ABSTRACT
With the increased demand for high quality maize seeds, the market absorbs innovations that add speed and reliability in the tests that enable differentiation of potential performance of seed lots. This study aims to evaluate the combination of the physiological tests with the expression of enzymes of respiratory route for differences in vigor levels in lots of hybrid seed corn. The percentage of germination, germination first count, emergence on tray, index of emergency speed, accelerated aging and respiration rate were evaluated by Pettenkoffer and Titulation method. Furthermore, the expression of alpha amylase, alcohol dehydrogenase, malate dehydrogenase and pyruvate decarboxylase was analyzed. With the results, it is determined that the vigor tests analyzed, together with the tests of enzymatic expressions of respiratory route were efficient to detect different levels of force in hybrid seed corn. Lot 1 had higher physiological quality while lot 2 had the lowest vigor compared to the other lots. With the vigor tests and expression of enzymes, it was possible to differentiate the quality of the seeds and therefore it can be recommended as reliable tests for defining the quality of seed lots.
Key words: Respiration, enzyme expression, vigor, viability.
The search for increase in productivity has raised the technological level in the maize crop fields. Consequently, there is increase in the demand for quality seeds and investment in the seeds sector; maize seeds utilized represented 91% in the last crop season (Abrasem, 2015).
One of the first requirements for the success of maize hybrid seed performance is their physiological quality (Marcos, 2005). The attributes which determine the seeds quality are related to genetic, physical, physiological and sanitary factors. These attributes can be characterized by germination, vigor and longevity tests which show their viability (Moterle et al., 2011; Nerling et al., 2013).
To meet the demand for maize, seeds analyses have become an indispensable tool for obtaining quality control data, mainly from the final maturation stage (Krzyzanowki et al., 1999).Many tests contribute to increased technology for seeds production, quick analysis of quality processing and consequently increased productivity and low costs. With this, seeds companies aim to upgrade tests, like germination and vigor tests, in order to have results with behavior equal to the seeds sown in the field (Santos et al., 2004).
The tests used to evaluate the physiological quality correspond to the somatory of two fundamental parameters: viability and vigor. Viability is valid if the germination test brings out maximum potential seeds and produces normal seedlings on favorable conditions (Carvalho and Nakagawa, 2000). Many a time, the seedlings that emerge from field can be considerably lower compared to those obtained from the germination tests in the laboratory (Bhering et al., 2003). This shows the necessity of obtaining complementary information. To complement this information, vigor tests are used, which evaluate the potential of seeds germination and the fast development of normal seedlings under a wide variety of environmental conditions (Aosa, 1983). Thus, apart from physiological tests, other techniques have been used to verify the degree of seeds deterioration, like respiratory and enzymatic analyses (Devi et al., 2007; Lamarca, 2009).
Respiratory activity can be evaluated by gas exchanges and consists of manometric O2 consumed or measured CO2 liberated. It is sensitive and requires few amounts of materials (Crispim et al., 1994). Furthermore, it presents a considerable quality seeds. It is an important complementary test compared to the traditional one for determining vigor of seeds lots (Mendes et al., 2009; Dode et al., 2012). Another way to verify the processes related to seeds quality is the evaluation of enzymatic activity of respiratory route. The alterations in the normal pattern of these enzymes lead to a respiratory disorders, causing problems in the seeds quality and vigor (Devi et al., 2007). However, for this work, important enzymes were selected which are related to the seeds respiratory activity
Thus, the objective of this work is to correlate the respiratory activity and enzymatic expression from respiratory route to determine the seeds’ physiological quality; specifically to differentiate the levels of vigor of hybrids maize seed lots.
The research was conducted in the Central Laboratory of Seeds of the Agricultural Department at Universidade Federal de Lavras (UFLA), in Lavras, MG. Five lots of hybrid maize seeds with different levels of vigor were used.
The seeds water content was determined by the oven method at 105°C for 24 h (BRASIL, 2009), using two replications of 50 seeds of each treatment. After this period, the seeds were taken to desiccators until the samples cooled down. Later, the seeds dry weight was obtained. The results were expressed in percentage.
