ABSTRACT
A galactose-specific lectin was purified from the skin mucus of African catfish (Clarias gariepinus) and the physicochemical properties determined. Phosphate buffered saline extract of the skin mucus of African catfish specifically agglutinated erythrocytes of rabbit and human blood group B, but did not agglutinate bat, rat, hen and human blood A and O erythrocytes. The haemagglutinating activity of the lectin was completely inhibited by lactose while galactose and melibiose inhibited the activity to some extent and was calcium-independent. The purified lectin has a native and subunit molecular weight of 63, 000 and 20, 000 Daltons, respectively suggesting a homotrimeric structure for the protein. The protein contained 126 amino acid residues per subunit. This was characterized by large amount of polar amino acids that constituted about 60% of the total amino acids. The lectin showed maximum activity over the pH range 6 – 9 and was heat stable up to 50°C. Ethylenediaminetetraacetic acid (EDTA) had no inhibitory effect on its haemagglutinating activity. Periodic acid–Schiff (PAS) staining showed that the lectin was not a glycoprotein. Chemical modifications of serine and arginine residues of the protein did not affect its haemagglutination activity while modifications of cysteine, tryptophan and histidine residues led to total loss of its activity. The study concluded that African catfish skin mucus lectin exhibited similar physicochemical properties with lectins from other fish skin mucus.
Key words: African catfish, Lectin, haemagglutination, skin mucus, galactose-specific, Clarias gariepinus
Lectins are heterogeneous class of proteins that bind specifically and reversibly to carbohydrates (Lis and Sharon, 1998). They are widely distributed in nature and can be found in almost all living organisms including bacteria, fungi, plants, invertebrates and vertebrates and may be either soluble or membrane-bound (Lis and Sharon, 1986; Drickamer, 1995; Khan and Khan, 2011). However, majority of the studies on lectin have been carried out on plant lectins, particularly on the seeds of legume species (Sharon and Lis, 1990; Chrispeels and Raikhel, 1991). They have attracted immense interest because of the various biological activities such as cell agglutination, antiviral, antitumor, antiproliferative, antifungal and immunomodulatory (Wang et al., 1996; Fang et al., 2010; Melo et al., 2011; Souza et al., 2011). They have become important class of proteins with a wide variety of biochemical uses which include their role in bioseparation and reversible immobilization.
Since the discovery of animal lectins, most animal lectins studied occur inside the body like in the plasma, cell cytoplasm or at the cell surface (Tasumi et al., 2004). However, some lectins outside the body have also been studied especially in fish. A large numbers of researchers have intensively purified lectins from skin mucus of various fish species (Alexander and Ingram, 1992; Suzuki et al., 2003; Tsutsui et al., 2007). Skin mucus lectins of several species of fish have been shown to bind microorganisms (Tasumi et al., 2002; 2004: Tsutsui et al., 2006, 2007). These findings suggest that lectins contribute to the self-defense mechanism against these microorganisms and also support the report that fish skin secretions contain immunoglogulins as well as various innate-defense factors such as complements, C-reactive protein, lysozyme, hemolysin, anti-microbial peptides and lectins (Suzuki et al., 2003; Argayosa et al., 2011; Benhamed et al., 2014).
African catfish (Clarias gariepinus) is essentially an omnivorous bottom feeder; however they are known to be very tolerant of extreme environmental conditions. To fight off pathogenic microorganisms, the epidermis and its secretion, the mucus acts as a barrier between the fish and the environment (Benhamed et al., 2014). The composition and rate of mucus secretion has been observed to change in response to microbial exposure or to environmental perturbations such as hyperosmolarity and acidity (Ellis, 2001; Subramanian et al., 2008). Consequently, study of the physical and biological properties of the lectin in the skin mucus of African catfish (C. gariepinus) would give insight to the roles of this molecule in host defense and in the known adaptability of the catfishes to diverse environmental conditions. Hence, this study reports the purification of a galactose-specific lectin from the skin mucus of the African catfish C. gariepinus and physicochemical properties of this lectin.
African catfish (C. gariepinus Burchell, 1822) was obtained from the Fishery Unit, Osin Farm Ltd, Yakoyo, via Ile Ife, Osun State, Nigeria. Fresh human blood was obtained from healthy donors and animal blood from rabbits, hen and Wistar albino rats supplied by the Animal Science, Department of Obafemi Awolowo University, Ile-Ife. Red blood cells were obtained from the blood samples and fixed with glutaraldehyde according to the method of Kuku and Eretan (2004). Sepharose 4B, divinyl sulphone, sugars and molecular weight standards were from Sigma Chemical Company, St. Louiz, MO, USA. All other reagents used were of analytical grades.
Extraction of crude lectin from fish skin mucus
Four African catfishes (each between 300 to 400 g body weights) were used per run of the purification procedure. The skin mucus was collected from the ventral part of the fish by gentle scraping of the skin using a soft rubber spatula with enough care not to damage the skin to avoid contamination with blood or secretion of epithelial cells/peripheral circulation and homogenized in five volumes of 10 mM phosphate buffer, pH 7.2 containing 50 mM NaCl. The homogenate was centrifuged for 30 min at 10,000 rpm using refrigerated centrifuge. The supernatant obtained constituted the skin mucus crude extract.
