ABSTRACT
The current study compares intranasal and oral midazolam for effect on sedation for patients requiring dental procedure. Eighty subjects between the ages of 5 and 12 years were received randomly either intranasal (0.2 mg/kg) or oral (0.5 mg/kg) midazolam. The observer assessed the children for sedation level at the following time points: Immediately before the drug was administered, and 20 and 30 min after drug administration. There were significant differences in sedation level among the both groups at the time of parental separation and at the time of induction. 39 (97.5%) and 40 (100%) of the forty patients who received oral midazolam were calm, drowsy or asleep at 20 and 30 min after drug administration, respectively. For patients who received intranasal midazolam, 32 (80%) and 33 (82.5%) of the forty patients were either calm or drowsy at 20 and 30 min after drug administration, respectively. None of the patients from the intranasal group was rated as ‘asleep’. Oral midazolam was found to be statistically more effective in providing a better sedation level at the time of parental separation and at the time of induction than intranasal administration. Our findings indicate a tendency for oral midazolam to be more effective as a premedication in children before general anesthesia.
Key words: Preoperative, midazolam, sedation, anesthesia, pediatrics.
The pre-anesthetic management in pediatric patients undergoing extensive dental treatment may be a challenge, particularly during parental separation and induction of anesthesia. The use of sedative premedication may help reduce the anxiety and minimizing psychological trauma related to anesthesia and surgery (Beeby and Hughes, 1980; Rosenbaum et al., 2009). MDZ is a potent, short-acting benzodiazepine sedative hypnotic, which has been used as a premedication for general anesthesia and routinely used in pediatric dentistry for dental procedures (Hartgraves Hartgraves and Primosch, 1994).
Midazolam has been used as a preoperative sedative agent via the intramuscular (Taylor et al., 1986), intranasal (Hartgraves and Primosch, 1994), oral (Hartgraves and Primosch, 1994; Cox et al., 2006), and rectal (Saint-Maurice et al., 1986) routes. The different routes of administration of midazolam (intranasal, oral, and rectal) for sedative premedication have been previously studied (Baldwa et al., 2012; Chhibber et al. 2011; Griffith et al., 1998; Lejus et al., 1997; Malinovsky et al., 1995). Studies by Kogan et al. (2002) and Yildirim et al. (2006) compared the intranasal and oral routes of administration of midazolam. The authors concluded that nasal midazolam induced sedation similar to that following oral administration of midazolam with a shorter delay of onset. Another study by Lee-Kim et al. (2004) demonstrated that the oral route of administration has higher level of sedative action and longer working time than intranasal.
The purpose of this study is to compare the effectiveness of oral and intranasal midazolam in achieving sedation in children prior to dental surgery.
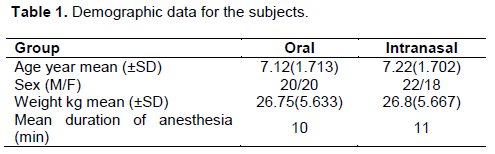
This study’s experimental protocol was approved by the local medical committee of King Hussein Medical Centre in Amman, Jordan. Informed consent was obtained from all parents of participating subjects. Eighty subjects between the ages of 5 and 12 years presenting for dental extraction under general anesthesia at King Hussein Medical Centre were included in this study between June 2013 and April 2014. All participants were in good health (ASA I). The demographic data for the children included in the study are shown in Table 1. The subjects were assigned randomly to receive either 0.5 mg/kg of oral midazolam or 0.2 mg/kg intranasal midazolam. Doses of 0.5 mg/kg for oral and 0.2 mg/kg for intranasal administration were chosen within the dose-exposure range found in preliminary studies (Acworth et al., 2001; Malinovsky et al., 1995). Half of the 80 patients received oral midazolam via a needleless syringe to the back of the mouth, whereas the other patients received intranasal midazolam as drops from a needleless syringe into the nostril.
Following drug administration, the child remained with the parent away from the treatment room for 20 min. The patient was then separated from the parent and transferred to the operatory. Vital signs were monitored continuously. Each patient was evaluated by observers for sedation level with assessments recorded immediately before the drug administration and at 20 and 30 min after drug administration.
Patient sedation was evaluated by observer using a five-point sedation scale:
1. Agitated, that is, clinging to parent and / or crying.
2. Alert, that is, a wake but not clinging to parent.
3. Calm, that is, sitting or lying comfortably with eyes spontaneously open.
4. Drowsy, that is, sitting or lying, comfortably with eyes closed but responding to minor stimulation.
5. Asleep, that is, eyes closed and not responding to minor stimulation.
The scale was devised by Wilton et al. (1988) to evaluate level of sedation of preschool children before anesthesia for surgery.
