termite mounds
Sampling of fungal combs
Several samples of fungal combs were collected from termite nests after digging and opening the termite mounds. Following the digging and opening of a mound, fungal combs were harvested aseptically by scrubbing them off the termite nests using a sterile spatula into sterile test tubes before transporting the collections to the laboratory for further experimental analysis. The fungal combs were named as either fungal comb 1 (FC1) or fungal comb 2 (FC2), depending on the type of mound from which they were collected. FC1s were obtained from the type 1 mounds while FC2s were obtained from the type 2 mounds as shown in Figure 1. A total of at least three mounds per type were used in the study.
Enrichment of the fungal combs
Approximately 10 g of composite fungal comb material per mound type were weighed and used to inoculate 100 ml of sterile carboxylmethylcellulose (CMC) or birchwood xylan broth (0.05 µg/ml MgSO4.7H2O, 0.005 µg/ml CaCI2, 0.005 µg/ml NaNO3, 0.009 µg/ml FeSO4. 7H20, 0.002 µg/ml ZnSO4, 0.012 µg/ml MnSO4, 0.23 µg/ml KCI, 0.23 µg/ml K2H2PO4, 0.2 µg/ml NH4NO3, 0.25 µg/ml yeast extract, 0.75 µg/ml peptone and 1% (w/v) of CMC or birchwood xylan substrate) supplemented with chloramphenicol to a final concentration of 200 µg/ml to inhibit the growth of bacteria. The inoculated broth was then incubated in a 2001651 Incubat bench-top incubator (JP Selecta SA., Barcelona, Spain) at 25±2°C for 72 h.
Isolation of fungi from the fungal combs
After enrichment, a 100 µl aliquot of the enriched CMC or birchwood xylan broth was used to spread plate fresh malt extract agar (MEA) supplemented with 200 µg/ml chloramphenicol, followed by incubation at 25±2°C for 72 h. The resultant single colonies were then picked up with sterile toothpicks and used to further sub-culture the isolates. The process was repeated several times until pure colonies were obtained.
Preservation of the pure fungal isolates
The pure colonies were allowed to grow and generate fully developed spores. Fungal isolates were then preserved by aseptically scraping off spores from surfaces of the media and suspending them in sterile distilled water. The preserves were then kept at room temperature till further use.
Screening of fungal isolates for lignocellulolytic enzymes
The capacity of the pure fungal isolates to produce cellulases or xylanases was evaluated using the plate assay technique (Teather and Wood, 1982). Spores of each of the obtained fungal isolates were used to individually inoculate fresh 1% (w/v) CMC or birchwood xylan agar supplemented with 200 µg/ml chloramphenicol, followed by incubation at 25±2°C for 7 days. Cellulase- or xylanase-producing isolates were then identified based on the formation of clear halos around the colonies after flooding plates for 10 min with 0.5% (w/v) of Congo red and de-staining for 15 min with 1 M sodium chloride (Adesina and Onilude, 2013).
Biochemical characterization of the fungal isolates
All fungal isolates that were found to have the ability to produce cellulases and xylanases in the screening process above were then subjected to characterization biochemically. The characterization process involved application of the spectrophotometric techniques, zymography analysis and enzyme kinetics of the lignocellulolytic extracts secreted by the fungal isolates.
Preparation of inoculum for solid state fermentation
Using a Neubauer haemocytometer or counting chamber, standard spore counts for each of the selected fungal isolates were performed using their respective spore suspensions preserved earlier on. The generated spore counts at 1 x 106 spores/ml were then used as inoculums for the solid state fermentation.
Production of lignocellulolytic enzymes in solid state fermentation
Solid state fermentation was undertaken to induce the production of lignocellulolytic enzymes. Approximately 7 g of wheat bran was weighed into 250 ml Erlenmeyer flasks after which 15 ml of nutrient solution (0.3 g peptone, 0.3 g malt extract, 0.3 g yeast extract in 100 ml distilled water) was added and sterilized in a SA-300VL autoclave (Sturdy Industrial Company Ltd., New Taipei City, Taiwan) at 121°C for 15 min. When the medium had cooled down, the flasks were separately inoculated with the prepared respective individual spore suspensions at a concentration of 1 x 106 spores/g of substrate used. The inoculated flasks were then incubated for 5 days at 28±2°C, for the solid-state fermentation to take place with wheat bran acting as the sole source of carbon.
Harvesting and storage of enzymes from the solid state fermentation
After fermentation, about 5 ml of 100 mM sodium citrate buffer, pH 5.0 was added to each gram of the fermented substrate, followed by shaking in a 2001651 Incubat bench-top incubator (JP Selecta SA., Barcelona, Spain) at 150 rpm for 2 h at 4°C. The obtained materials were then centrifuged at 2500xg at 4°C for 20 min in an LSE high speed microcentrifuge (Corning Inc., Amsterdam, Netherlands). The resultant supernatant, containing the crude enzymes, was collected and stored at -20°C until needed.
Spectrophotometric assaying for the cellulase and xylanase activities
Cellulase and xylanase activities were determined by mixing together 0.25 µl of the crude enzyme extract, 0.25 µl of the 1% (w/v) CMC or birchwood xylan substrate and 0.25 µl of the 100 mM sodium citrate buffer; pH 5.0, followed by incubation at 50°C for 5 min. Each enzyme activity was then terminated by adding 75 µl of the 3,5-dinitrosalicylic acid to the reaction mixture followed by boiling at 100°C for 10 min in a WS 27-2 Shel-Lab water bath (Sheldon Manufacturing Inc., Cornelius, Oregon). For each test sample, an appropriate blank, whereby the crude enzyme extract in the reaction mixture was replaced with an equal volume of water was also prepared. After cooling, the color developed was then measured at 540 nm, using a Spectronic Helios Epsilon spectrophotometer (Thermo Electronic Scientific Instruments LLC., Middleton, Wisconsin). Alongside this assay, some glucose and xylose standards (Supplementary Material) were prepared and used to plot a standard curves from where concentrations of the metabolized substrates (CMC or birchwood xylan) were then estimated (Mandels, 1969).
