Seaweeds are a source of natural antioxidants having potential application in oxidative stress and associated diseases. In this work, anti-atherogenic properties associated with the antioxidant activity from the hydrophilic extracts of Halimeda incrassata were studied. The phenolic content assessed in the aqueous extract and fraction phenolic acids (FPA) was 0.13 ± 0.05 and 0.47 ± 0.09 mg of gallic acid equivalents (GAE)/g dry seaweed, respectively. In DPPH•, radical scavenging assay fractions exhibited a dependent concentration. The seaweeds extract inhibited the desoxirribose oxidation in the presence or absence of EDTA (IC50 = 1.91± 0.09 mg/mL) (IC50 = 2.95 ± 0.01 mg/mL). In vivo antioxidant properties of FPA-H.incrassata were investigated in rats with a CCl4-induced liver injury. Pre-treatment with H. incrassata led to approximately 50% reductions in liver TBARS levels. The treatment with H. incrassata FPA also increased the activity of the CAT enzyme, which in turn resulted in an enhanced antioxidant defense. The expression of Catalase by PCR-RT technique demonstrated a higher gene expression when compared with that which was observed in the CCl4-treated group. Antiatherogenic properties were studied in the inhibition of lipoprotein oxidation mediated by Cu2+ or HRP/H2O2, free radical scavenging, and metal ion chelation, and it was dose dependent with a higher concentration needed for the aqueous extract than for the FPA fraction. Antioxidant activity was also improved in macrophages as evaluated in the cell supernatant (by TBARS formation); and by luminol enhanced chemiluminescence after cell activation with zymosan; and a degree of cell lipoperoxidation was decreased by the Halimeda incrassata extract. The results of this work add to the antioxidant potential of the seaweed for its application in oxidative stress associated conditions.
Oxidative stress is involved in a variety of pathologies and indeed it is presently considered a common factor in chronic non transmissible diseases as like atherosclerosis (Sanchez-Recalde and Kaski, 2001). Hence, among the most interesting alternatives for the modulation of oxidative stress as a target to halt the development of atherosclerosis, antioxidant effects have been sought at different stages of the disease: LDL oxidation, macrophage activation, foam cell formation, smooth muscle cell migration and advanced plaque remodeling (Kaliora et al., 2006); and then multiple mechanisms have been proposed for targeting an atherogenesis associated oxidative stress (Stocker and Keaney, 2004).
Seaweeds have combinations of highly developed antioxidant defense systems, allowing them to preserve structural integrity before different kinds of environmental stresses. However, they possess a wide diversity of bioactive compounds from amino acids, such as micosporines, polysaccharides, carotenoids, terpenoids, and an especially high phenolic content (Dutra-Rocha et al., 2007). Their unique composition makes them attractive candidates for their application in the antioxidant field. A correlation has also been found between the consumption of phenolic compounds in general, and seaweeds in particular, and the incidence of cardiovascular diseases (Bocanegra et al., 2009).
In the quest for more potent antioxidants from natural sources, our group has been especially interested in studying the beneficial properties of seaweed from the Halimeda genus for an application in biomedicine in hepato-, neuro- and athero-protection. Several lines of results have documented the ability of a hydrophilic extract from this seaweed to target free-radical mediated processes in vitro cell culture and in vivo experimental models (Rivero et al., 2003; Fallarero et al., 2003; Linares et al., 2004; Mancini-Filho et al., 2009; de Oliveira e Silva et al., 2012). In previous studies, in vitro Halimeda spp seaweeds have been described as having a relationship between antioxidant activity and antiatherogenic properties (Costa-Mugica et al., 2012). Additionally, it has been shown that Halimeda spp. has a high phenolic content (Vidal et al., 2009; Vidal et al., 2011), together with low amounts of other antioxidants, such as ascorbate, β-carotene, chlorophylls, and selenium, and that these compounds may be able to explain antioxidant properties.