The germination test was conducted with four replications of 50 seeds; they were sown in moistened germitest paper in the proportion of 2.5 ml of water per g of paper. Seeds were kept in germinator, regulated with temperature of 25°C and the evaluations of normal seedlings were done in two counts: the first on the fourth day and the last one on the seventh day after sowing. The results were expressed in medium percentage of normal seedlings of the four replications (BRASIL, 2009).
For the calculation of emergence speed index, daily evaluations were done from the beginning of seedlings emergence, counting of the number of emerged seedlings until the stabilization of the stand. At the final test, with the daily data of the number of emerged seedlings, the emergence speed index was calculated, according to the formula proposed by Maguire (1962):
E.S.I.= (E1/N1)+ (E2/N2)+ ... + (En/Nn),
where: E.S.I = emergence speed index; E1, E2 and En = number of normal seedlings computed in the first count, second count and in the last count; N1, N2 and Nn = number of sowing days at 1ª, 2ª and at last count.
In the germination test, there were normal seedlings on the fourth day after sowing. For the accelerated aging test, plastic transparent boxes (11.5 x 11.5 x 3.5 cm) like mini-cameras were used, where the seeds were distributed to form a uniform layer. 40 ml of distillated water was added, creating an environment of 100% of air relative humidity. The boxes were closed and kept in aging chamber (B.O.D.), regulated at 41°C for 96 h. The respiratory activity was evaluated according to the physic-chemical methods given by Pettenkoffere and Titulation, which evaluates the quantity of CO2 liberated and O2 consumed respectively from seeds respiration (Crispim et al., 1994). The results obtained were correlated with the other tests to determine the seeds’ physiological quality. For the Pettenkofer method, the respiratory activity was determined using four flasks: the first two contained sodium hydroxide (NaOH), the third was conditioned with seeds analyzed and the fourth had barium hydroxide (Ba(OH)2).The flasks were closed with silicone, and connected by a tube; the air flux was controlled through a tap. After two hours of seeds exposure, two aliquots of supernatant were taken for titulation. In each aliquot were added two drops of phenolphthalein reagent color and later submitted for titulation with hydrochloric acid (HCl). The volume of HCl used until the “turn point” was proportional to the quantity of BaCO3 presented in the solution, which is also proportional to the quantity of CO2 from the seeds respiratory activity. From the stoichiometric calculations, it was possible to obtain the quantity of liberated CO2 during the process of seeds respiration. The result was expressed in quantity of liberated carbon dioxide per g of seeds per hour.
For the titulation method transparent plastic boxes were used (gearbox type) for supporting the seeds. In the deep of each gerbox were placed 40 ml of KOH solution at 0.1 N (Figures 3 and 4) and after were closed to avoid gas exchanges with the environment. Each of the four replications of 50 seeds was placed on blotter paper moistened with 2x the seeds’ weight. The gearboxes were kept in cold chamber (type B.O.D.) for a period of 24 h at constant temperature of 25°C.
After this period, drops of phenolphthalein reagent color were added in a sample of 25 ml of KOH solution, per replication; and were submitted for titulation with HCl 0.1 N. In the “turning point” the volume of HCl spent in each tested replication was registered. This volume of HCl which is directly related with the quantity of CO2 fixed for the KOH solution is from respiration.
For the Pettenkofer and titulation methods, the results were expressed in mg of CO2 and mg of O2 per g of dry seeds respectively, according to the following formulas:
Formula already simplified for Pettenkofer:
(W-L) x C /MS
Where, W: white reading test; L: reading of HCl volume spent to neutralize the KOH submitted for respiration; C: correction factor (3.52); MS: seeds dry matter.
Formula already simplified for titulation:
(Lb-Ls) x 1.105/h*g
Where, Lb: white reading test (mL); Ls: sample reading (mL); H: length of stay on the device (hours); G: mass of used seeds (g).