Protein concentration determination
Protein concentration of the crude extract and other fraction were determined by method of Lowry et al. (1951) using Bovine Serum Albumin (BSA) as standard.
Purification of Lectin
The crude extract of the skin mucus of the African catfish was applied on to a Sephadex G-150 column (2.5 x 40 cm) previously equilibrated with 10 mM phosphate buffer, pH 7.2 containing 50 mM NaCl. The protein was eluted with the same buffer. Fractions of 4 ml were collected, elution was monitored at 280 nm and the fractions were assayed for hemagglutinating activity. The peak with lectin activity was pooled, dialysed and chromatographed on a column (0.5 x 20 cm) of lactose-sepharose 4B equilibrated with 10 mM phosphate buffer containing 50 mM NaCl (pH 7.0). The unbound proteins were washed off from the column with 100 ml of equilibrating buffer. The adsorbed protein was eluted with 0.2 M lactose in 10 mM phosphate buffer after which it was exhaustively dialyzed to remove the bound sugar.
Haemagglutinating activity
Agglutination of the red blood cells by the crude extract and the various fractions that were obtained during purification was estimated as described by Pattanapanyasat et al. (2010). A serial two-fold dilution of the lectin solution in U-shaped microtitre plates (100 μl) was mixed with 50 μl of a 2% suspension of human and various animals erythrocytes in phosphate buffered saline, pH 7.2 at room temperature (All erythrocytes were fixed with 1% glutaraldehyde). The plate was left undisturbed for 1 h for agglutination to take place. The haemagglutination titre of the lectin expressed as the reciprocal of the highest dilution exhibiting visible agglutination of erythrocytes was reckoned as one haemagglutinating unit. Specific activity was the number of haemagglutination units per mg protein.
Blood group specificity
The blood group specificity of the crude extract was established using erythrocytes from different blood groups of the ABO system and other animals.
Sugar inhibition test
The hemagglutination inhibition tests to investigate inhibition of lectin-induced hemagglutinations by various carbohydrates were performed in a manner analogous to the hemagglutination test (Tsivileva et al., 2001). A serial dilution of the sample was made until the end-point causing haemagglutination was obtained. 50 μl of the 0.2 M sugar solution was added to each well and allowed to stand for 30 min at room temperature and then mixed with 50 μl of 2% rabbit erythrocyte suspension. The haemagglutination titres obtained were compared with a non-sugar containing blank. 0.2 M of each sugar in PBS was prepared. The sugars tested include: D(+)-glucose, D(+)-mannose, D(+)-arabinose, D(+)-glucosamine hydrochloride, D(-)-sorbose, sorbitol, mannitol, maltose, sucrose, fructose, lactose, 1-O-methyl-α-glucopyranoside, rhamnose, raffinose, galactose, dulcitol, cellobiose, 2-deoxy-α-D-glucose, melibiose, L-fucose, melezitose and N-acetylglucosamine.
Polyacrylamide gel electrophoresis
Polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate (SDS) was performed on 10% gels in Tris-glycine buffer, pH 8.9. The proteins were stained with Coomassie Brilliant Blue R, while the presence of covalently bound sugar in the lectin was detected by staining the gels with periodic acid Schiff reagent (PAS staining) (Packer et al., 2002).
Molecular weight determination
The purified lectin was subjected to Sodium Dodecyl Sulfate – Polyacrylamide gel electrophoresis (SDS–PAGE) for subunit molecular weight determination in accordance with the procedure of Weber and Osborn (1975) using the following protein markers: Bovine serum albumin (Mr 66,000), Ovalbumin (Mr 45, 000), Pepsin (Mr 38,000), Trypsinogen (Mr 24, 000), β-Lactoglobulin (Mr 14, 000). Gel filtration on a Sephadex G-100 column (2.5 × 100 cm), which had been calibrated with molecular weight markers was carried out to determine the native molecular weight of the lectin. 5 ml of each standard protein was applied to the column and ran separately using a 10 mM phosphate buffer, pH 7.2 as eluants at a flow rate of 10 ml/h. Fractions of 5 ml were collected and the elution of the standard proteins was monitored at 280 nm. The void volume (Vo) of the column was determined using Blue dextran (elution monitored at 620 nm). The molecular weight markers used were: Creatine phosphokinase (Mr 81,000; 5 mg/ml), bovine serum albumin (Mr 66,000; 5 mg/ml), Ovalbumin (Mr 45,000; 5 mg/ml), Chymotrysinogen (Mr 25,000; 5 mg/ml), and Lysozyme (Mr 14,000; 5 mg/ml).
Effect of temperature on haemagglutinating activity
The effect of temperature on the haemagglutinating activity was determined as described by Sampaio et al. (1998). Aliquots of lectin were incubated at different temperatures (30 to 90°C) for 30 min then rapidly cooled in ice and assayed for agglutinating activity. Agglutinating activity of the lectin sample kept at 20°C for 30 min was used as control.
