Full Length Research Paper
ABSTRACT
Vegetable production is an important economic activity in Cameroon. Due to poor health status of the seeds, the yield is decreasing. Sources, preservation, and quality status of some locally cultivated vegetable seeds was investigated. Seeds were obtained from local farmers, agro-shops, and local markets in four villages of Bamessing, Bamali, Bamunka and Bambalang. The seed sources and preservation methods were recorded on the field using questionnaire and verbal interview; and the quality status of the seeds was determined in the laboratory. Fungal and bacterial identification were done using Potato Dextrose Agar and Nutrient Agar, respectively. Blotter and germination methods were done to ascertain the viability of the seeds. 48% of the respondents indicated they obtained seeds from local farmers, 37% from local market and 15% from agro shops. Ocimum gratissimum was highly used by farmers in all villages to preserve seeds. The fungi species identified included Saccharomyces cerevisiae, Aspergillus niger, Fusarium oxysporum, Penicillium digitatum and Rhizopus nigricans and the bacteria identified included both short, long rods bacilli with some coccobacilli. The blotter method gave 87.78 and 56.67% germination in Brassica rapa and Solanum nigrum, respectively with B. rapa being the first to germinate with the least number of days.
Key words: Bacteria, fungi, locally cultivated vegetables seeds, sources, quality.
INTRODUCTION
Seeds are small embryonic plants enclosed in a covering called the seed coat, usually with some stored food. The health status of a seed refers primarily to the presence or absence of disease-causing organisms such as fungi, bacteria, and viruses. Some animal pests such as nematodes and insects, and physiological conditions such as trace elements deficiency may also affect the health status of seeds (PROTA, 2004). Whether seeds are stored for sale or sown for crop production, information about their quality status is very important. Several authors have examined the importance of locally cultivated vegetable seeds of Africa as valuable sources of food, income and traditional medicine (Mihretu, 2019). Many locally cultivated leafy vegetables are collected in their natural growing habitats as wild species (Schippers, 2000). Out of the 150 food-plants commonly consumed by man, 115 are locally cultivated African species (Kiambi and Atta-Krah, 2003).
Cameroon is endowed with enormous agricultural potentials, which contribute significantly to poverty reduction and sustainable development. About 75% of the active population of Cameroon is involved in agricultural production which accounts for about 50% of the total exports (MINEFI, 2002). Thus, the health of these locally cultivated vegetable seeds must be taken into consideration when working on crop production. Also, a very nutritive herbaceous leafy vegetable called Cleome gynandra is locally cultivated in many parts of sub-Sahara Africa (Wasonga et al., 2015; Zharare, 2012); and it is mainly cultivated by subsistence farmers or semi-domesticated (Muasya et al., 2009). Quality seed is a key element for a successful crop production (Kameswara et al., 2017). Seed quality is determined by several internal and external factors that influence seed development and maturation. Seed samples of most African locally cultivated vegetables collected from farmer’s stores and other sources had a germination percentage in the range of 15 to 92% (Abukutsa-Onyango, 2003). Both fungal and bacterial growth affects both the seeds and the vegetable plant itself, including its roots and leaves. Some of these pathogens affecting locally cultivated leafy vegetables in Ndop are Fusarium, Phytophthora, Pythium, powdery mildew, leaf spots of cabbage, gray mold and Rhizoctonia. Ralstonia solanacearum is a bacterial pathogen-causing wilt in which the plant wilts and dies. Choanephora (a fungal disease), causes white rust, which appears like blisters on the leaves. The disease Anthracnose affects eggplant in Ndop (Khoo et al., 2022).
The seed borne disease can affect the next generation of seeds if care is not taken. They affect the quality status of seeds in different ways like seed rots, seedling decay, discoloration, reduced size of seeds and twisting of seeds (Berinnyuy and Fontem, 2011). Fungi are considered as the most important pathogens for seeds (Baskin and Baskin, 2001), thus reducing their survival and germination rate is very important (Schafer and Kotanen, 2004). The effects of infective fungi on seeds and seedlings include poor germination, low seedling vigour and even complete failure of seedling establishment, leading to low yield quantity and quality (Dykstra and Braumandl, 2006). Most researchers concentrate on the medicinal values of seeds, the food properties, and seed dormancy. However, based on available literature, very little or no work has been done on seed sources, preservation, and quality status of locally cultivated vegetable seeds and Ndop Central, North-West Region of Cameroon is not an exception. In this respect, this study was set to determine the sources of seeds, their modes of preservation and the quality status of seeds in Ndop Central, North-West Region of Cameroon.