For the enzymatic evaluations, two samples of 25 seeds of each treatment were collected. These seeds were sown in germitest paper and after 72 h, they were macerated with PVP and liquid nitrogen in ice. Later they were stored at -86°C, until the extraction moment. For the enzymes extraction, the extraction buffer (Tris HCl 0.2 M pH 8 + 0.1% of β-mercaptoethanol) was added in the proportion of 250 Ï»L for 100 mg of seeds powder. The material was homogenized in vortex and kept in refrigerator during 12 h followed by the centrifugation at 14000 rpm for 30 min at 4°C. Then, 60 µL of supernatant in polyacrilamide gel was applied. The electrophoretic run was realized in a discontinuous polyacrylamide gel system at 7.5 (separating gel) and 4.5% (concentrating gel) using Tris-glycine pH 8.9 as standard buffer in the gel electrode system. The running was performed at 150 V for 5 h.
At the end of running, the gels were revealed for the enzymes alpha amylase (α-AMI- EC 3.2.1.1.), malate dehydrogenase (MDH- EC 1.1.1.37.), alcohol dehydrogenase (ADH - EC 1.1.1.1) and pyruvate decarboxylase according to the protocols established by Alfenas, (2006). Complete randomized experimental design was used for the five materials, with four replications. The data were statistically interpreted using variance analyses for all the tests with the aid of SISVAR® statistical program (Ferreira, 2011). For comparing the averages, the Scott-Knott test at 5% of probability was used. The simple linear correlation coefficient (r) of Pearson was determined between the values obtained in the tests used for the evaluation of the physiological quality of seeds. The evaluation of the gels was realized on transilluminator, being considered the variation of intensity of bands.
The medium water content of the seeds in the test was 13.1, with maximum variation of 1%. Minimum variation of water content is important between the materials to avoid increased deterioration process and formation of products which cause immediate damages, like free radicals, found in the final result (Marcos, 2005).
Vigor tests use specific situations of stress to preview the relative behavior of lots in field. This is because a variable number of tests must be used for the results to be coherent and consistent (Carvalho and Nakagawa, 1988; Woodstock, 1973). Thus, various tests were applied to maize seed of 5 lots. For the tests used to evaluate the characteristics of the seeds’ vigor were observed significant differences between the lots, with exception of the results observed in the germination test (Table 1).
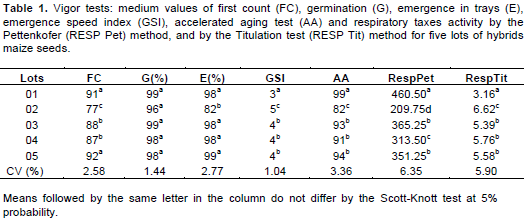
The results of vigor tests in all the seeds were consistent. This means that the lots were classified in a pattern in most part of the tests. Similar results were found by Pereira (2012), on evaluating the physiological quality of pepper and chili seeds through vigor tests and respiratory activity. This observation was different from the one related by Caliari and Silva (2001) and Castro (2011) who studied 45 and 16 lots of maize seeds, respectively, and obtained different results based on the vigor test used. The first germination count, realized to facilitate the conduction of germination test, can be considered a vigor test, because the germination speed is one of the first characteristics to be affected by the deterioration process of seeds (Vieira et al., 1994). This test is based on the principle that the lots with highest percentage of normal seedlings on the fourth day of germination are the most vigorous. It is a quick and an important test, since the uniformity and emergence speed of seedlings bare the most important compounds inside the actual concept of seeds vigor (Willyder, 2010).
In this work, it was possible to observe that the first count of germination (FC) differentiated the lots into three levels, separating lots 01 and 05 as having better quality and lot 02 as having lower quality; however, there was no difference for the germination (G%) between the lots (Table 1). The emergence test on trays (E%) separated the lots only in two levels which were statistically different: lot 02 was the less vigorous and the others were considered statistically equal. For the emergence speed index (ESI), lot 01 presented less number of days for medium emergence of seedlings. However, it is possible to infer that this lot presented better quality. Lot 02 necessitated more days for seeds germination, being the less vigorous lot as compared to the others.
The accelerated aging test (AA) is based on the increase of seeds deterioration by the exposure of these seeds to adverse conditions of high temperature and humidity (40 to 45°C and 100% UR). These are the most related environmental factors related to seeds deterioration (Delouche and Baskin, 1973). For this test, three levels of differentiation were determined in similar way to the others tests: lot 01 presented better quality and lot 02 presented lower quality.