Concentration of the enzyme extracts secreted by the fungal isolates
About 10 ml of the crude enzyme extracts previously clarified for spectrophotometric assays were concentrated through precipitation with 80% (w/v) ammonium sulphate salt, whereby the mixtures were swirled overnight at 150 rpm at 4°C. The mixtures were then centrifuged for 20 min at 2500xg at 4°C to pellet out the concentrated proteins. The resultant pellets were then re-dissolved in 1 ml of the 100 mM citrate buffer pH 5.0, to obtain some 10 times enzyme concentrates. The resultant concentrated enzyme extracts were then dialyzed against a 100 mM sodium citrate buffer pH 5.0 at 4°C for 18 h or until the dialysate weights had remained constant. The resultant enzyme concentrates or dialysates were then kept at –20°C for further use.
Resolution and analysis of the total protein content in the concentrated enzyme concentrates
The total protein contents in each of the resultant enzyme concentrate or dialysate were resolved by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and in accordance with the method of Laemmli (1970). Briefly, 40 µl of the enzyme concentrate were suspended in 1X protein loading buffer (20% (v/v) glycerol, 100 mM Tris-HCL, 2% (w/v) sodium dodecyl sulphate (SDS), 0.1% (w/v) bromophenol blue and 20% (v/v) β-mercaptoethanol) and boiled for 5 min on an Accu-Block Digital dry bath (Labnet International Inc., Woodbridge, New Jersey). The samples were then loaded (for concentration purposes) onto a 5% (v/v) stacking gel (4.8% (v/v) acrylamide solution, 0.1% (w/v) SDS, 125 mM Tris-HCl, 0.05% (w/v) ammonium persulphate (APS) and 0.4% (v/v) tetramethylethylenediamine (TEMED)), before being resolved on a 12% (v/v) running gel (12% (v/v) acrylamide solution, 0.1% (w/v) SDS, 375 mM Tris-HCl, 0.05% (w/v) APS and 0.2% (v/v) TEMED). Actual protein resolution was achieved by running the loaded gel at 120 V for 1 h using a Mini Protean vertical gel system (Bio-Rad Laboratories Inc., Hercules, California) submerged in 1X protein running buffer (1.44% (w/v) glycine, 0.3% (w/v) Tris-HCl, 0.1% (w/v) SDS) and against an unstained standard molecular weight marker (Fermenters Int., Burlington, Canada). The resolved gel was then stained with a Coomassie staining solution (0.5% (w/v) Coomassie brilliant blue powder, 10% (v/v) ethanol, 10% (v/v) methanol and 10% (v/v) acetic acid) for 30 min, shaking at 100 rpm on an Ultra-Rocker platform (Bio-Rad Laboratories Inc., Hercules, California). The stained gel was then similarly de-stained with a de-staining solution (10% (v/v) ethanol, 10% (v/v) methanol, 10% (v/v) acetic acid). The de-stained and resolved gel was then visually analyzed for presence of the various protein bands against the standard molecular weight marker (Fermenters Int.,) and images captured by a Chemi-Doc Imaging system (Bio-Rad Laboratories Inc., Hercules, California).
Zymography of cellulases and xylanases
This was undertaken according to the method of Téllez-téllez et al. (2013) and Pointing (1999), whereby a 12% (v/v) polyacrylamide gel incorporated with either 0.1% (w/v) CMC or birchwood xylan was prepared, followed by an electrophoretic resolution of the enzyme concentrates against a standard molecular weight marker (Fermenters Int.,). After electrophoresis, the molecular weight marker was cut off the gel and separately stained with Coomassie brilliant blue while the remainder of the gel was separately washed; firstly, with a mixture (1:1) of 100 mM sodium phosphate buffer; pH 7.2 and 40% (v/v) isopropanol for 1 h, to remove SDS; and secondly, with sodium phosphate buffer only for an hour to remove isopropanol. The washed gel was then re-natured through submergence in a 1:1 mixture ratio of 50 mM sodium phosphate buffer and 1 mM EDTA at 4°C for 1 h. The re-natured gel was then incubated at 50â°C for 1 h to allow the cellulases or xylanases to degrade their provided CMC or birchwood xylan substrate. After this, the gel was then stained for 30 min with 0.1% (w/v) Congo red, followed by de-staining for 20 min with 1 M sodium chloride. Furthermore, the gel was immersed in 0.5% (v/v) acetic acid for 1 h, to get better clearing contrast at the areas of enzyme activity. Finally, the previously cut off molecular weight marker was re-aligned to the main gel and images captured by a Chemi-Doc Imaging system (Bio-Rad Laboratories Inc.,).
Enzyme kinetics of the cellulases and xylanases
Determination of the kinetic rates of the cellulases and xylanases in each of the generated enzyme concentrate were undertaken via the Hanes-Woolf plot method. This final step on the generated enzyme concentrates was carried out so as to relate activities of these two specific enzymes in the obtained fungal isolates to their close counterparts in other known and/or related microbial systems. In this regard, reaction set ups with increasing concentrations (0.125, 0.25, 0.50 and 1.00 mM) of the substrate (CMC for cellulases and birchwood xylan for xylanases) were prepared, followed by measurement of enzyme activity (initial velocity) for each of the used substrate concentrations as already been described with the spectrophotometric method. Using the obtained initial velocities and the used substrate concentrations for each enzyme, a Hanes-Woolf plot was then sketched, followed by determination of the reaction kinetics constants (Km and Vmax) for each of the assessed enzymes (Kwezi et al., 2011; Meier et al., 2010). From this sketch, Km was determined as the negative value of the x-intercept (x = -Km, when y = 0) of a linear fit of the plotted data while Vmax was calculated from the y-intercept (y = Km/Vmax, when x = 0) of the same linear fit.
Morphological characterization of the fungal isolates
All fungal isolates that were initially found to have the ability to produce cellulases and xylanases during both the screening and biochemical characterization processes above were subjected to further characterization morphologically. The characterization process involved use of both the macroscopic (visual) and microscopic examinations of cultures after the fungal isolates had been grown and generated on MEA for 5 days at 25±2°C. Targeted macroscopic features include colony size, colony colour, reverse colony colour, texture and zonation while microscopic examination was undertaken using the scotch tape method, whereby mycelia and spores were taken from the edge of the colonies using a scotch tape and then stained with methylene blue on microscopic slides. The method used herein was a direct modification of that of Rodriguez-Tudela and Aviles (1991)and Harris (2000) and it resulted in a tentative identification of the various selected fungal isolates.