Effect of pH on haemagglutinating activity
The effect of pH on the haemagglutinating activity was measured by incubating the samples in the following buffers at different pH values; 0.2 M citrate buffer, pH 3 to 6; 0.2 M Tris-HCl buffer, pH 7 and 8; and 0.2 M glycine-NaOH buffer, pH 9 to 11. After 1 h, the haemagglutination activity of the lectin was determined. The control values were the agglutination titre of the lectin in PBS, pH 7.2.
Effect of EDTA and divalent cations
The effect of ethylenediaminetetraacetic acid (EDTA) and divalent cations on the lectin activity was carried out as described by Wang et al. (1996). The purified lectin sample was dialysed against 10 mM EDTA for 24 h and the hemagglutination activity of the demetallized lectin was determined. The treated lectin was then incubated with 50 μl each of the following cations: 10 mM MgSO4, BaCl2, MnCl2, FeCl3, CaCl2 and SnCl2 for 2 h in order to evaluate their capacity to restore haemagglutination.
The Ouchterlony double diffusion experiment
1.5% (w/v) agar solution in PBS containing 0.01% (w/v) sodium azide was prepared. The solution was slowly heated until the agar had completely dissolved and poured into clean Petri dishes. A well was made at the centre of each Petri dish and four other wells equidistant from the centre were made around it. 50 μl of the lectin sample was placed in the centre well, and 50 μl of the polysaccharide (250, 100, 50 and 10 mg/ml of each polysaccharide) was placed in the surrounding wells. The polysaccharides tested were, inulin, dextrin, glycogen, starch and the polysaccharide (galactomannan) from Afzelia africana seeds.
Amino acid analysis
The purified lectin was subjected to analysis of amino acid content. The amino acid composition of the lectin sample was determined using methods described by Spackman et al. (1958). The sample was hydrolyzed, evaporated in a rotary evaporator and loaded into the Technicon sequential Multi-sample Amino Acid Analyzer (TSM). The nitrogen content of the sample was determined by Kjeldhal method.
Effect of chemical modification of amino acid residues on hemagglutinating activity
Modification of tryptophan residues with N-bromosuccinimide (NBS) was carried out according to the method of Spande and Witkop (1967). 10 μl aliquot of 10 mM NBS (in water) was added to 100 μllectin sample (1 mg/ml in 50 mM sodium acetate buffer, pH 6.0) with rapid mixing, 10 μl of the reagent was then added to the lectin sample every 10 min at 20°C for 1 h. Excess reagent (NBS) was removed by dialyzing the solution against distilled water after which 100 μl aliquot was removed from the dialyzed solution and assayed for residual hemagglutinating activity. Lectin incubated with PBS in the absence of NBS served as control.
Reduction of the thiol groups was carried out by incubating 100 μl of the lectin (1 mg/ml) in 50 mM phosphate buffer (pH 8.0) with 10 μl of 0.1 mM 5, 5'- dithiobis-(2-nitrobenzoic acid) (DTNB) at 27°C (10 μl of the reagent was added at 15 min intervals for 1 h). Excess reagent was removed followed by determination of residual hemagglutinating activity. Lectin incubated in the absence of DTNB served as control.
For serine modification, the lectin (100 μg) in 100 μl of 50 mM Tris-HCl buffer (pH 7.4) was incubated with10 μl of 5 mM phenyl methyl sulfonyl fluoride (PMSF) at 27°C (10 μl of the reagent was added at 15 min intervals for 1 h). Excess reagent was removed followed by determination of residual hemagglutinating activity. Lectin incubated in the absence of PMSF served as control (Habeeb, 1972).
Phenylglyoxal was used for modification of arginine residues (Riordan, 1979). 100 μl of 1 mg/ml lectin sample in PBS, pH 7.5 was incubated with 10 μl of 10 mM phenyglyoxal (in 0.1 M sodium carbonate, pH 8.0) at room temperature for 1 h (with addition of 10 μl of reagent every 15 min). The modified lectin sample was dialyzed exhaustively to remove excess reagent and assayed for hemagglutinating activity as described above.
Histidine residues were modified with diethyl pyrocarbonate according to the method of Ovaidi et al. (1967). The lectin (100 µl, mg/ml) in 0.1 M phosphate buffer (pH 7.2) was mixed with 20 mM diethyl pyrocarbonate freshly prepared in absolute ethanol for 1 h. Excess reagent was removed from the solution by dialysis followed by determination of residual haemagglutinating activity. Lectin sample in the absence of diethyl pyrocarbonate served as control.
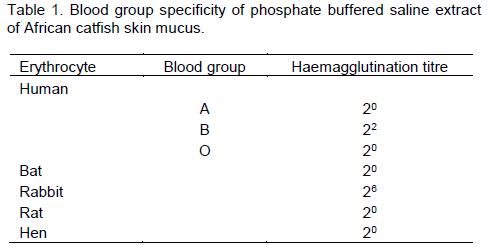
It was observed that African catfish skin mucus lectin agglutinated only erythrocytes from human blood Group B, but exhibited no haemagglutinating activity when it was checked against human A and O blood cells. The haemagglutinating activity was more pronounced with rabbit blood erythrocytes but showed no specificity for rat, bat and hen erythrocytes (Table 1).