Regarding the respiration test done for the seeds, determined by the Pettenkofer method, it was possible to separate statistically the five lots of maize seeds into three levels of vigor. According to Mendes et al. (2009) this test is an alternative or an important complement to the traditional tests for the determinations of seeds lots vigor, because it is a practical, simple and cheap test. So, in seeds with high vigor were observed the higher values of respiration by the liberation of CO2 characterizing the integrity of mitochondria. According to Bewley and Black (1994), the integrity of mitochondria in the viable embryos increases from the beginning of imbibition process, which becomes more efficient in ATP production. Thus, vigorous seeds breathe more compared to those seeds with less vigor, in the same period of time.
Castro (2011) observed this differentiation in maize seeds; Pereira (2012) in pepper and chili seeds; Dranski et al. (2013), in canola seeds and Venske et al. (2014) in cotton seeds. By the titulation method, it was also possible to separate statically the five lots of maize seeds in three levels of vigor. Thus, in seeds with high vigor was observed higher consumed O2 and consequently increase in the CO2 production. The high consumed O2 was observed for lot 1 and consequently higher vigor of seeds, contributing also to the integrity of mitochondria (Bewley and Black, 1994). For lot 02, there was lower consumption of O2 as compared to the other lots (Table 1).
In relation to the simple linear correlation analysis (Table 2) between the results observed in the tests used for the evaluation of physiological quality of maize seeds, there was significant correlation between the results of respiratory activity measured by the Pettenkofer method and those observed in the first count of germination test, with (r) of 0.8626, germination with (r) de 0.9136, emergence speed index with (r) of -0.9092 and aging with (r) of 0.9831.
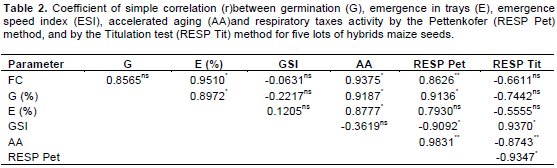
By the titulation method, there was significant correlation between the results of respiratory activity and those results observed in the tests of emergence of seedling speed index with (r) of 0.9370 and for the aging with (r) of -0.8743 and the Pettenkofer method with (r) of –0.9347. Crispim et al. (1994) observed significant correlations between the results observed in the titulation methods and other tests used for the evaluation of physiological quality of soybean seeds. Pereira (2012) also observed correlation between the results obtained in the physiological tests and Pettenkofer and titulation tests for pepper and chili seeds, reinforcing the importance of evaluating the seeds respiratory activity for complementing the germination and vigor tests. In relation to the enzymatic analysis, the alpha amylase expression (Figure 1) was higher in lot 01 and their lower expression was in lot 02, indicating a correlation of the expression of this enzyme with the vigor tests (Table 1). Alpha amylase enzyme is a hydrolytic enzyme that acts on starch; it assists in the supply of energy present mainly during the beginning of seeds germination, contributing to higher vigor of seeds. Consequently, it provides substrates for plant use and thus, ensures higher quality of seeds emergence as it occurs in lot 01 (Nedel et al., 1999).
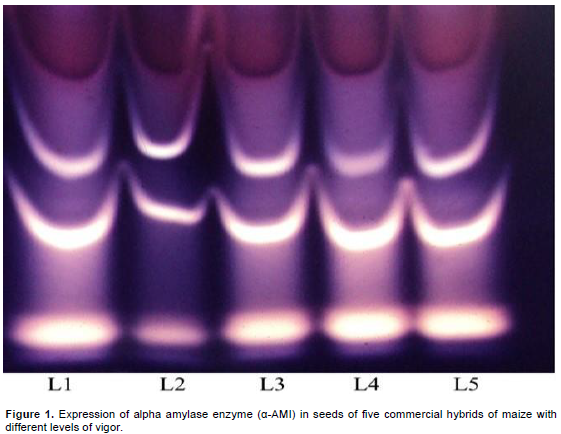
In maize, the alpha amylase enzyme, when promotes the starch hydrolyses, makes available the carbohydrates required for the embryo development, contributing to the germinative process (Franco et al., 2002). However, Oliveira (2013) points out that beyond the amylases genes, various others genes can be involved in the character control of seeds’ physiological quality, for example, genes related directly with respiration.