MATERIALS AND METHODS
Study area
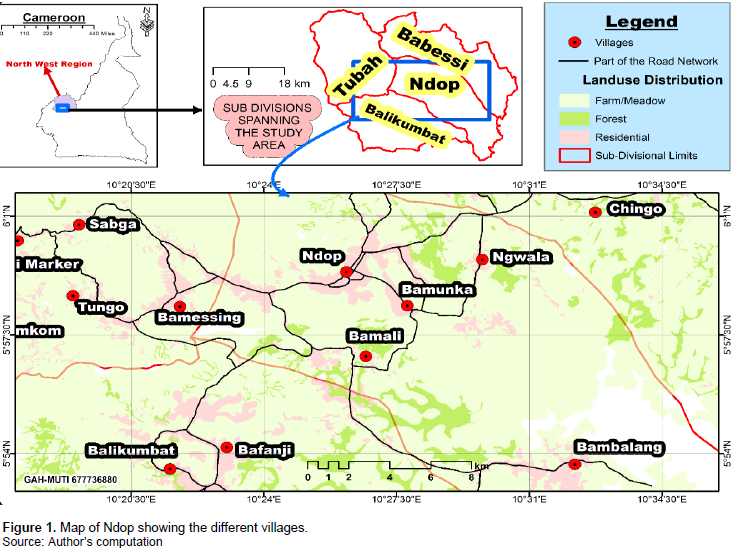
Ndop is situated in the Northwest Region of Cameroon, precisely in the Ngoketunjia Division between latitudes 05°15′ to 06°11′N and longitude 10°15′ to 10°50′E. It is located between the Bamenda Mountains and the Oku Massif part of the Cameroon volcanic line and bordered to the West by the Eastern escarpment (Sabga) and is characterized by colluvial and alluvial flood plains (1150 to 1200 masl). It is about 45 km from the regional capital of Northwest of Cameroon, Bamenda. It is bounded to west by Mezam Division, to the North by Babessi Subdivision, to the south by Balikumbat and to the east by River Noun and part of the West Region (Lambi, 1999). Ndop Central Subdivision has a total surface area of 1126 km2 with a total population of about 104,361 inhabitants as of 2017 (Council Development Plan). This population is unevenly distributed in the four villages (Bamessing, Bamali, Bamunka and Bambalang) consisting of 71 quarters. The map of the study area is as shown in Figure 1.
Questionnaire administration
Socio economic surveys were carried out in the study area to identify the different seed sources, and preservation methods. The different seed sources and preservation methods used by the different stakeholders (farmers, agricultural shop owners) were studied. In the study area, a total of 240 semi-structured, open- and closed-ended questionnaires were administered to inhabitants of the four villages that made up the Ndop Central Subdivision (Bamessing, Bamali, Bamunka and Bamabalang). Before administering questionnaires and conducting verbal interviews, the consent of the participants was obtained. Sixty questionnaires were randomly distributed to locally cultivated vegetable seeds dealers (farmers, seed sellers in local markets and agro shop owners) in each village. Each questionnaire was made up of 47 questions. These questions were grouped in three sections A, B and C. Section A comprised of demographic information, section B comprised of seed sources and section C focused on preservation methods. These questionnaires were distributed at home, on the farmlands and even in the markets where the farmers were found. There were verbal interviews, as well, on the field even in the market during seed collection for better understanding of seed sources and preservation methods. Of the 47 questions per questionnaire, 36 were used for evaluation concerning the seed sources and preservation methods; while 11 were used to obtain demographic information like the gender of the seed dealer, their age, marital status, highest educational qualification, occupation, family size, length of time that the farmer has been dealing with vegetable seeds among other questions. Field observations were complementary to the questionnaire and pictures were taken in almost all the stages.