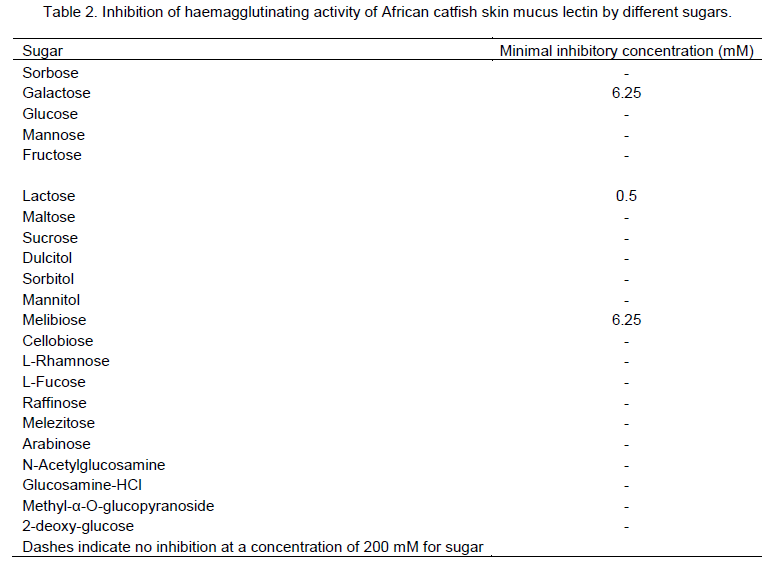
The hapten inhibition studies to define the sugar specificities of the crude extract showed that lactose completely inhibited the haemagglutinating activity with minimum inhibitory concentration of 0.5 mM (Table 2). Other galactose–containing sugar like melibiose and galactose also inhibited the lectin activity significantly. Dulcitol, mannitol, cellobiose, L-fucose, raffinose, melezitose, arabinose and methyl-α-O-glucopyranoside slightly inhibited the lectin activity. However, sucrose, glucose, maltose, cellibiose, glucosamine hydrochloride, 2-deoxy-glucose, sorbose, mannose, fructose, sorbitol, L-rhamnose and N-acetylglucosamine showed no inhibitory activity against African catfish skin mucus lectin.
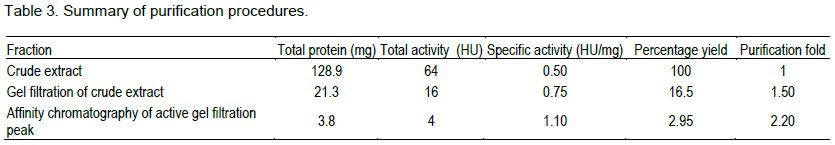
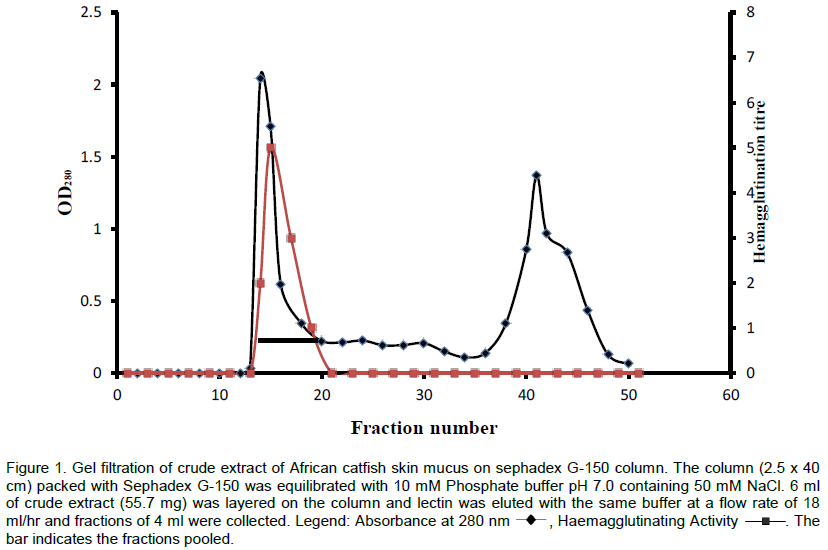
The lectin was isolated and purified to homogeneity from the skin mucus by a two step purification procedure comprising of gel filtration on Sephadex G-100 and affinity chromatography on Lactose-Sepharose 4B and the bioactivity of the lectin was measured by haemagglutination during each purification step. The level of purification was monitored by increase in its specific activity (Table 3). A typical elution profile on gel filtration is as shown in Figure 1. Two protein peaks were obtained, only the first peak exhibited haemagglutinating activity. The lectin active peak was further purified by affinity chromatography on Lactose-Sepharose 4B column (Figure 2). The lectin activity resided in the fraction adsorbed in the immobilized sugar, which was eluted with 0.2 M lactose in 10 mM phosphate buffer pH 7.2. The final preparation gave a distinct single protein band in SDS-PAGE.