In relation to the malate dehydrogenase expression presented in Figure 2, it was observed higher expression of this enzyme for lots 1 and 5, and lower expression in lot 02, indicating again, a correlation of this enzyme expression with the vigor tests realized. The malate dehydrogenase enzyme has an important function in the production of NADH for the Krebs Cicle, participating in the respiratory process. Thus, it is present in different cellular compartments and so, expressing higher intensity of bands in seeds lots with higher respiratory taxes (Shatters et al., 1994).
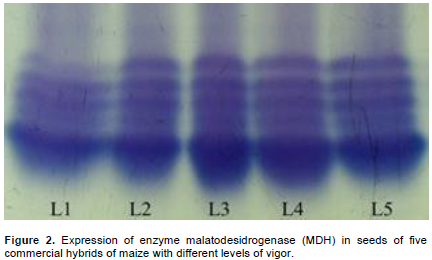
The correlation of these results can be associated to the activity of this enzyme. MDH enzyme acts in the generation of energy to important metabolic process, like seeds germination; it participates in the movement of malate through the mitochondrial membrane generating energy and fixing CO2 in plants (Taiz and Zeiger, 2009). It was not possible to infer differences in the expression of alcohol dehydrogenase enzyme activity (Figure 3). Thus, in this work, alcohol dehydrogenase was not effective for determining the quality of lots. According to the results obtained in Table 1, the lots presented significant differences in the characteristics of vigor evaluated, which cannot be observed in bands of the enzyme alcohol dehydrogenase. On the other hand, Castro (2011) observed that the electrophoretic profile of ADH enzyme for maize lots submitted for respiratory activity evaluation presented reduction in the intensity for the lot with lower physiological quality.
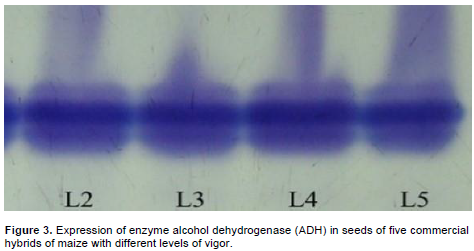
These results can be justified once the ADH enzyme is involved in the process of converting acetaldehyde to ethanol and the accumulation of acetaldehyde has been related to the deterioration of seeds. Zhang et al. (1994) report that, when the activity of alcohol dehydrogenase enzyme decreased, seeds becomes more susceptible to the deleterious effects of acetaldehyde, which can be an important factor that accelerates the deterioration of seeds during storage. Analyzing the expression of pyruvate decarboxylase enzyme (Figure 4), it was observed higher expression of this enzyme in lot 02, which according to the vigor tests presented in Table 1, corresponded to the lot with lower quality. Thus, in seeds with low vigor, the respiratory activity can be compromised affecting aerobic route and consequently the anaerobic route. Consequently, toxic products such as acetaldehyde and ethanol are accumulated. In this process, the pyruvate decarboxylase enzyme converts pyruvate from glycolysis to acetaldehyde which is reduced to ethanol in anaerobic route (Ferreira and Borghetti, 2007).

With this, the analyses of expression profiles of enzymes involved in the respiratory process are an important tool that complements the physiological tests for the separation of seeds lots in respect to their physiological quality. Alterations in the activities of these enzymes influence the metabolic processes of synthesis that are linked to respiratory processes and consequently influence the seed vigor and seedling development which depend on adequate availability of accumulated reserves (Marini et al., 2013).
The physiological tests and the enzymatic analyses applied to five evaluated lots were efficient to detect different levels of quality in hybrid maize seeds. Through the physiological and enzymatic tests, it was possible to verify that lot 1 presented higher vigor, while lot 2 presented lower vigor compared to the other lots. There are correlations between the vigor of maize seeds and the expression of enzymes from respiration route, except the alcohol dehydrogenase enzyme, because it was not possible to infer differences in its expression.
The authors have not declared any conflict of interests.
The authors thank the Foundation for Research Support of the State of Minas Gerais (FAPEMIG), the Coordination for the Improvement of Higher Education Personnel (CAPES) and The National Council for Scientific and Technological Development (CNPq), for financial support and scholarships.