Statistical analysis
All spectrophotometric enzyme assaying data in this work are means of triplicate assays (n = 3) subjected to analysis of variance (ANOVA) (Super-Anova, Statsgraphics Version 7; Statsgraphics Corp., The Plains, VI, USA). Where ANOVA revealed significant differences between treatments, means were separated by the post hoc Student–Newman–Keuls (SNK) multiple range test (p < 0.05)
Screening of the fungal isolates with lignocellulolytic capacity
The Congo red assay as a detection method principally
depends on the formation of a complex between the reactive dye, Congo red and a polysaccharide such as cellulose or xylan. Microbes that secrete cellulases or xylanases will degrade the polysaccharide and a clear halo zone will then appear after addition of the dye and de-staining with NaCl on agar plates due to the disappearance of the concerned polysaccharide around the growing colony as shown in Figure 2. The Figure shows a representation of the determined cellulolytic and xylanolytic activities for the isolated and screened fungal isolates in this study while Table 1 below presents a detailed representation of all the fungal isolates isolated and screened in the study.
Biochemical characterization of the fungal isolates with lignocellulolytic capacity
After screening, all fungal isolates that could demonstrate both the cellulolytic and xylanolytic activities were selected and then further subjected to characterization biochemically. The process involved use of the spectrophotometric method, zymography analysis and enzyme kinetics of the lignocellulolytic extracts secreted by the obtained fungal isolates. Figure 3 shows the obtained spectrophotometric results while Figure 4 and Table 2 present the zymographic outcomes, then Figure 5 and Table 3 showing the enzyme kinetics results.
Morphological characterization of the fungal isolates with lignocellulolytic capacity
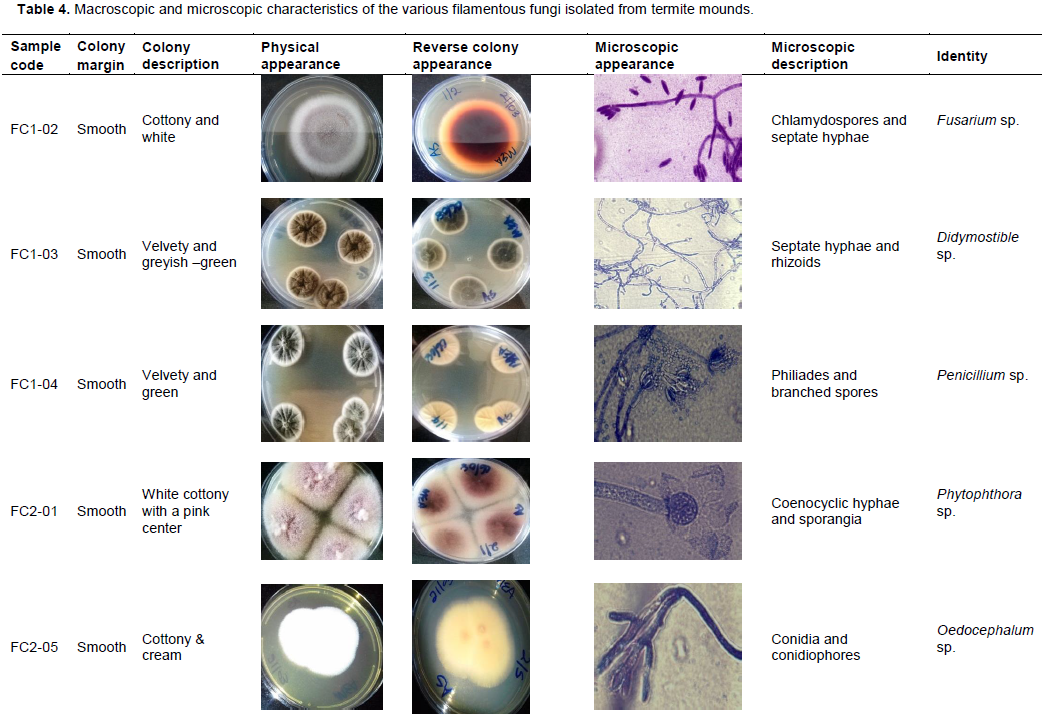
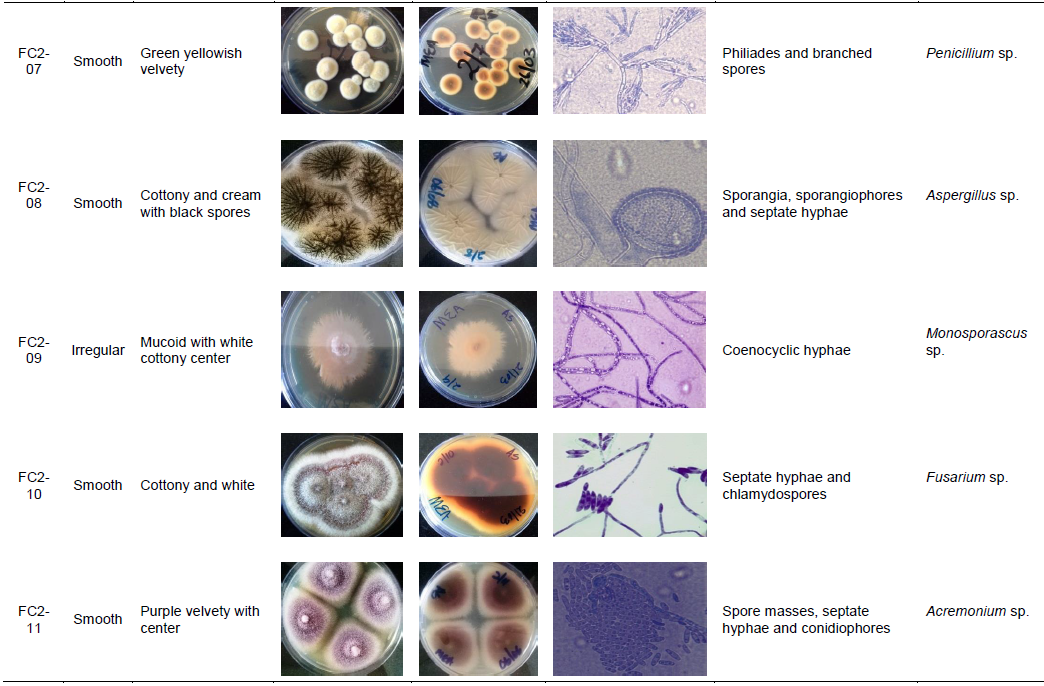
After biochemical characterization, the same set of fungal isolates that had demonstrated both the cellulolytic and xylanolytic activities were further subjected to morphological characterization, which literally involved the physical examination of fungal structures macroscopically and microscopically as described in Table 4 whilst they are still growing on the MEA. From this approach, identification of the fungal isolates into tentative and specific groups was technically achieved as indicated in Table 4.