Seed sources
Different locally cultivated vegetable seeds were collected on the field from different sources (farmers at home, neighbours, agro-shops, local markets) in the four different villages. Each stakeholder gave only the quantity they could afford (records on the quantity was not noted) except in Agro shops where about 5 g per seed type was purchased. Different homes per village were visited. Information was obtained during discussion with the farmers, from whom the seeds were collected, concerning their seeds sources; especially during questionnaire administration. Some seeds were collected from the Bamali market (Metarh Panneh) and some from Ndop main market, which are held daily. Then some of the seeds like those of Abelmochus esculentus and Solanum nigrum were purchased from agricultural seed stores in Ndop town (Bamunka).
Seed preservation
With seed preservation, farmers were asked whether they preserve vegetable seeds or not. If they preserve, why do they preserve vegetable seeds? Is it to avoid contamination by pathogens? to ensure high viability? Or to ensure seed diversity, etc. Farmers were asked to list the different ways they used to preserve seeds. They were also asked which plant species they use for preservation, and which one is best for preserving the locally cultivated vegetable seeds.
Identification of different pathogens that affect the seed quality
An agar plate method, using Nutrient Agar (NA), was used for bacteria identification and Potato Dextrose Agar (PDA) for fungi identification.
Bacteria identification
To begin with bacterial identification, the NA was prepared according to an agar dilution method of Sharma and Trivedi (2002); and allowed to cool and solidify before the different vegetable seeds were plated into them. They were then transferred to an incubator where they were incubated at a temperature of 35.5°C for 24 h with alternating period of 12 h, with daylight and 12 h of darkness, for bacterial identification.
Fungi isolation and identification
For fungi identification, PDA medium was prepared where 9.8 g of PDA was weighed on a sensitive electronic scale balance and then poured into a conical flask containing 200 mL of distilled water according to the method of Sharma and Trivedi (2002).
The seeds were surface sterilized by immersing in 1% sodium hypochlorite (NaClOH) for 3 min, then in 70% alcohol for a minute and then rinsed in three changes of sterile distilled water for a minute each. The different seeds depending on their sizes were platted in different numbers in the Petri-dishes with the prepared PDA. After plating, the Petri-dishes were sealed with paraffin and incubated at 25°C for 7 days which is in line with Leslie and Summerell (2006).
After 7 days of incubation, wet mounts were made from the growth at the margins. Methylene blue was used in staining the slide and a cover slide was used to cover it before it was mounted and observed under the microscope with an eyepiece lens of 10x and objective lens of 40x magnifications. Upon observation, pictures of each different fungus were taken and used for identification. This identification process was followed by a sub-culturing process, which was aimed at getting pure cultures. Identification was according to Leslie and Summerell (2006).
Determination of viability of locally cultivated vegetable seeds in Ndop
Two methods (blotter method and germination method) were used and the procedure was according to International Rules for Seed Testing Association (ISTA, 2001).
Data analysis
Data obtained from the questionnaires was analyzed using descriptive statistics in Microsoft Excel 2019. Data collected on the seed sources and preservation methods was represented in tables and as bar charts.
RESULTS AND DISCUSSION
Seed sources and preservation
During this survey, it was observed that most of the seeds of locally cultivated vegetables in Ndop came from local growers, agricultural seed stores and local markets. Figure 2 demonstrates that 48% of the respondents indicated that the seeds they use came from local farmers and this represented the highest percentage in this study. On the other hand, the least source of seed was from the agro shops as only 15% of the respondents indicated agro shops as their source of seeds.
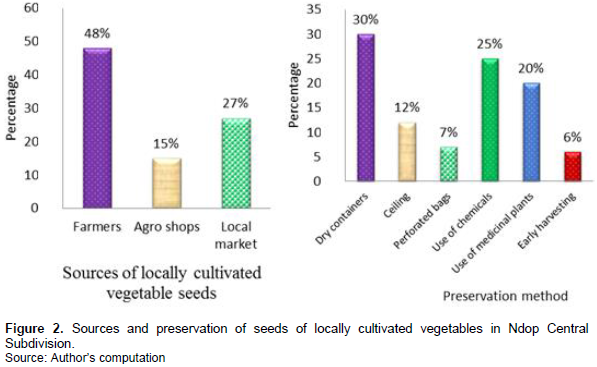
The high percentage (48%) was because the population of Ndop finds it easy to get seeds from the local farmers. This is in line with the results of Debruyne et al. (2018) who found out the various sources of seeds used by the farmers include agricultural shops that retail seeds to the farmers and farmers who keep their seeds for the next growing season.