REFERENCES
|
ABRASEM (2015). Associação Brasileira De Sementes E Mudas. Anuário 2015. Brasília, 2015.
|
|
|
|
Alfenas AC (2006). Eletroforese e marcadores bioquímicos em plantas e microrganismos. Viçosa: UFV, 627 p.
|
|
|
|
|
AOSA (1983). Seed Vigor Testing Handbook. Association of Official Seed Analysts. Contribution (32).
|
|
|
|
|
Bewley JD, Black M (1994). Physiology and biochemistry of seed in relation to germination: viability, dormancy and environmental control. Berlin: Springer-Verlag. 375 p.
|
|
|
|
|
Bhering MC, Dias DCFS, Barros DI, Dias LS, Tokuhisa D (2003). Avaliação do vigor de sementes de melancia (Citrulluslunatus Schrad.) pelo teste de envelhecimento acelerado. Rev. Bras. Sementes 25(2):1-6.
Crossref
|
|
|
|
|
BRASIL (2009). Ministério da Agricultura, Pecuária e Abastecimento. Regras para análise de sementes. Ministério da Agricultura, Pecuária e Abastecimento. Secretaria de Defesa Agropecuária. Brasília, DF: Mapa/ACS, 395 p.
|
|
|
|
|
Caliari MF, Silva WR (2001). Interpretação de dados de testes de vigor na avaliação da qualidade fisiológica de sementes de milho. Rev. Bras. Sementes 23(1):239-251.
Crossref
|
|
|
|
|
Carvalho NM, Nakagawa J (1988). Sementes: ciência, tecnologia e produção. Campinas: Fundação Cargill 424 p.
|
|
|
|
|
Carvalho NM, Akagawa J (2000). Sementes: ciência, tecnologia e produção. Jaboticabal: Funep, 4. ed., 588 p.
|
|
|
|
|
Castro MB (2011). Avaliação da qualidade fisiológica de sementes de milho por meio da atividade respiratória. Dissertação (Mestrado em Fitotecnia) - Universidade Federal de Lavras, Lavras, 67 p.
|
|
|
|
|
Crispim JE, Martins JC, Pires JC, Rosolem CA, Cavariani C (1994). Determinação da taxa de respiração em sementes de soja pelo método da titulação. Pesqui. Agropec. Bras. 29(10):1517-1521.
|
|
|
|
|
Delouche JC, Baskin CC (1973). Accelerated aging techniques for predicting the relative storability of seed lots. Seed Sci. Technol. 1(3):427-452.
|
|
|
|
|
Devi R, Muhjral N, Gupta AK, Kaur N (2007). Cadmium induced changes in carbohydrate status and enzymes of carbohydrate metabolism, glycolysis and pentose phosphate pathway in pea. Environ. Exp. Bot. 61:167-174.
Crossref
|
|
|
|
|
Dode JDS, Meneghello GE, De Moraes DM, Peske ST (2012). Teste de respiração para avaliar a qualidade fisiológica de sementes de girassol. Rev. Bras. Sementes 34(4):686-691.
Crossref
|
|
|
|
|
Dranski JAL, Pinto Jr AS, Herzog NFM, Malavasi UC, Malavasi MDM, Guimarães VF (2013). Vigor of canola seeds through quantification of CO2 emission. Ciênc. Agrotecnologia 37:229-236.
Crossref
|
|
|
|
|
Ferreira AG, Borghetti F (2007). Germinação: do básico ao aplicado. Porto Alegre: ARTMED 323 p.
|
|
|
|
|
Ferreira DF (2011). Sisvar: a computer statistical analysis system. Ciênc. Agrotecnologia (UFLA). 35(6):1039-1042.
|
|
|
|
|
Franco OL, Rigden DJ, Melo FR, Grossi-de-sa MF (2002). Plant – amylase inhibitors and their interaction with insect a-amylases. Eur. J. Biochem. 269:397-412.