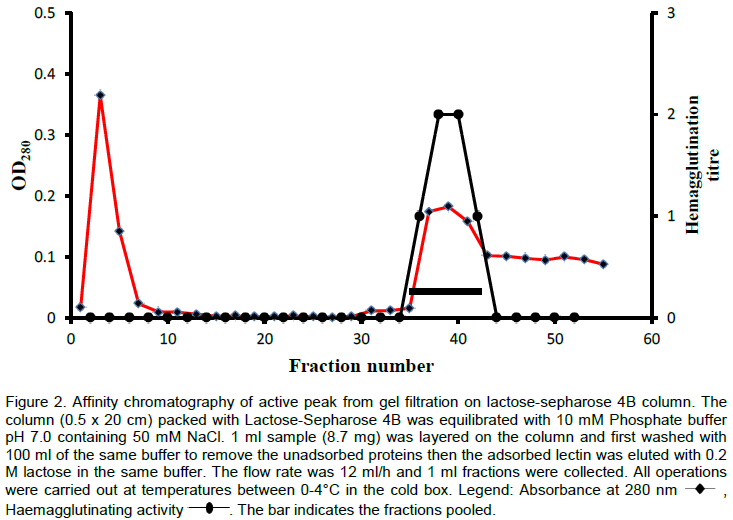
The molecular weight of the native protein was estimated to be 63,000 Daltons by gel filtration while the subunit molecular weight of 20,000 Daltons was obtained by SDS-PAGE suggesting a trimeric structure for the lectin. The minimum chemical molecular weight estimated from the amino acid composition was approximately 18,000 Daltons. SDS-PAGE revealed a homogenous single band of protein stain. The amino acid composition analysis revealed that the purified lectin is made up of 126 amino acids residues (Table 4).
The haemagglutinating activity of African catfish skin mucus lectin was not affected by EDTA even at high concentration. Also, addition of divalent metal ions showed no effect on the activity of the lectin either before or after dialysis against EDTA.
African catfish skin mucus lectin was negative with Periodic acid-Schiff’s reagent staining, suggesting that it is not glycosylated. The lectin when examined for possible interaction with some polysaccharides by Ouchterlony double diffusion experiment did not form any precipitin line with any of the polysaccharides/glycoprotein used.
The involvement of some amino acids residues in the haemagglutinating activity of African catfish skin mucus lectin was investigated using specific modifying reagents. Phenylglycoxal and Phenylmethane sulphonyl fluoride (PMSF) did not produce any significant alteration in the haemagglutinating activity of the mucus lectin. However, total loss of haemagglutinating activity was observed when African catfish skin mucus lectin was treated with 5, 5’–dithiobis-(2-nitro benzoic acid) (DTNB), N-bromosuccinimide (NBS) and Diethyl pyrocarbonate (DEPC) (Table 5).
Recently, a number of lectins have been purified from the skin mucus of several species of fish including Japanese eel (Anguilla japonica) (Tasumi et al., 2002), Ponyfish (Leiognathus nuchalis) (Okamoto et al., 2005), Torafugu (Takifugu rubripes) (Tsutsui et al., 2006) and Conger eel (Conger myrister) (Tsutsui et al., 2007). In addition, studies have also shown that skin mucus contains a number of novel lectins (Suzuki et al., 2003; Tasumi et al., 2004; Tsutsui et al., 2005).
We successfully purified and characterized a lectin from the skin mucus of African catfish, an important commercial tropical freshwater fish, by a combination of gel filtration on Sephadex G-150 and affinity chromatography on Lactose-Sepharose 4B gels. It was noted that the skin mucus lectin exhibited blood group specificity similar to that of lectin found in Pufferfish and Japanesse eel (Tsutsui et al., 2003; Tasumi et al., 2004). African catfish skin mucus lectin could not agglutinate erythrocytes from all blood samples tested. However, it showed strong specificity towards blood Group B erythrocyte but could not agglutinate other human erythrocytes. The lectin could not agglutinate erythrocytes from hen, rat and bat but showed high preference for the rabbit erythrocytes.
The inhibitory analysis showed that glucose and its derivatives had no inhibitory effect on the skin mucus lectin activity. Among the carbohydrates tested, galactose, lactose and melibiose strongly inhibited the haemagglutinating activity. This inhibitory effect on lectin activity gives support to the classification of this lectin as a member of galactose-specific lectins. Similarly, lectins from Conger eel and Japanesse eel have also been classified as galactose-specific (Tasumi et al., 2004; Muramoto et al., 1999). The strong inhibition by lactose and melibiose over galactose indicated that catfish skin mucus lectin may possess an extended sugar-binding site. The result also suggested that this lectin does not differentiate between α and β-galactoside since lactose and melibiose inhibited the lectin activity to a similar extent. The importance of C-4 hydroxyl group orientation is indicated by the failure of mannose or glucose to act as inhibitor.