It was also realized that most of the farmers in all the villages preserve their seeds in dry containers like calabashes, clay pots, bottles; which are the most preferred storage methods as indicated by 30% of the respondents, or in dry porous and well aerated bags, where they store seeds like okra, pumpkin, and okongobong. In some cases, they hang the bag containing the seeds of especially S. nigrum, Amaranthus tricolor, cowpea and Chinese cabbage on the ceiling in their external kitchen.
The second means of preservation was the use of chemicals as indicated by 25% of the famers. For example, chemicals like cypercot (some farmers even showed some of the remaining chemicals in a container) that they used in seed preservation. Fungicide like mancozeb 800 g/kg and wood ash were also mentioned as other chemicals used in preserving locally cultivated vegetable seeds in Ndop Central. Still as part of preservation, many farmers at home said hey preserved their seeds by preventing rats and cockroaches from defecating on them, or just running across, the exposed seeds. They reported that once a rat jumps across, or over, the seeds (especially the seeds of huckleberry) will not geminate. When asked how they managed to solve this problem; they said to avoid the problem, the farmer should be the first to jump over the seeds while saying “As I jump over this my seeds, any rat jumping over it after me will have no effect on the seeds”.
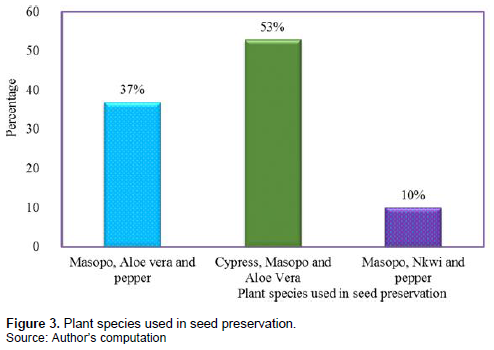
Concerning the use of medicinal plants in seed preservation in Ndop, the following plants were listed to be used in seed preservation by 20% of the farmers: Ocimum gratissimum, Aloe vera, Cupressus species, Capsicum annum (pepper) and Triumfetta annua (“Nkwi”). From these, O. gratissimum, A. vera and Cupressus spp., were highly used to preserve seeds of locally cultivated vegetable seeds as indicated by 53% of the farmers; and the least used were T. annua and C. annum as indicated by just 10% of the farmers (Figure 3). The farmers mentioned the fact that if the seeds are not well preserved, they will be destroyed by pests like weevil, cockroaches and white ants, which will lead to low viability; and as a result, poor yield will be realized from the farm. Some said there will also be low seed diversity if there is no proper preservation.
Bacterial identification
Gram positive short Bacillus rods and coccobacillus were identified on S. nigrum from farmers at home. Gram positive short bacillus rods was identified on A. esculentus from farmers at home, Gram positive short bacillus rods and coccobacillus were identified on the seeds of Cucurbita moschata, Gram positive short bacillus rods were identified on the seeds of Vernonia hymenolepis. Gram positive coccobacillus was identified on the seeds of Phaseolus spp., and Gram-positive short bacillus rods were identified on the seeds of Telfairia occidentalis (Table 1).
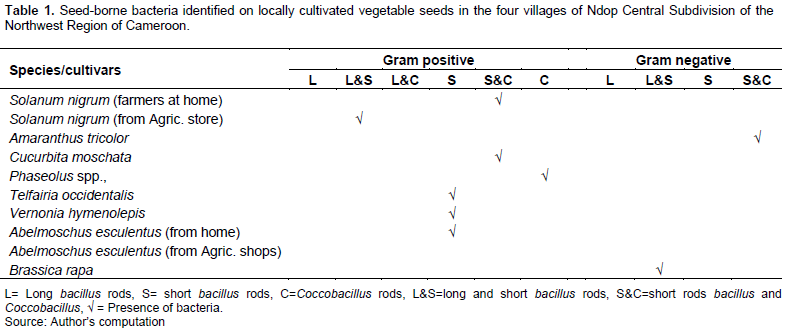
These results are in accordance with the work of Leff and Fierer (2013) who found various bacteria communities associated with surfaces of fresh fruits and vegetables. It was realized that only the seeds of A. tricolor and Brassica rapa contain Gram negative bacteria; whereas A. tricolor contained Gram negative short bacillus rods and coccobacilli and B. rapa had Gram negative long and short bacillus rods.