All children received a standardized GA by the same anesthesiologist. The anesthesiologist used mask induction with sevoflurane, oxygen and nitrous oxide. Thereafter, 2 g/kg of fentanyl and 0.5 mg/kg of atracurium were injected to facilitate tracheal intubation. Sevoflurane was the inhalational anesthetic used for maintenance of anesthesia. Patients’ electrocardiogram, arterial blood pressure, pulse oximetry, were monitored as part of standard GA procedure following surgery, the patient was taken to the post anesthesia care unit (PACU), where the patient was monitored continuously for 1 h. The means for weight and age were analyzed using a paired t test.
Findings for sedation levels were analyzed for statistically significant differences between the groups at 20 and 30 min after midazolam administration using the Mann-Whitney U test. Mann-Whitney U test at the 95% significance level was used to compare the effectiveness of the two routes of midazolam administration. P < 0.05 was considered significant. The independent variable in the study was drug administration route (oral or intranasal). The dependent variable in the assessment of the effectiveness of each route was the sedation level.
Both groups were comparable with respect to age, weight, and duration of anesthesia as shown in Table 1. The children’s reaction to being separated from their parent(s) 20 min after receiving premedication is displayed in Table 2. Changes in sedation levels following oral and intranasal midazolam at 30 min after premedication administration are shown in Table 3.
A significant difference was observed during the 20 and 30-minute time-period between the 2 regimens: Intranasal midazolam showed more children agitated and alert, while oral midazolam presented more children quiet or asleep.
For patients who received intranasal midazolam, 32 (80 %) of the forty patients were calm, drowsy or asleep and 8 (20%) were rated as agitated, alert at 20 min, However, no significant changes in sedation scores were noted at 30 min as the number of agitated or alert participants decreased from 8 to 7 (17.5 %) while the number of calm and drowsy participants increased from 32 to 33 (82.5%). None of the patients from the intranasal group were rated as asleep.
For patients who received oral midazolam, 39 (97.5%) of the forty patients were calm, drowsy or asleep and one patient (2.5%) was rated as alert at 20 min, and none of the children in the oral midazolam group was rated as agitated or alert at 30 min. This difference was statistically significant between the group that received the oral midazolam and the group that received intranasal midazolam at the time of parental separation, z = −1.997 (p = 0.046), and at the time of induction, z = −2.386 (p = 0.017).
During the premedication time, none of the patients in the study had an incidence of bradycardia, hypotension or desaturation episodes. During the procedure time, blood pressure and pulse oximetry values for all subjects were in the normal range as shown in Table 4.
All children in the both groups recovered spontaneous ventilation. The time taken for discharge from recovery room was 28 to 50 min which was similar in both groups.
Several studies have suggested that midazolam is an effective premedication for children when administered intramuscularly (Taylor et al., 1986), rectally (Saint-Maurice et al., 1986), intranasal (Hartgraves and Primosch, 1994), or orally (Hartgraves and Primosch, 1994; Cox et al., 2006).
Studies by Kogan et al. (2002) and Yildirim et al. (2006) found that both intranasal and oral midazolam produces good levels of sedation and anxiolysis, but no significant difference in the effects of sedation was observed between the oral midazolam group and the intranasal midazolam group.
A study by Lee-Kim et al. (2004) demonstrated no statistical difference for overall behavior between the oral midazolam group and the intranasal midazolam group however intranasal subjects showed more movement and less sleep toward the end of the dental procedures, and faster onset time but shorter working time than oral midazolam group.
When assessing the level of sedation, both routs were effective but the difference in sedation level between the 2 routes of administration was significant at the time of parental separation and at the time of anesthesia.
The improvement and success in pediatric sedation over time for patients receiving oral midazolam may have been affected by the method of drug administration and the amount of drug absorption. Unlike oral administration, where its effect last for longer time, too rapid an administration via the intranasal route could result in loss of the premedication into the oral cavity. The result is less drug absorption into the nasal mucosa and, therefore, a lower blood level of the drug and a decrease in sedation with time progression.
The results of the present study must be interpreted in light of the small number of participants enrolled. Further investigation with a greater number of patients might yield more meaningful results.