Thus, in view of these previous considerations, the aim of this study was to evaluate the antioxidant properties of the hydrophilic fractions obtained from the green seaweed Halimeda incrassata, in free radical scavenging, and in animal models, and a possible relation to lipoprotein oxidation, and macrophage oxidative stress, as pathological stages of atherosclerosis.
Seaweed collection and hydrophilic extract preparation
The seaweed Halimeda incrassata (Ellis) Lamouroux (Chlorophyta, Bryopsidales) was collected during December 2011 in the Bajo de Santa Ana, La Habana, Cuba. Voucher specimens were authenticated by Dr. A. M. Suárez from the Seaweeds Laboratory at the Marine Research Center of the University of Havana. Freshly collected specimens were washed with distilled water and dried at room temperature for 3-5 days. After milling and sieving, the dry powder was used to obtain the hydrophilic extracts. The dry seaweed powder was extracted with distilled water (1:5 w/v) at room temperature and centrifuged at 800 g and at 4ºC for 20 min. The supernatant was recovered, lyophilized, and kept at -20ºC until use. The weight yield of the final extract in terms of dry seaweed was 5%. The lyophilized material was dissolved in distilled water at known concentrations for the different studies. The lyophilized material was denominated as being an aqueous extract.
Polyphenolic rich fractions were obtained according to Krygier et al. (1982). Tetrahydrofurane extraction was performed on the dry seaweed in order to obtain a fraction rich in free phenolic acids (FPA). The weight yield of the final dry fraction in terms of dry seaweed was 0.8%.
Total phenolic concentration
The total phenolic content was determined by the Folin-Ciocalteau assay as previously described by Vidal et al. (2009) and expressed as µg of Gallic Acid Equivalents (GAE) /g of sample. The calibration curve was obtained in the range of 100-1000 µg gallic acid/ml.
DPPH• radical scavenging assay
The free radical scavenging activity of the Halimeda incrassata extract was done similar to Goupy et al. (1999). Briefly, 0.6 ml of the extract (10 to 40 μg GAE) was mixed with 0.6 mL of a methanolic solution of DPPH• (60 μM). Absorbance was measured in time. Radical scavenging activity was calculated relative to the reference absorption as a Percentage Inhibition (PI) (%) = (1-Asample/Areference) × 100. IC50 was the antioxidant quantity needed (mg aqueous extract or fraction dry) to scavenge 50% of DPPH•.
Hydroxil scavenging and Fe3+ chelating capacity
Antioxidant protection in 2-desoxi-D-ribose oxidation by hydroxil radicals was quantified by the malondialdehyde formation according to Aruoma (1994). The reaction mix was 850 µL phosphate buffer (KH2PO4/KOH) 10 mM, pH 7.4, with or without 50 µL EDTA 100 µM and 50 µl FeCl3 25 µM. After 1 min, 50 µl 2-desoxi-D-ribose 2.8 mM and H2O2 2.8 mM were added. Reaction was initiated with 50 µL 100 µM ascorbic acid and stopped after 1 h at 37oC. The degree of oxidation was assessed by a TBARS formation. Samples were incubated with 1 ml TBA 1%, 1mL TCA 2.8%, at 80°C for 20 min. Absorbance was monitored at 532 nm. Without EDTA, the assay indicated the Fe chelating capacity of the extract, whereas with EDTA, it measured the hydroxil radical scavenging activity.
Antioxidant activity protecting against liver damage in CCl4 –induced Wistar rats
Animals and treatment schedule
Male Wistar rats from the University of São Paulo, Brazil, weighing 120 g–150 g, were maintained under a controlled diet, with cycles of 12 h of light/dark, at 25 °C and 60% humidity. The rats had free access to water and to a standard food diet according to the care guidelines for laboratory animals used in research. The animal studies were approved by the Institutional Ethical Committee for Animal Experimentation from the Faculty of Pharmaceutical Sciences (USP), Brazil.