The activity of the skin mucus lectin does not require Ca2+ or any other divalent cation for its haemagglutinating activity. It is therefore calcium-independent lectin. Tsutsui et al. (2003) reported that the haemagglutinating activity of pufflectin-s (skin mucus lectin of Pufferfish, Takafugu rubripe) was not altered by the addition of either EDTA or CaCl2, indicating that pufflectin-induced agglutination does not require calcium. The skin mucus lectins of conger eel and Japanese eel have also been reported to be calcium-independent (Tasumi et al., 2002; Suzuki et al., 2003; Tsutsui et al., 2007).
Based on the SDS-PAGE and gel filtration results we conclude that African catfish skin mucus lectin is a homomeric protein with three identical subunits. The molecular weight calculated from the deduced amino acid composition was 18,000 Daltons. This calculated value is in good agreement with that measured by SDS-PAGE. This is in conformity with what was obtained for starfish lectin (Kakiuchi et al., 2002) and salmon serum lectin (Ewart et al., 1999). Though, many of the skin mucus lectins isolated so far have been shown to be homodimer (Tasumi et al., 2004), our result indicated that skin mucus lectin from African catfish is a unique one with three subunits of identical size. The amino acid composition analysis revealed that the purified lectin is made up of 126 amino acids residues, which is very close to what was obtained for skin mucus lectins from pufferfish and conger eel, (Tsutsui et al., 2003, 2007). The lectin from African catfish skin mucus was characterized by low content of arginine, aspartic acid, serine, glycine, valine, isoleucine and leucine. The number of half-cystine is considerably high compared to other sulphur containing amino acid (methionine) suggesting the presence of disulphide bridges within the native structure of the African catfish skin mucus. There is also abundance of charged and uncharged polar amino acids like tyrosine, glutamic acid, lysine, histidine and cysteine, which together represents about 60% of the total amino acids of the lectin. In general, the hydrophobic amino acid content of this lectin is very high and is more than half of the total amino acids suggesting that the protein may be more of a globular protein than a fibrous.
The thermostability of various lectins appears to differ widely. Some are relatively stable while others are much less so. The lectin isolated was thermally stable over a wide range of temperature between 25 and 50°C; however after 50°C, it started losing activity very rapidly. At 60°C, the lectin completely lost its activity. Also, the lectin was found stable between pH 5.0 and 10.0 but maximally active between pH 6.0 to 9.0. Some of the fish skin mucus lectins that have been isolated were heat and pH stable over the same range of pH and temperature obtained for African catfish skin mucus lectin (Tateno et al., 1998; Tasumi et al., 2004; Dutta et al., 2005).
The carbohydrate content varies from lectin to lectin and in some cases, it could be as high as 30%, however in some cases it is totally absent. The lectin was not glycosylated according to the Periodic acid-Schiff’s staining which is comparable to the result obtained by Tsutsui et al. (2003) for Pufflectin-s. The agreement of calculated and measured molecular weights also indicates that this lectin lacks attached carbohydrate. The lectin did not show any precipitin line with any of the polysaccharides/glycoprotein used. This could however be due to many factors such as the concentration of the protein as well as the molecular size of the polysaccharides. This could also be explained based on the theory that a lectin may either fail to precipitate a polysaccharide or form precipitin bands in agar gel because the lectin may not be specific for that polysaccharide.
Identification of specific amino acids involved in the biological activity of proteins provides information about the relationship between its structure and the role played by amino acid side chains in its activity. A common strategy for identifying the amino acids is to treat the protein with specific affinity modifying reagents. This investigation provides clues about the amino acids involved in the biological activity. The outcome of this investigation indicated that arginine and serine are not playing any important role in the activity of the lectin. The results also strongly suggest that cysteine, tryptophan and histidine were either located at the sugar-binding site or were involved in the maintenance of the lectin active conformation. Previous studies have shown that tryptophan is indispensable for the haemagglutinating activity of some lectins especially galectins. Tryptophan residue was implicated in sugar binding activity of both Congerin I and II because its modification by 2-nitrophenyl sulfenyl chloride (NPS-CL) led to about 87% loss of the haemagglutinating activity of the congerins (Muramoto et al., 1999). The loss of activity may also be attributed to deprotonation of some reactive groups at the active site like indole group of tryptophan at low pH, consequently leading to the disruption of binding forces and the loss of activity. Also, congerins haemagglutinating activity decreased significantly when histidine residue was modified with DEPC (Muramoto et al., 1999). The cysteine residue seems to play a structural role rather than sugar binding considering the homotrimeric structure of the lectin.
The lectin from skin mucus of African catfish showed similar biological and molecular properties with the skin mucus lectins from several species of fish. However, some minor differences still exist which give each lectin unique characteristic. Further line of research should involved deducing the structure of this lectin for proper classification and also investigation of the physiological function of this lectin to clarify its involvement in the mucosal defense mechanism of African catfish.
The authors have not declared any conflict of interests.