Most studies nowadays have focused on cellulases and xylanases. This is due to a high demand of these enzymes in world-wide markets for food and beverage industries (Kuhad et al., 2011). Cellulases decompose cellulose, a recalcitrant polymer formed from D-glucose residues linked by β-1,4-glycosidic bonds. On the other hand, xylan, a complex polysaccharide consisting of a backbone of xylose residues linked by β-1,4-glycosidic bonds is degraded by xylanases. In food and beverage industries, cellulases are required for their ability to increase the yield of potato pulp macerations, guava juice clarifications and tomato juice filtrations (Karmakar and Ray, 2011). Xylanases, on the other hand, are remarkable in the baking industries, where they have a role in increasing the specific volume of bread (Beg et al., 2001; Polizeli et al., 2005)while in biscuit making, they are recommended for making cream crackers lighter (Harris and Ramalingam, 2010). These enzymes can release antioxidants from fruit and vegetable pomaces, improve starch and protein extraction yields, modify the viscosity of fruit purees, control the bitterness of citrus fruits, and having an essential role in the improvement of texture and quality of bakery products (Kuhad et al., 2011; Polizeli et al., 2005).
Frequently, microorganisms are used as the main source of industrial enzymes because they can be easily cultured in large quantities in a very short period of time. Moreover, microbial proteins are often more stable than those from other sources, such as animal or plant origins (Tasia and Melliawati, 2017). Fungi are well-known as agents of decomposition of organic matter in general and lignocellulosic substrate in particular (Jahangeer et al., 2005). Therefore, commercial enzymes that are on the market are often of fungal origin, including cellulases and xylanase (Polizeli et al., 2005). Fungal cellulases and xylanases can be produced in large amounts and often are less complex than their bacterial counterparts. This then makes it ideal to clone fungal genes and produce their corresponding enzymes through recombination methods for advanced up-scaling and subsequent downstream processing (Acharya and Chaudhary, 2012). Filamentous fungi from termite mounds have a very good potential in cellulase and xylanase production for industries since they are already adapted to their environ-ments of lignocellulosic composition (Robl et al., 2013;
Sunitha et al., 2013). They spend the entire or part of their life cycles colonizing dead plant material, essentially causing its sequential degradation and decomposition (Mattéotti et al., 2011; Ni and Tokuda, 2013). This aspect thus highlights how termite mound fungi are potential producers of enzymes for the food and feed industry (Bignell, 2010; Eggleton, 2010; Liu et al., 2011). The aim of this study therefore, was mainly to isolate cellulase- and xylanase-producing filamentous fungi from termite mounds and assess their potentials for use in food and beverage industries.
In that regard then, culture enrichment and repeated sub-culturing techniques were used to isolate the intended filamentous fungi from termite mounds, whereby both types of termite mounds were targeted as shown in Figure 1. These types mounds are commonly termed type 1 as indicated in Figure 1a and type 2 as indicated in Figure 1b termite mounds. From this process, a total of 17 isolates were obtained; 6 from the type 1 mounds and 11 from the type 2 mounds as presented in Table 1. This was then followed by a concerted screening of such isolates for lignocellulolytic capacity, using the agar plate- based clearing assay technique and specifically, the Congo red assay as indicated in Figure 2. The agar plate-based clearing assay technique has previously been used for the screening of cellulase- and xylanase-producing fungi and is notably reported as a suitable preliminary assay (Abdel-Sater and El-Said, 2001; Adesina and Onilude, 2013; Reddy et al., 2015). In this case, two aspects were essential; firstly, to test the ability of the isolates to grow on the provided substrates (CMC and xylan) and secondly, to check if such growing isolates could hydrolyze the substrates. As is presented in Table 1, out of the 6 isolates from the type 1 termite mounds, 3 (50%) could grow on CMC and xylan while out of the 11 isolates from the type 2 termite mounds, 7 (64%) could grow on both substrates. These outcomes therefore, notably indicated to us that filamentous fungi from termite mounds have a great potential to produce cellulases and xylanases for the subsequent degradation of cellulose and hemicellulose respectively. Sizes of the areas of substrate hydrolysis were observed to be in the range 4-13 mm for the cellulolytic activity as shown in Figure 2a and Table 1, and 1-12 mm for the xylanolytic activity as shown in Figure 2b and Table 1, something which is not very much different from what was previously exhibited by plant endophytes with a massive ability to degrade cellulose and hemicellulose that generally had diameters in the range of ≥20 mm (Tasia and Melliawati, 2017). Thus, the abilities of fungi to degrade cellulose and hemicellulose may be gained as a result of adaptation of these microbes to their habitats, which is a set of lignocellulosic materials (Bhagobaty and Joshi, 2012).
At this point and irrespective of whether an isolate could hydrolyze the provided substrates or not, all isolates (10) that had shown the capability to grow on CMC and/or xylan as shown in Table 1 were further assessed for their ability to secrete cellulases and xylanases through spectrophotometry (a more sensitive technique than the agar plate assay). This aspect whereby some fungal isolates can grow on CMC or xylan but failing to produce clearing zones on the media has commonly been observed in many studies (Adesina and Onilude, 2013)wherein the common practice would be to select only those isolates with the clearing zones and leaving behind those without (Reddy et al., 2015), which is inappropriate. Gradually, it then became clear that as a solid-based method, the Congo red assay has some inherent technical challenges that influence the formation of clearing zones on media and hence, such an aspect has to be taken into consideration each time one is dealing with this kind of an assay. For instance, besides the growth kinetics of isolates, the relative diffusion of enzymes in the media also depends upon several factors such as the percentage of the agar, the molecular weight size of the enzyme itself and the temperature used for growth (Adesina and Onilude, 2013; Sridevi and Charya, 2011). Therefore, in order to undertake the intended spectrophotometric assays in this study, the obtained 10 isolates were first grown on wheat bran followed by collection of their secreted extracellular enzyme extracts and the extracts then tested for both the cellulolytic and xylanolytic activity analysis. As is shown in Figure 3, all the isolates had positive results, indicating the ability of the termite mound filamentous fungi to secrete cellulases as described in Figure 3a and hemicellulases as described in Figure 3b that in turn accelerate the natural decomposition of lignocellulosic materials in termite nests.