Fungi
Fungi were observed after incubation for seven days in the laboratory, all the seeds showed fungal growth although the seeds of okra from agricultural shops showed very little trace of fungi on them. The seeds with the least growth of fungi after A. esculentus from agricultural shops were the seeds of S. nigrum from agricultural shops. This was followed by the seeds of A. tricolor, which equally showed a small growth of fungi. Cowpea and T. occidentalis showed the highest growth of fungi which appeared dark in colour and highly concentrated. Finally, other seeds like those of pumpkin,
Chinese cabbage and those of sweet bitter leaf were molded, and appeared whitish or slightly yellow in colour. Fusarium spp., S. cerevisiae, was identified on the seeds of A. tricolor (Table 2).
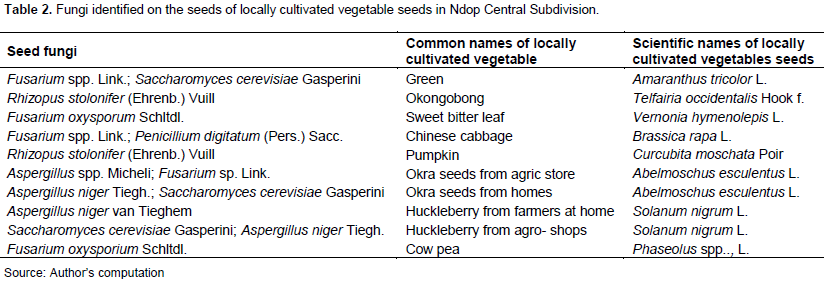
These results are in line with the work of Hamim et al. (2014) who carried out test on seed borne infection and germination of seeds of seven vegetables viz. amaranth, red amaranth, spinach, okra, cucumber, tomato and eggplant. Eleven fungi species were detected: Alternaria species, Aspergillus flavus, Aspergillus niger, Phomopsis vexans, Curvularia species, Fusarium spp., Penicillium species, Rhizopus species, Colletotrichum dematium, Macrophomina phaseolina and Cladosporium species. Six fungi were detected in amaranth, six fungi in red amaranth, four fungi in spinach, six fungi in okra, four fungi in cucumber, four fungi in tomato and five fungi in eggplant seeds. On Solanum nigrum from agricultural shops, S. cerevisiae and A. niger were identified. This concurs with the work of Tsopmbeng and Fomengia (2015), who carried out research on fungi associated with seeds of Huckleberry (S. nigrum) grown in the Western Highlands of Cameroon. Fusarium oxysporum was also identified on cowpea seeds. This result is in line with the work of Assunção et al. (2003) who carried out an experiment in field microplots to evaluate the influence of Fusarium wilt intensity on cowpea yield losses.
Pumpkin seeds had mold fungus on them. This result is similar to that of Weidenbörner (2001) who carried out 75 surface disinfected and 75 non-disinfected Austrian pumpkin seeds. A. esculentus seeds from agricultural shops contained Aspergillus and Fusarium spp. This result matches with the work of Kibria et al. (2015) who carried out work on the management of seed-borne fungal pathogens of okra collected from seed companies in India. A. esculentus collected from homes contain A. niger. This result is consistent with the work of Jalander and Gachande (2012) who found out that culture filtrates of A. niger caused reduction in seed germination and root and shoot elongation, when the filtrate of A. niger was found to be inhibitory.
Seed viability
For the blotter method, B. rapa showed the highest germination percent of 87.78% and the 2nd and 3rd were S. nigrum from farmers at home and S. nigrum from agricultural shops while the least germination was observed for T. occidentalis with 0% germination. For the germination method, the highest percentage of germination was recorded from A. esculentus from agricultural shops (77.78%) followed by Phaseolus species, (75.55%); and least percentage germination was recorded from Amaranthus tricolor (Table 3).