REFERENCES
|
Alexander JB Ingram GM (1992). Noncellular nonspecific defense mechanism of fish. Ann. Rev. Fish Dis. 2:249-279.
Crossref
|
|
|
|
Argayosa AM, Bernal RAD, luczon AU, Arboleda JS (2011). Characterization of mannose-binding protein isolated from the African catfish (Clarias gariepinus) serum. Aquaculture 310:274-280.
Crossref
|
|
|
|
|
Benhamed S, Guardiola FA, Mars M, Esteban MA (2014). Pathogen bacteria adhesion to skin mucus of fishes. Vet. Microbiol. 171:1-12.
Crossref
|
|
|
|
|
Chrispeels MJ, Raikhel NV (1991). Lectins, Lectins Genes, and Their Role in Plant Defense. Plant Cell 3:1-9.
Crossref
|
|
|
|
|
Drickamer K (1995). Increasing diversity of animal lectin structures. Curr. Opin.Struc. Biol. 5:612-616.
Crossref
|
|
|
|
|
Dutta S, Sinha B, Bhattacharya B, Chatterjee B, Mazumder S (2005). Charaterization of a galactose binding serum lectin from the Indian catfish, Clarias batrachus: Possible involvement of fish lectins in differential recognition of pathogens. Comp. Biochem. Physiol. 141C:76-84.
|
|
|
|
|
Ellis AE (2001). Innate host defense mechanisms of fish against viruses and bacteria. Develop. Compar. Immunol. 25:827-839.
Crossref
|
|
|
|
|
Ewart KV, Johnson SC Ross NW (1999). Identification of a pathogen-binding lectin in Salmon serum. Comp. Biochem.Physiol 123C:9-15.
|
|
|
|
|
Fang EF, Lin P, Wong JH, Tsao SW, Ng TB (2010). A lectin with anti-HIV-1 reverse transcriptase, antitumor, and nitric oxide inducing activities from seeds of Phaseolus vulgaris cv. extralong autumn purple bean. J. Agric. Food Chem. 58:2221-2229.
Crossref
|
|
|
|
|
Habeeb AFSA (1972). Reaction of protein sulfhydryl groups with Ellman's reagent. Meth. Enzymol. 25:457-464.
Crossref
|
|
|
|
|
Kakiuchi M, Okino N, Sueyoshi N, Ichinose S, Omori A, Kawabata S, Yamaguchi K, Ito M (2002). Purification, characterization, and cDNA cloning of alpha-N-acetyl-galactosamine-specific lectin from starfish, Asterina pectinifera. Glycobiol. 12:85-94.
Crossref
|
|
|
|
|
Khan F, Khan MI (2011). Fungi Lectins: Current molecular and biochemical perspectives. Int. J. Biol. Chem. 5:1-20.
Crossref
|
|
|
|
|
Kuku A, Eretan OB (2004). Purification and Partial Characterisation of a Lectin from the fresh leaves of Kalanchoe crenata (And.) Haw. J. Biochem. Mol. Biol. 37(2):229-233.
Crossref
|
|
|
|
|
Lis H, Sharon N (1986). Lectins as molecules and as tools. Ann. Rev. Biochem. 55:35-67.
Crossref
|
|
|
|
|
Lis H, Sharon N (1998). Lectins: carbohydrate-specific proteins that mediate cellular recognition. Chem. Rev. 98:637-674.
Crossref
|
|
|
|
|
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951). Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275.
|
|
|
|
|
Melo CML, Porto CS, Melo-Junior MR, Mendes CM, Cavalcanti CCB, Ceolho LCBB, Porto ALF, Leao AMAC, Correia MTS (2011). Healing activities induced by cramoll 1, 4 lectin in healthy and immunocompromised mice. Int. J. Pharmaceut. 408:113-119.
Crossref
|
|
|
|
|
Muramoto K, Kagawa D, Sato T, Ogawa T, Nishida Y, Kamiya H (1999). Functional and structural characterization of multiple galectins from the skin mucus of conger eel, Conger myriaster. Comp. Biochem.Physiol. 123B:33-45.
Crossref
|
|
|
|
|
Okamoto M, Tsutsui S, Tasumi S, Suetake S, Kikuchi K, Suzuki Y (2005). Tandem repeat of L-Rhamnose-binding lectin from the skin mucus of ponyfish, Leiognathus nuchalis. Biochem. Biophys. Res. Comm. 333:463-469.
Crossref
|
|
|
|
|
Ovaidi J, Libor S, Elodi P (1967). Spectrophotometric determination of histidine in proteins with diethylpyrocarbonate. Acta Biochim. Biophys. Sinica 2:455-458.
|
|
|
|
|
Packer NH, Ball MS, Devine PL, Patton WF (2002). Detection of Glycoproteins in Gels and Blots. In: Walker JM, Totowa NJ, The Protein Protocols Handbook 2nd edition. Humana Press, pp. 762-763.
|
|
|
|
|
Pattanapanyasat K, Noulsri E, Lerdwana S, Sukapirom K, Onlamoon N, Tassaneetrithep B (2010). The Use of Glutaraldehyde-Fixed Chicken Red Blood Cells as Counting Beads for Performing Affordable Single-Platform CD4+ T-Lymphocyte Count in HIV-1-Infected Patients. J. Acq. Immun. Def. Synd. 53:47-54.