Additionally, when the same secreted extracellular enzyme extracts were subjected to SDS-PAGE as shown in Figure 4a followed by analysis by zymography, it emerged that several protein fractions of various molecular weight sizes as presented in Table 2 were responsible for the degradation of CMC shown in Figure 4b and birchwood xylan shown in Figure 4c, demonstrating the ability of the tested fungal isolates to secrete various isoforms of cellulases and xylanases that then degrade cellulose and hemicellulose respectively. The molecular weight sizes of the various isoforms of cellulases and xylanases observed in this study ranged from 18-90 kDa for cellulases described in Figure 4b and Table 2 and 20-85 kDa for xylanases described in Figure 4c and Table 2, which is in good agreement with findings of other previous studies. For cellulases, molecular weight sizes of 25-50 kDa were reported in Trichoderma reesei and Phanerochaete (Nayebyazdi et al., 2012), 20-45 kDa in Aspergillus niger (Ncube et al., 2012), 25-100 kDa in endophytes (Elisashvili et al., 2015) and 30-250 kDa in most fungi (Kuhad et al., 2011; Li et al., 2011; Liming and Xueliang, 2004; Santa-Rosa et al., 2018; Yasmin et al., 2013; Ritter et al., 2013; Zhang and Zhang, 2013). For xylanases, molecular weight sizes of 20-50 kDa were reported in endophytes (Polizeli et al., 2005), 18-52 in Aspergillus aculeatus (Fujimoto et al., 1995), 29 kDa in Hypocrea lixii (Sakthiselvan et al., 2014), 19 kDa in Aspergillus fumigatus (Silva et al., 1999) and 45-70 kDa in Neocallimastix frontalis (de Segura and Fevre, 1993).
Finally, the secreted extracellular enzyme extracts were used to determine the relative cellulolytic and xylanolytic kinetic rates (Km and Vmax) of each of the obtained fungal isolate via the Hanes-Woolf plot method as indicated in Figure 5. This was done so as to relate the enzymatic activities of the obtained fungal isolates to other known and/or closely related microbial systems found in the termite gut. For the cellulases on one hand, Km values in the range of 0.125-2.011 mM and Vmax values in the range of 1,546.34-3,205.13 µmol/s were obtained as presented in Figure 5a and Table 3. These values are in close agreement with the kinetic rates of various recombinant cellulases previously expressed and purified from termite guts and termite gut symbionts, whose Km values ranged from 1.90-4.66 mM and Vmax values
ranging from 0.838-1,429.000 µmol/s as shown in Table 5. For the xylanases on the other hand, Km values in the range 0.248-4.302 mM and Vmax values in the range of 880.04-2,177.79 µmol/s were obtained as shown in Figure 5b and Table 3. Once again, these values are also in close agreement with the kinetic rates of various xylanases and recombinant xylanases previously purified and/or expressed and purified from termite gut bacteria, whose Km values ranged from 3.92-9.00 mM and Vmax values ranging from 256.0-7,575.0 µmol/s as described in Table 6.
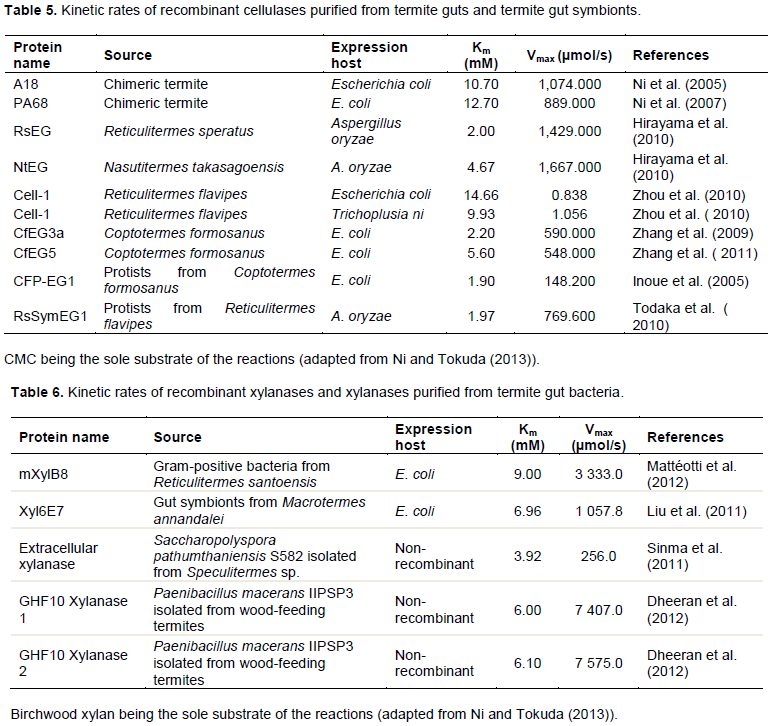
Apparently, when comparing the kinetic ratios (that is, Vmax/Km) of cellulases with those of xylanases within a single organism, it emerged from our work that the ratios of cellulases were always higher than those of xylanases for all the obtained fungal isolates as described in Figure 5 and Table 3. This scenario is not unusual because previously, a recombinant protein from Clostridium thermocellum, CtCel5E, that had a dual function as a cellulase and xylanase, displayed a Km value of 2.1 mM and a Vmax of 1 564 µmol/s for the cellulase and a Km value of 4.6 mM and a Vmax of 883.5 µmol/s for the xylanase (Yuan et al., 2015). Notably, all the kinetic values of CtCel5E together with most of those proteins in Tables 5 and 6 were generally lower than those of our own enzyme extracts probably due to three possible reasons. Firstly, most of the proteins, including CtCel5E were recombinant while our own extracts were not. Secondly, the source of some of the proteins, including CtCel5E was bacterial or prokaryotic whilst that of our own was fungal or eukaryotic, of which fungi are naturally known to be superior producers of lignocellulolytic enzymes (Favaro et al., 2013; Ramanjaneyulu et al., 2015). Lastly and in the event that substrate concentration was a limiting factor in the study, the cellulose content of most lignocellulosic substrates is always higher than that of hemicellulose, for example, rice straw, switch grass and sugarcane bagasse, all have a 35% cellulose and 25% hemicellulose content (Chen, 2014; Koshy and Nambisan, 2012; Shawky et al., 2011).