Hepatic injury was induced in the rats by an intraperitoneal administration of a single dose of 3 mL CCl4 (mixed 1:1 with olive oil) on day 21. Gallic Acid (GA) was used as a reference.
The animals were grouped as follows:
Group I: Control, treated daily with vehicle (1 ml, p.o.) for 21 days.
Group II: Treated daily with vehicle (1.0 mL, p.o.) for 21 days, followed by treatment with CCl4.
Group III: Treated daily with an aqueous extract of Halimeda incrassata (300 mg/kg, p.o.) for 21 days, followed by treatment with CCl4.
Group IV: Treated daily with ferulic acid (20 mg/kg, p.o.) for 21 days, followed by treatment with CCl4.
At the end of the treatment (day 22), a blood sample and the liver of each animal was collected. ASAT and ALAT were determined by commercial laboratory kits (LABTEST).
TBARS assay
As a marker of lipid peroxidation, the TBARS contents were measured in the liver homogenates and serum of the animals using the method of Ohkawa et al. (1979). The results were expressed as nmol/mg protein.
Glutathione (GSH) analysis
The hepatic total of GSH content was measured using the method of Ellman (1959) as the change in absorbance was monitored at 410 nm for 5 min, and the GSH level was calculated by using pure GSH as standard.
Determination of superoxide dismutase (SOD) activity
The cytoplasmatic SOD activity was evaluated according to McCord and Fridovich (1969) by using 100 mM cytochrome C, 500 mM xanthine, 1 mM EDTA, and 200 mM KCN in 0.05 M potassium phosphate, pH 7.8. The xanthine oxidase (same volume in the blank) was placed in a glass tube along with 15 μl of the cytosolic fraction from each liver tissue. The results were expressed as U/mg protein. One unit (U) was the enzyme activity that induced 50% of inhibition of the xanthine reaction at 25 °C, pH 7.8.
Determination of catalase (CAT) activity
The activity was evaluated by the decomposition of hydrogen peroxide caused by the cytoplasmatic enzyme CAT according to Beutler (1975), through the decrement of the optic density at 230 nm (coefficient of the molar extinction 0.0071 mM–1 cm –1) at 37 °C. One U of CAT corresponded to the enzyme activity that hydrolyzed 1 molecular weight of H2O2 per minute at 37 °C, pH 8.0. The activity was expressed as U/mg of protein.
Expression of hepatic enzymes in rats by RT/PCR
RNA Extraction: CAT and SOD gene evaluation
RNA was extracted from the rat liver utilizing a 100 mg sample and 1 mL of trizol reagent (Invitrogen). The extract was kept at room temperature for 5 min with the addition of 200 μl chloroform (Merck). The samples were mixed by vortexing for 15 s and kept at room temperature for 5 min. After this, they were centrifuged at 12,000 × g for 15 min at 4 °C. 400 μl of the supernatant was removed, avoiding the interphase, and mixed with 500 μl of isopropanol by vortexing for 5 s. These samples were then centrifuged at 12,000 × g for 5 min at 4 °C, discarding the supernatant. To the resulting pellet, 1000 μl of ethanol (75%) was added and gently mixed, followed by centrifugation at 7500 × g for 10 min at 4°C, discarding the supernatant. Finally, 20 μl of distilled water, RNAse-free, was added and incubated at 50 °C for 10 min. This material was then stored at -70°C.
Reverse transcription
2 μg of RNA were added to 1.0 μl of primers (SOD or CAT), 1.0 μl of 10 mM dNTP, and 4.0 μL of sterile distilled water. The reaction was started by heating at 65°C for 5 min; then it was quickly chilled on ice. Next, 4.0 μl of 5× first strand buffer (Invitrogen), 2.0 μl of 0.1 M DTT (Invitrogen), and 1.0 μl of RNAseOut Ribonuclease inhibitor (Invitrogen) were added and incubated at 37°C for 2 min. After that, 1.0 μl (200 U) of reverse transcriptase (M-MLV RT-Invitrogen) was added and incubated at 37°C for 50 min. The reaction was stopped by heating at 70 °C for 15 min. The PCR product (cDNA) was stored at -70°C.