Crossref
|
|
|
|
|
Krzyzanowki FC, Vieira RD (1999). Deterioração controlada. In: Krzyzanowki FC, Vieira RD, França Neto JB (Ed.). Vigor de sementes: conceitos e testes. Londrina: ABRATES, pp. 61-68.
|
|
|
|
|
Lamarca EV (2009). Taxas respiratórias e velocidade de deterioração de sementes de CaesalpiniaechinataLam. em função de variações hídricas e térmicas. Tese. Instituto de Botânica da Secretaria do Meio Ambiente. São Paulo, Brasil. 106 p.
|
|
|
|
|
Maguire JD (1962). Speed of germination-aid in selection and evaluation for seedling emergence and vigor. Crop Science, Madison 2(1):176-177.
Crossref
|
|
|
|
|
Marcos FJ (2005). Fisiologia de sementes de plantas cultivadas. Piracicaba: FEALQ. 495 p.
|
|
|
|
|
Marini P, Moraes CLM, Larré CF, Lima MC, Moraes DMD, Amarante LD (2013). Indicativos da perda de qualidade de sementes de arroz sob diferentes temperaturas através da atividade enzimática e respiratória. Interciencia 38:54-59.
|
|
|
|
|
Mendes CR, Moraes DM, Lima MGS, Lopes NF (2009). Respiratory activity for the differentiation of vigor on soybean seeds lots.Rev. Bras. Sementes 31:171-176.
Crossref
|
|
|
|
|
Moterle LM, Dos Santos RF, Scapim CA, Braccini AL, Bonato CM, Conrado T (2011). Efeito de biorregulador na germinação e no vigor de sementes de soja. Rev. Ceres 58(5):651-660.
Crossref
|
|
|
|
|
Nedel FB, Rocha M, Pereira J (1999). Anos de vida perdidos por mortalidade: um dos componentes da carga de doenças. Rev. Saude Publica 3:460-469.
Crossref
|
|
|
|
|
Nerling D, Coelho CMM, Nodari RO (2013). Genetic diversity for physiological quality of seeds from corn (Zea mays L.) intervarietal crossbreeds. J. Seed Sci. 35(4): 449-456.
Crossref
|
|
|
|
|
Pereira EM (2012). Avaliação da qualidade fisiológica de sementes de pimenta e pimentão por meio da atividade respiratória. 69 p. Dissertação (Mestrado em Fitotecnia)–Universidade Federal de Lavras, 2012.
|
|
|
|
|
Santos CMR, Menezes NL, Villela FV (2004). Alterações fisiológicas e bioquímicas em sementes de feijão envelhecidas artificialmente. Rev. Bras. Sementes 26(1):110-119.
Crossref
|
|
|
|
|
Shatters RGJR, Abdelghany A, Elbagoury O (1994). Soybean seed deterioration and response to priming: changes in specific enzyme activities in extracts from dry and germinating seeds. Seed Sci. Res. 4(1):33-41.
Crossref
|
|
|
|
|
Taiz L, Zeiger E (2009). Fisiologia Vegetal.4.ed. Porto Alegre: Artmed 819 p.
|
|
|
|
|
Venske EV, Abreu JJDS, Sousa ADM, Martins LF, Moraes DMD (2014). Atividade respiratória como teste de vigor em sementes de algodão. Rev. Bras. Ciênc. Agrárias 9:174-179.
|
|
|
|
|
Vieira RD, Carvalho NM, Sader R (1994). Testes de vigor e suas possibilidades de uso. In: Vieira RD, Carvalho NM. Testes de vigor em sementes. Jaboticabal: FUNEP. pp. 31-47.
|
|
|
|
|
Willyder LRP (2010). Testes de vigor em sementes de milho. 50 p. Dissertação (Mestrado em Fitotecnia) - Universidade Estadual Paulista, Faculdade de Ciências Agrárias e Veterinárias, Jaboticabal, 2010.
|
|
|
|
|
Woodstock LW (1973). Physiological and biochemical tests of seed vigor. Seed Sci. Technol. 1(1):127-157.
|
|
|
|
|
Zhang M, Maeda Y, Furihata Y, Nakamaru Y, Esashi Y (1994). A mechanism of seed deterioration in relation to the volatile compounds evolved by dry seeds themselves. Seed Sci. Res. 4(01):49-56.
Crossref
|
|