Overally, when the obtained 10 sets of the fungal isolates were further subjected to macroscopic and microscopic analysis whilst they were still growing on MEA, a total of eight distinct groups of filamentous fungi, capable of degrading cellulose and hemicellulose (Hyodo et al., 2003; Khokhar et al., 2012)were identified with their identities technically established as Fusarium, Didymostible, Penicillium, Phytophthora, Oedocephalum, Aspergillus, Monosporascus and Acremonium as shown in Table 4. In this regard, it was noted that termite mounds typically have a very high level of fungal diversity and such diversity not being only rich in terms of the number and types of filamentous fungi but also in terms of the number and nature of isoforms of the lignocellulolytic enzymes as described in Figure 4 and Table 2. Taken together, findings of this study conceivably establish that filamentous fungi from termite mounds are a good source of cellulases and xylanases that can be recommended for use in the food and beverage industries. However, before these enzymes are used in industrial systems, further detailed studies to improve their activities and/or performances need to be undertaken.
The authors have not declared any conflict of interests.
REFERENCES
|
Abdel-Sater M, El-Said A (2001). Xylan-decomposing fungi and xylanolytic activity in agricultural and industrial wastes. International Biodeterioration and Biodegradation 47(1):15-21.
Crossref
|
|
|
|
Acharya S, Chaudhary A (2012). Bioprospecting thermophiles for cellulase production: a review. Brazilian Journal of Microbiology 43(3):844-856.
Crossref
|
|
|
|
|
Adesina F, Onilude A (2013). Isolation, identification and screening of xylanase and glucanase-producing microfungi from degrading wood in Nigeria. African Journal of Agricultural Research 8(34):4414-4421.
Crossref
|
|
|
|
|
Beg Q, Kapoor M, Mahajan L, Hoondal G (2001). Microbial xylanases and their industrial applications: A review. Applied Microbiology and Biotechnology 56(34):326-338.
Crossref
|
|
|
|
|
Bhagobaty R, Joshi S (2012). Enzymatic activity of fungi endophytic on five medicinal plant species of the pristine sacred forests of Meghalaya, India. Biotechnology and Bioprocess Engineering 17(1):33-40.
Crossref
|
|
|
|
|
Bhat M (2000). Cellulases and related enzymes in biotechnology. Biotechnology Advances 18(5):355-383.
Crossref
|
|
|
|
|
Bignell DE (2010). Morphology, physiology, biochemistry and functional design of the termite gut: An evolutionary wonderland. In Biology of termites: A modern synthesis. Springer. pp. 375-412.
Crossref
|
|
|
|
|
Chen H (2014). Chemical composition and structure of natural lignocellulose. In Biotechnology of lignocellulose. Springer. pp. 25-71.
Crossref
|
|
|
|
|
de Segura BG, Fevre M (1993). Purification and characterization of two 1, 4-beta-xylan endohydrolases from the rumen fungus Neocallimastix frontalis. Applied Environmental Microbiology 59(11):3654-3660.
|
|
|
|
|
Dheeran P, Nandhagopal N, Kumar S, Jaiswal YK, Adhikari DK (2012). A novel thermostable xylanase of Paenibacillus macerans IIPSP3 isolated from the termite gut. Journal of Industrial Microbiology and Biotechnology 39:851-860.
Crossref
|
|
|
|
|
Eggleton P (2010). An introduction to termites: Biology, taxonomy and functional morphology. In Biology of termites: A modern synthesis. Springer, pp. 1-26.
Crossref
|
|
|
|
|
Elisashvili V, Kachlishvili E, Asatiani MD (2015). Shiitake medicinal mushroom, Lentinus edodes (higher basidiomycetes) productivity and lignocellulolytic enzyme profiles during wheat straw and tree leaf bio-conversion. International Journal of Medicinal Mushrooms 17:77-86.
Crossref
|
|
|
|
|
Favaro L, Jooste T, Basaglia M, Rose SH, Saayman M, Görgens JF, van Zyl WH (2013). Designing industrial yeasts for the consolidated bioprocessing of starchy biomass to ethanol. Bioengineered 4(2):97-102.
Crossref
|
|
|
|
|
Fujimoto H, Ooi T, Wang SL, Takizawa T, Hidaka H, Murao S, Arai M (1995). Purification and properties of three xylanases from Aspergillus aculeatus. Bioscience, Biotechnology, and Biochemistry 59(3):538-540.
Crossref
|
|
|
|
|
Harris AD, Ramalingam C (2010). Xylanases and its application in food industry: A rview. Journal of Experimental Sciences 25:77-85.
|
|
|
|
|
Harris JL (2000). Safe, low distortion tape touch method for fungal slide mounts. Journal of Clinical Microbiology 38(12):4683-4684.
|
|
|
|
|
Hirayama K, Watanabe H, Tokuda G, Kitamoto K, Arioka M (2010). Purification and characterization of termite endogenous β-1,4-endoglucanases produced in Aspergillus oryzae. Bioscience, Biotechnology, and Biochemistry 74:1680-1686.
Crossref
|
|
|
|
|
Hyodo F, Tayasu I, Inoue T, Azuma JI, Kudo T, Abe T (2003). Differential role of symbiotic fungi in lignin degradation and food provision for fungusâ€growing termites (Macrotermitinae: Isoptera). Functional Ecology 17(2):186-193.
Crossref
|
|
|
|
|
Inoue T, Moriya S, Ohkuma M, Kudo T (2005). Molecular cloning and characterization of a cellulase gene from a symbiotic protist of the lower termite, Coptotermes formosanus. Gene 349:67-75.