PCR reaction for amplification
5 μl of cDNA was amplified in a volume of 50 μl containing 5 μl 20 mM Tris-HCl (hydroxymethyl aminomethane-hydrochloride) buffer, pH 8.4, 500 mM KCl, 1.5 μl 50 mM MgCl2, 1 μl 10 mM dNTP, 35.1 μl diethyl pyrocarbonate (DEPEC), 1.0 μL of primers (SOD or CAT), and 0.4 μL (5 U/μl) Taq polymerase. This reaction mixture was warmed by a thermal cycler (Bio-Rad) at 94°C for 3 min and 35 cycles of 45 s at 94°C, 30 s at 55°C, 1.3 min at 72°C, and 72°C for 10 min. Finally, the mixture was cooled at 4°C for an indeterminate time. The PCR amplified product was analyzed by a 2.0% agarose gel (Sigma) electrophoretic run (60 V). The bands, stained with 0.5 μg/mL ethidium bromide, were documented by a fluorescent table (Vilber-Lourmat) and photographed by a digital camera (Sony). The bands were revealed with –262 bp (C to T) from the primers used to CAT genotyping and +242 bp (C to T) from the primers used to SOD genotyping (Promega, Madison/USA):
Primer SOD 1 – sequence (5’ to 3’): TCT AAG AAA CAT GGC GGT CC.
Primer SOD 2 – sequence (5’ to 3’): CAG TTA GCA GGC CAGCAG AT.
Primer CAT 1 – sequence (5’ to 3’): GCG AAT GGA GAG GCA GTG TAC.
Primer CAT 2 – sequence (5’ to 3’): GAG TGA CGT TGT CTT CAT TAG CAC TG.
Effect of hydrophilic fractions on the inhibition of LDL oxidation mediated by Cu2+ and HRP/H2O2
Oxidation experiments were conducted with heparin precipitated LDL (hep-LDL), a model of LDL that has interacted with extracellular matrix, and is, therefore, more prone to oxidation (Upritchard and Sutherland, 1999). Lipoproteins were isolated from normolipemic human serum by the method of Wieland and Seidel (Wieland and Seidel, 1983). 5 ml sodium citrate buffer (64 mmol/L, pH 5.12) containing heparin (50 000 UI/L) was added to 0.5 mL serum. After incubating for 10 min at room temperature the sample was centrifuged at 3000 rpm for 15 min. The Hep-LDL precipitate was washed 3 times with a Hepes buffer (5 mM Hepes, 20 mM NaCl, 4 mM CaCl2 and 2 mM MgCl2, pH 7.2), then by centrifuging at 3000 rpm for 15 min; the hep-LDL was was next dissolved in a 0.5 mL phosphate buffer, pH 7.4, with NaCl 4%. The Hep-LDL fraction was next divided into aliquots and kept at 4ºC. The cholesterol content was determined by an enzymatic assay (Boehringer Mannhein Diagnostics) and the protein content by the Lowry method.
In brief, for the oxidation, LDL (0.2 μmol cholesterol) was diluted in a phosphate buffer and incubated in the presence or absence, of hydrophilic fractions (aqueous extract and FPA fraction) for 6 h at 37oC, with 10 μM Cu2+ , or HRP (119 U)/H2O2 (12.9 µM). The maximum degree of oxidation was determined by TBARS as described in Frostegard et al. (1990) and expressed as nmoles MDA equivalents/ mg protein, using TMP as standard.
Antioxidant activity of hydrophilic extracts in macrophages
Cell experiments were done with the macrophage RAW 264.7 cell line. Cells were cultured in DMEM containing fetal bovine serum (FBS), 2 mM L-glutamine and streptomycin/penicillin in 5% CO2.