The dental anesthesiologist noted that the intranasal route of midazolam administration could produce a burning sensation when the liquid is administered. Furthermore, the drug can have a noxious taste when administered via the intranasal route and more can be lost through the oronasal pathway, rendering the intranasal midazolam less effective.
Time points should be appropriate to achieve onset time of premedication. Further investigation with a greater number of time points to determine the minimum time interval between oral midazolam or intranasal midazolam premedication and separation from parents to ensure a smooth separation should be conducted.
Oral midazolam could be more effective as a premedication than the intranasal route was noted in the present study. When used before general anesthesia, the oral route allowed for a better sedation level at the time of parental separation and anesthesia than the intranasal route.
The authors have not declared any conflict of interest.
REFERENCES
|
Acworth JP, Purdie D, Clark RC (2001). Intravenous ketamine plus midazolam is superior to intranasal midazolam for emergency paediatric procedural sedation. Emerg. Med. J. 18:39-45.
Crossref
|
|
|
|
Baldwa NM, Padvi AV, Dace NM, Garasia MB. (2012). Atomised intranasal midazolam spray as premedication in pediatric patients: comparison between two doses of 0.2 and 0.3 mg/kg. J. Anesth. 26:346-50.
Crossref
|
|
|
|
|
Beeby DG, Hughes JOM (1980). Behaviour of unsedated children in the anaesthetic room. Br J Anaesth. 52:279-281.
Crossref
|
|
|
|
|
Chhibber AK, Fickling K, Lustik SJ (2011). Pre-Anesthetic Midazolam: A Randomized Trial with three Different Routes of Administration. J. Anesth. Clin. Res. 2:118.
Crossref
|
|
|
|
|
Cox RG, Nemish U, Ewen A, Crowe MJ (2006). Evidence-based clinical update: Does premedication with oral midazolam lead to improved behavioral outcomes in children. Canad. J. Anesth. 53(12):1213-1219.
Crossref
|
|
|
|
|
Griffith N, Howell S, Mason DG (1998). Intranasal midazolam for premedication of children undergoing day-case anaesthesia: comparison of two delivery systems with assessment of intra-observer variability. Br. J. Anaesth. 81(6):865-869.
Crossref
|
|
|
|
|
Hartgraves PM, Primosch RE (1994). An evaluation of oral and nasal midazolam for pediatric dental sedation. J. Dent Child. pp. 61175-61181.
|
|
|
|
|
Kogan A, Katz J, Efrat R, Eidelman LA (2002). Premedication with midazolam in young children: A comparison of four routes of administration. Paediatr Anaesth. 12:685-689.
Crossref
|
|
|
|
|
Lee-Kim SJ, Fadavi S, Punwani I, Koerber A (2004). Nasal versus oral midazolam sedation for pediatric dental patients. J. Dent. Child. 71:126-130.
|
|
|
|
|
Lejus C, Renaudin M, Testa S, Malinovsky JM, Vigier T, Souron R (1997). Midazolam for premedication in children: nasal vs. rectal administration. Eur. J. Anaesthesiol. 14:244-249.
Crossref
|
|
|
|
|
Malinovsky JM, Populaire C, Cozian A, Lepage JY, Lejus C, Pinaud M (1995). Premedication with midazolam in children: effect of intranasal, rectal and oral routes on plasma midazolam concentration. Anaesthesia. 50:351-354.
Crossref
|
|
|
|
|
Rosenbaum A, Kain ZN, Larsson P, Lönnqvist PA, Wolf AR (2009). The place of premedication in pediatric practice. Paediatr. Anaesth. 19(9):817-828.
Crossref
|
|
|
|
|
Saint-Maurice C, Meistleman C, Rey E, Esteve C, De Lauture D, Olive G (1986). The pharmacokinetics of rectal midazolam for premedication in children. Anesthesiol. 65:536-538.
Crossref
|
|
|
|
|
Taylor MB, Vine PR, Hatch DJ (1986). Intramascular midazolam premedication in small children. Anaesth. 41:21-26.
Crossref
|
|
|
|
|
Wilton NCT, Leigh J, Rosen DR, Pandit UA (1988). Preanesthetic sedation of preschool children using intranasal midazolam. Anesthesiol. 69:972-975.
Crossref
|
|
|
|
|
Yildirim SV, Guc BU, Bozdogan N, Tokel K (2006). Oral versus intranasal midazolam premedication for infants during echocardiographic study. Adv. Therapy 23:719-724.
Crossref
|
|