Crossref
|
|
|
|
|
Jahangeer S, Khan N, Jahangeer S, Sohail M, Shahzad S, Ahmad A, Khan SA (2005). Screening and characterization of fungal cellulases isolated from the native environmental source. Pakistan Journal of Botany 37(3):739.
|
|
|
|
|
Karmakar M, Ray R (2011). Current trends in research and application of microbial cellulases. Research Journal of Microbiology 6(1):41-53.
Crossref
|
|
|
|
|
Khokhar I, Haider MS, Mushtaq S, Mukhtar I (2012). Isolation and screening of highly cellulolytic filamentous fungi. Journal of Applied Sciences and Environmental Management 16(3).
Crossref
|
|
|
|
|
Koshy J, Nambisan P (2012). Pre-treatment of agricultural waste with Pleurotus sp. for ethanol production. International Journal of Plant, Animal, and Enviromental Sciences 2(2).
|
|
|
|
|
Kuhad RC, Gupta R, Singh A (2011). Microbial cellulases and their industrial applications. Enzyme Research 20:11-24.
Crossref
|
|
|
|
|
Kwezi L, Ruzvidzo O, Wheeler JI, Govender K, Iacuone S, Thompson PE, Irving HR (2011). The phytosulfokine (PSK) receptor is capable of guanylate cyclase activity and enabling cyclic GMP-dependent signaling in plants. Journal of Biological Chemistry 286(25):22580-22588.
Crossref
|
|
|
|
|
Laemmli UK (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680-685.
Crossref
|
|
|
|
|
Li DC, Li AN, Papageorgiou AC (2011). Cellulases from thermophilic fungi: recent insights and biotechnological potential. Enzyme Research 2011:1-9.
Crossref
|
|
|
|
|
Liming X, Xueliang S (2004). High-yield cellulase production by Trichoderma reesei ZU-02 on corn cob residue. Bioresource Technology 91(3):259-262.
Crossref
|
|
|
|
|
Limayem A, Ricke SC (2012). Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects. Progress in Energy and Combustion Science 38(4):449-467.
Crossref
|
|
|
|
|
Liu N, Yan X, Zhang M, Xie L, Wang Q, Huang Y, Zhou Z (2011). Microbiome of fungus-growing termites: A new reservoir for lignocellulase genes. Applied Environmental Microbiology 77(1):48-56.
Crossref
|
|
|
|
|
Makonde HM, Mwirichia R, Osiemo Z, Boga HI, Klenk HP (2015). Pyrosequencing-based assessment of bacterial diversity and community structure in termite guts, mounds and surrounding soils. SpringerPlus 4(1):471.
Crossref
|
|
|
|
|
Mandels M (1969). The production of cellulases. Advanced Chemistry 95:391-414.
Crossref
|
|
|
|
|
Mattéotti C, Bauwens J, Brasseur C, Tarayre C, Thonart P, Destain J (2012). Identification and characterization of a new xylanase from Gram-positive bacteria isolated from termite gut (Reticulitermes santonensis). Protein Expression and Purification 83:117-127.
Crossref
|
|
|
|
|
Mattéotti C, Haubruge E, Thonart P, Francis F, De Pauw E, Portetelle D, Vandenbol M (2011). Characterization of a new β-glucosidase/β-xylosidase from the gut microbiota of the termite (Reticulitermes santonensis). FEMS Microbiology Letters 314(2):147-157.
Crossref
|
|
|
|
|
Meier S, Ruzvidzo O, Morse M, Donaldson L, Kwezi L, Gehring C (2010). The Arabidopsis wall associated kinase-like 10 gene encodes a functional guanylyl cyclase and is co-expressed with pathogen defense related genes. Plos One 5(1):e8904.
Crossref
|
|
|
|
|
Mtui GY (2012). Lignocellulolytic enzymes from tropical fungi: Types, substrates and applications. Scientific Research and Essays 7(15):1544-1555.
Crossref
|
|
|
|
|
Nayebyazdi N, Salary M, Ghanbary MAT, Ghorbany M, Bahmanyar MA (2012). Investigation of cellulase activity in some soil borne fungi isolated from agricultural soils. Annals of Biological Research 3(12):5705-5713.
|
|
|
|
|
Ncube T, Howard RL, Abotsi EK, van Rensburg ELJ, Ncube I (2012). Jatropha curcas seed cake as substrate for production of xylanase and cellulase by Aspergillus niger FGSCA733 in solid-state fermentation. Industrial Crops and Products 37(1):118-123.
Crossref
|
|
|
|
|
Ni J, Takehara M, Watanabe H (2005). Heterologous over-expression of a mutant termite cellulase gene in Escherichia coli by DNA shuffling of four orthologous parental cDNAs. Bioscience, Biotechnology, and Biochemistry 69:1711-1720.
Crossref
|
|
|
|
|
Ni J, Tokuda G (2013). Lignocellulose-degrading enzymes from termites and their symbiotic microbiota. Biotechnology Advances 31(6):838-850.
Crossref
|
|
|
|
|
Ni J, Tokuda G, Takehara M, Watanabe H (2007). Heterologous expression and enzymatic characterization of beta-glucosidase from the drywood-eating termite, Neotermes koshunensis. Applied Entomology and Zoology 42:457-463.
Crossref
|
|
|
|
|
Ohkuma M (2003). Termite symbiotic systems: Efficient bio-recycling of lignocellulose. Applied Microbiology and Biotechnology 61(1):1-9.
Crossref
|
|
|
|
|
Pointing SB (1999). Qualitative methods for the determination of lignocellulolytic enzyme production by tropical fungi. Fungal Diverssity 2:17-33.
|
|
|
|
|
Polizeli M, Rizzatti A, Monti R, Terenzi H, Jorge J, Amorim D (2005). Xylanases from fungi: Properties and industrial applications. Applied Microbiology and Biotechnology 67(5):577-591.
Crossref
|
|
|
|
|
Ramanjaneyulu G, Reddy GPK, Kumar KD, Reddy BR (2015). Isolation and screening of xylanase producing fungi from forest soils. International Journal of Current Microbiology and Applied Sciences 4:586-591.
|
|
|
|
|
Reddy GPK, Narasimha G, Kumar KD, Ramanjaneyulu G, Ramya A, Kumari BS, Reddy BR (2015). Cellulase production by Aspergillus niger on different natural lignocellulosic substrates. International Journal of Current Microbiology and Applied Sciences 4(4):835-845.
|
|
|
|
|
Ritter CET, Camassola M, Zampieri D, Silveira MM, Dillon AJP (2013). Cellulase and xylanase production by Penicillium echinulatum in submerged media containing cellulose amended with sorbitol. Enzyme Research 2013:1-9.