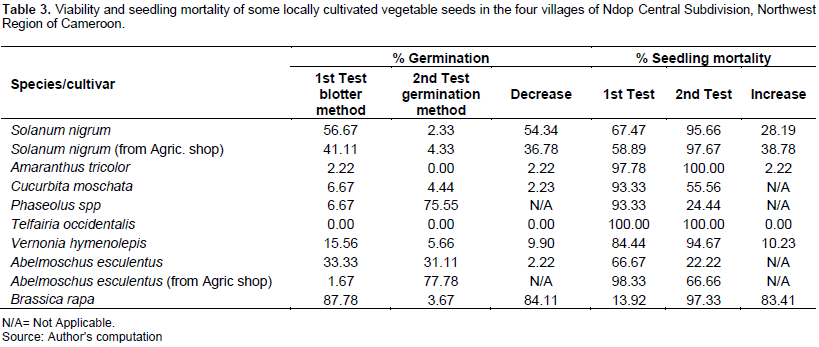
It can therefore be seen that the viability of the various locally cultivated vegetable seeds was very low as seed deterioration can also arise from poor storage conditions. This result is comparable with the work of Hamim et al. (2014). The highest total seed borne fungal infection was found in okra (26.75%), while the lowest was found in cucumber (13.50%). The highest percent germination was recorded in cucumber (87%), while the lowest was in okra (49.5%). Maximum germination failure was recorded in okra (50.5%) and the lowest was recorded in cucumber (13%). Maximum normal seedlings were recorded in cucumber (77%) and the minimum was in okra (45%). The highest number of abnormal seedlings was found in okra (4%) and lowest in eggplant (2%). The highest number of diseased seedlings was found in okra (9%) and lowest in cucumber (2%). Maximum numbers of dead seeds were found in red amaranth (48%), while lowest in cucumber (18%).
CONCLUSION
The bacterial and fungal species identified in this study could be regarded as the causative agents of the diseases affecting locally cultivated vegetable seeds in the Ndop Central Subdivision. Results obtained indicate the need for an emerging suitable management strategy to control and eradicate seed borne diseases caused by bacteria and fungi. The high nature of the values of percentage of seed mortality of the locally cultivated vegetable seeds is a call for concern regarding the sources of seeds and the preservation methods. The blotter and germination methods gave a low viability of locally cultivated vegetable seeds. Hence, there is need for seed quality status check and handling before planting.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGEMENT
The authors are thankful to the Life Sciences Laboratory, Faculty of Science in the University of Buea, where the laboratory work was done.
REFERENCES
|
Abukutsa-Onyango MO (2003). Diversity of cultivated African Leafy vegetables in three communities in Western Kenya. African Journal of Food, Agriculture, Nutrition and Development 7(3)1-13. |
|
|
Assunção IP, Michereff SJ, Mizubuti ESG, Brommonschenkel SH (2003). Influence of Fusarium Wilt Intensity on cow pea yield. Fitopatologia Brasileira 28:615-619. |
|
|
Baskin CC, Baskin JM (2001). Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination. Academic Press, San Diego, p. 666. |
|
|
Berinnyuy JE, Fontem DA (2011). Evaluating post-harvest opportunities and constraints to utilization and market of African vegetables Cameroon. African Journal of Food, Agriculture, Nutrition and Development 11(2)1-17. |
|
|
Debruyne S, Ruiz-González A, Artiles-Ortega E, Ampe B, Van Den Broeck W, De Keyser E, Vandaele L, Goossens K, Fievez V (2018). Supplementing goats with coconut medium chain fatty acids in early life influences growth and rumen papillae development until 4 months after supplementation but effects on in vitro methane emissions and the rumen microbiota are transient. Journal of Animal Sciences 96(5):1978-1995. |
|
|
Dykstra PR, Braumandl TF (2006). Historic influence of the mountain pine beetle on stand dynamics in Canadas rocky mountain parks. Mountain Pine Beetle Initiative Working Paper Pacific Forestry Centre 89 pp. |
|
|
Hamim I, Mohanto DC, Sarker MA, Ali MA (2014). Effect of seed borne pathogens on germination of some vegetable seeds. Journal of Phytopathology and Pest management 1(1):34-51. |
|
|
International Rules for Seed Testing Association (ISTA) (2001). International Rules for Seed Testing. Rules Amendments. Seed Science and Technology 29:1-127. |
|
|