Crossref
|
|
|
|
|
Riordan JF (1979). Arginyl residues and anion binding sites in proteins. Mol. Cell. Biochem. 26:71-92.
Crossref
|
|
|
|
|
Sampaio AH, Rogers DJ, Barwell CJ (1998). A Galactose-Specific Lectin from the Red Marine Alga. Ptilota filicina. Phytochem. 48:765-769.
Crossref
|
|
|
|
|
Sharon N, Lis H (1990). Leguminous lectins: A large family of homologous proteins. FASEB J. 4:3198-3208.
|
|
|
|
|
Souza JD, Silva MBR, Argolo ACC, Napleao TH, Sa RA, Correia MTS, Paiva PMG, Silva MDC, Coelho LCBB (2011). A new Bauhinia monandra galactose-specific lectin purified in milligram quantities from secondary roots with antifungal and termicidal activities. Int. Biodeterior. Biodegrad. 65:696-702
Crossref
|
|
|
|
|
Spackman DH, Stein EH, Moore S (1958). Automatic Recording Apparatus for Use in the Chromatography of Amino Acids. Anal. Chem. 30:1191-1198.
Crossref
|
|
|
|
|
Spande TF, Witkop B (1967). Determination of the tryptophan content of proteins with N-bromosuccinimide. Meth. Enzymol. 11:498-506.
Crossref
|
|
|
|
|
Subramanian S, Ross NW, MacKinnon SL (2008). Comparison of the biochemical composition of normal epidermal mucus and extruded slime of hagfish (Myxine gluinosa L.). Fish Shell. Immunol. 25:625-632.
|
|
|
|
|
Suzuki Y, Tasumi S, Tsutsui S, Okamoto M, Suetake H (2003). Molecular diversity of skin mucus lectins in fish. Comp. Biochem. Physiol. 136B:723-730.
Crossref
|
|
|
|
|
Tasumi S, Ohira T, Kawazoe I, Suetake H, Suzuki Y, Aida K (2002). Primary structure and characteristics of a lectin from skin of mucus of Japanese eel Anguilla japonica. J. Biol.Chem. 277:27305-27311.
Crossref
|
|
|
|
|
Tasumi S, Yang WJ, Usami T, Tsutsui S, Ohira T, Kawazoe I, Wilder MN, Aida K, Suzuki Y (2004). Characteristics and primary structure of galectin in the skin mucus of Japanese eel, Anguilla japonica. Comp. Biochem. Physiol. 28C:325-335.
|
|
|
|
|
Tateno H, Saneyoshi A, Ogawa T, Muramoto K, Kamiya H, Saneyoshi M (1998). Isolation and characterization of rhamnose-binding lectins from eggs of steelhead trout (Oncorhynchus mykiss) homologous to low density lipoprotein receptor superfamily. J. Biol. Chem. 273:19190-19197.
Crossref
|
|
|
|
|
Tsivileva OM, Nikitina VE, Garibova LV, Ignatov VV (2001). Lectin activity of Lentinus edodes. Int. Microbiol. 4:41-45.
|
|
|
|
|
Tsutsui S, Iwamoto K, Nakamura O, Watanabe T (2007). Yeast-binding C-type lectin with opsonic activity from conger eel (Conger myriaster) skin mucus. Mol. Immunol. 44:691-702.
Crossref
|
|
|
|
|
Tsutsui S, Okamoto M, Tasumi S, Suetake H, Kikuchi K, Suzuki Y (2006). Novel mannose-specific lectins found in torafugu Takifugu rubripes: A review. Comp. Biochem. Physiol. 1D:122-127
|
|
|
|
|
Tsutsui S, Tasumi S, Suetake H, Kikuchi K, Suzuki Y (2005). Demonstration of the mucosal lectins in the epithelial cells of internal and external body surface tissues in pufferfish (Fugu rubripes). Dev. Comp. Immunol. 29:243-253.
Crossref
|
|
|
|
|
Tsutusi S, Tasumi S, Suetake H, Suzuki Y (2003). Lectins homologus to those of monocotyledonous plants in the skin mucus and intestine of pufferfish, Fugus rubripes. J. Biol. Chem. 278:20882-20889.
Crossref
|
|
|
|
|
Wang HX, Liu WK, Ng TB, Ooi VEC, Chang ST (1996). The Immunomodulatory and Antitumor Activities of Lectins from the Mushroom Tricholoma mongolicum. Immunopharmacol. 31:205-211.
Crossref
|
|
|
|
|
Weber K, Osborn M (1975). Protein and sodium dodecyl sulphate: molecular weight determination on polyacylamide gels and related
|
|
|
|
|
procedures. In Neurath H, Hill RL (Eds) "The Proteins" 3rd edition Academic Press, New York, pp. 179-223.
|
|