Crossref
|
|
|
|
|
Robl D, da Silva Delabona P, Mergel CM, Rojas JD, dos Santos Costa P, Pimentel IC, Padilla G (2013). The capability of endophytic fungi for production of hemicellulases and related enzymes. BMC Biotechnology 13(1):94.
Crossref
|
|
|
|
|
Rodriguez-Tudela J, Aviles P (1991). Improved adhesive method for microscopic examination of fungi in culture. Journal of Clinical Microbiology 29(11):2604-2605.
|
|
|
|
|
Sakthiselvan P, Naveena B, Partha N (2014). Molecular characterization of a xylanase-producing fungus isolated from fouled soil. Brazilian Journal of Microbiology 45(4):1293-1302.
Crossref
|
|
|
|
|
Santa-Rosa PS, Souza AL, Roque RA, Andrade EV, Astolfi-Filho S, Mota AJ, Nunes-Silva CG (2018). Production of thermostable β-glucosidase and CMCase by Penicillium sp. LMI01 isolated from the Amazon region. Electronic Journal of Biotechnology 31:84-92.
Crossref
|
|
|
|
|
Sharma V, Sumbali G (2013). An overview of the symbiotic interaction between ants, fungi and other living organisms in ant-hill soils. International Journal of Environmental Sciences 4(3):432.
|
|
|
|
|
Shawky BT, Mahmoud MG, Ghazy EA, Asker MM, Ibrahim GS (2011). Enzymatic hydrolysis of rice straw and corn stalks for monosugars production. Journal of Genetic Engineering and Biotechnology 9(1):59-63.
Crossref
|
|
|
|
|
Silva CHC, Puls J, Sousa MV, Ferreira-Filho EX (1999) Purification and characterization of a low molecular weight xylanase from solid-state cultures of Aspergillus fumigatus Fresenius. Revista de Microbiologia 30:114-119.
Crossref
|
|
|
|
|
Sinma K, Khucharoenphaisan K, Kitpreechavanich V, Tokuyama S (2011). Purification and characterization of a thermostable xylanase from Saccharopolyspora pathumthaniensis S582 isolated from the gut of a termite. Bioscience, Biotechnology, Biochemistry 75:1957-1963.
Crossref
|
|
|
|
|
Sridevi B, Charya MS (2011). Isolation, identification and screening of potential cellulase-free xylanase producing fungi. African Journal of Biotechnology 10(22):4624-4630.
|
|
|
|
|
Subramaniyan S, Prema P (2002). Biotechnology of microbial xylanases: Enzymology, molecular biology, and application. Critical Reviews in Biotechnology 22(1):33-64.
Crossref
|
|
|
|
|
Sunitha V, Nirmala Devi D, Srinivas C (2013). Extracellular enzymatic activity of endophytic fungal strains isolated from medicinal plants. World Journal of Agricultural Sciences 9(1):1-9.
|
|
|
|
|
Taprab Y, Ohkuma M, Johjima T, Maeda Y, Moriya S, Inoue T, Kudo T (2002). Molecular phylogeny of symbiotic Basidiomycetes of fungus-growing termites in Thailand and their relationship with the host. Bioscience, Biotechnology, and Biochemistry 66(5):1159-1163.
Crossref
|
|
|
|
|
Tasia W, Melliawati R (2017). Cellulase and xylanase production from three isolates of indigenous endophytic fungi. Paper presented at the IOP Conference Series: Earth and Environmental Science, pp. 1-5.
|
|
|
|
|
Teather RM, Wood PJ (1982). Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Applied Environmental Microbiology 43(4):777-780.
|
|
|
|
|
Téllez-téllez M, Díaz R, Sánchez C, Díaz-godínez G (2013). Hydrolytic enzymes produced by Pleurotus species. African Journal of Microbial Research 7:276-281.
|
|
|
|
|
Todaka N, Lopez CM, Inoue T, Saita K, Maruyama J, Arioka M (2010). Heterologous expression and characterization of an endoglucanase from a symbiotic protist of the lower termite, Reticulitermes speratus. Applied Biochemistry and Biotechnology 160:1168-1178.
Crossref
|
|
|
|
|
Yasmin S, Mattoo RL, Nehvi FA (2013). Isolation, characterization and molecular weight determination of cellulase from Trichoderma viride. African Journal of Biotechnology 12(28):4512-4518.
Crossref
|
|
|
|
|
Yuan SF, Wu TH, Lee HL, Hsieh HY, Lin WL, Yang B, Huang CH (2015). Biochemical characterization and structural analysis of a bifunctional cellulase/xylanase from Clostridium thermocellum. Journal of Biological Chemistry 290(9):5739-5748.
Crossref
|
|
|
|
|
Zhang DH, Lax AR, Bland JM, Allen AB (2011). Characterization of a new endogenous endo-β-1,4-glucanase of Formosan subterranean termite (Coptotermes formosanus). Insect Biochemistry and Molecular Biology 41:211-218.
Crossref
|
|
|
|
|
Zhang DH, Lax AR, Raina AK, Bland JM (2009). Differential cellulolytic activity of native-form and C-terminal tagged-form cellulases derived from Coptotermes formosanus and expressed in E. coli. Insect Biochemistry and Molecular Biology 39:516-522.
Crossref
|
|
|
|
|
Zhang XZ, Zhang YHP (2013). Cellulases: Characteristics, sources, production, and applications. Bioprocessing Technology in Biorefinery for Sustainable Production of Fuels and Chemical Polymers 8:131-146.
Crossref
|
|
|
|
|
Zhou X, Kovaleva ES,Wu-Scharf D, Campbell JH, Buchman GW, Boucias DG (2010). Production and characterization of a recombinant β-1,4-endoglucanase (glycohydrolase family 9) from the termite Reticulitermes flavipes. Archives of Insect Biochemistry and Physiology 74:147-162.
Crossref
|
|