Jalander V, Gachande BD (2012). Effects of aqueous leaf extracts of Datura sp against two plant pathogenic fungi. International Journal of Food Agriculture and Veterinary Sciences 2(3):131-134. |
|
|
Kameswara RN, Dulloo ME, Engels JMM (2017). A review of factors that influence the production of quality seed for long-term conservation in genebanks. Genetic Resources and Crop Evolution 64:1061-1074. |
|
|
Khoo YW, Tan HT, Khaw YS, Li S, Chong KP (2022). First report of anthracnose on purple dream Solanum melongena in Malaysia caused by Colletotrichum siamense. Plant Disease. |
|
|
Kiambi DK, Atta-Krah K (2003). Plant genetic resources in the global and African setting. Proceedings of, The First Plant Resources of Tropical Africa (PROTA) International Workshop, 23rd to 25th September 2002, Nairobi, Kenya. University of Wageningen. |
|
|
Kibria HGM, Ahsan SM, Ahmed T (2015). Management of seed borne fungal pathogens of okra collected from seed companies. Asian Journal of Medical and Biological Research 1 (3):628-640. |
|
|
Lambi CM (1999). The Bamendjin Dam of the Upper Noun Valley of Cameroon, No Human Paradise Environmental Education Project, University of Strathclyde, Glasgow, Scotland, p. 67. |
|
|
Leff JW, Fierer N (2013). Bacterial communities associated with the surfaces of fresh fruits and vegetables. PloS One 8(3):e59310. |
|
|
Leslie JF, Summerell BA (2006). Fusarium laboratory workshops-A recent history. Mycotoxin Research 22(2):73-74. |
|
|
Mihretu FB (2019). Challenges and opportunities of vegetable quality seed production and seed system in Ethiopia. International Journal of Research Studies in Agricultural Sciences 5(8):15-25. |
|
|
MINEFI (2002). Annual report from the Ministry of the Economy, Finance and Industry describes the action and results of the 25 departments and services of Minéfi, in central administration as well as at decentralized or international level. |
|
|
Muasya AM, David AS, Erik FS (2009). Isolepis tenella, a New Combination in Cyperaceae. Journal for Botanical Nomenclature 16(1): 89-90. |
|
|
PROTA (2004). Plant Resources of Tropical Africa: Vegetables. Grubben GJH, Denton OA (Editors). PROTA Foundation, Netherlands/Backhuys Publishers, Leiden, Netherlands/CTA Wageningen, Netherlands, 667 p. |
|
|
Schafer M, Kotanen PM (2004). Impacts of naturally occurring soil fungi on seeds of Meadow plants. Plant Ecology 175:19-35. |
|
|
Schippers RR (2000). African Indigenous Vegetables. An Overview of the Cultivated Species. Natural Resources Institute/ACP-EU Technical Centre for Agricultural and Rural Cooperation, Chatham, 214p. |
|
|
Sharma N, Trivedi PC (2002). Screening of leaf extracts of some plants for their nematicidal and fungicidal properties against Meloidogyn incognita and Fusarium oxysporium. Asian Journal Experimental Sciences 16: 21-28. |
|
|
Tsopmbeng NG, Fomengia DN (2015). Fungi associated with seeds of huckleberry (Solanum scabrum Mill) grown in the Western Highlands of Cameroon. Journal of Agriculture and Technology 11(3):791-801. |
|
|
Wasonga DO, Ambuko JL, Cheminingwa GN, Odeny DA, Crampton BG (2015). Morphological Characterization and Selection of Spider Plant (Cleome gynandra). Accessions from Kenya and South Africa. Asian Journal of Agricultural Sciences 7(4):36-44. |
|
|
Weidenbörner M (2001). Pumpkin seeds- the mycobiota and potential mycotoxins. European Food Research and Technology 212(3):279-281. |
|
|
Zharare GE (2012). Differential requirements for breaking seed dormancy in biotypes of Cleome gynandra and two Amaranthus species. African Journal of Agricultural Research 7(36):5049-5059. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0