TBARS formation by cells
For the assessment of antioxidant activity, cells were pre-incubated for 24 h with an aqueous extract of Halimeda Incrassata. Lipoperoxidation levels were evaluated in the supernatant by a TBARS assay as in Frostegard et al. (2003).
ROS production
ROS production by cells was determined in conditions similar to Kopprasch et al. (2008). Luminol 4 µM was added to cells in a 50 mM Hepes buffered DMEM. After adding seaweed, aqueous extracts cells were stimulated with opsonized zymosan (OZ) 1 mg/mL. Chimioluminiscent response was measured in time, and amplitude of the curve was taken as maximum ROS production. Experiments were done with a Lumi-Aggregometer from Chrono-Log Corporation with AGGRO/LINK software version 5.2.3.
Statistical analysis
Values are given as mean + standard deviation (s.d.) of experiments that were done in triplicate, and performed at least two independent times. In studies of the antioxidant activity in the cell systems, statistical significance was determined by ANOVA with a Tukey posttest. Significant differences were concluded for p < 0.05. Data were processed using Microcal Origin and GraphPad Prism software.
Over the last few years, seaweeds have been widelyinvestigated as a source of bioactive compounds, with different attributes, and in this context, the genus Halimeda has been studied for different pharmacological properties, including antioxidant activity (Moo-Puc et al., 2008; Nor et al., 2010).
Phenolic content
The phenolic content, as assessed in the Aqueous Extract and by the Fraction Phenolic Acids (FPA), was 0.13 ± 0.05 and 0.47 ± 0.09 mg Gallic Acid Equivalents (GAE)/ g dry seaweed, respectively. Both fractions had a high phenolic content.
The phenolic content found is in the range of the one for seaweeds of Halimeda spp. worked by our research group (12-13) and higher than for other seaweeds informed in the literature like Fucus vesiculosus (Phaeophyceae) and Caulerpa racemosa (Chlorophyta) (Jimenez-Escrig et al., 2001).
The phenolic contribution to antioxidant activity was evaluated in the hydrophilic fractions. When comparing different fractions from the seaweed Eisenia bicyclis (Phaeophyceae), Kim et al. (2011) found that the highest phenolic content was associated with the antioxidant activity and the hepatoprotective effect, against tert-butyl hyperoxide damage (t-BHP) in hydrophilic fractions from the seaweed.
In our previous work, Vidal et al. (2009) identified 8 phenolic acids in Halimeda opuntia and H. monile (Chlrophyta) respectively. They reported that salicylic, cinnamic, gallic, pirogalic and cafeic acids were the principal polyphenolic compounds in both seaweeds. In Halimeda incrassata, it was identified that there were major polyphenolicqq compounds of salicylic and ferulic acids, and they suggested that their levels were related to the antioxidant activity of the seaweed (Vidal et al., 2011).
Likewise, the antiatherogenic activity of phenolic compounds has been studied when considering their antioxidant properties as being the main mechanism of action (Bocanegra et al., 2009; Jimenez-Escrig et al., 2001)
Antioxidant activity in vitro: DPPH• radical scavenging and hydroxyl scavenging and Fe3+ chelating capacity
DPPH• radical scavenging was determined for the Aqueous Extract and FPA. Both fractions exhibited a concentration dependent on free radical scavenging activity (Figure 1). The FPA fraction had an IC50 value of 0.46 mg of dry residue (27.1 µg of polyphenol); while the Aqueous Extract had an IC50 value of 5.75 mg of lyophilized substance (14.8 µg of polyphenol).
DPPH• radical scavenging has been widely used to study the activity of antioxidant molecules and plant extracts. It is considered, that presently, more than 90% of antioxidant studies use this method in combination with other assays (Goupy et al., 1999). In this work, free radical scavenging by DPPH• assay indicated a dose-dependent effect, and in comparison with Halimeda incrassata, a six-fold lower activity for Caulerpa racemosa, a more than 30-fold decrement in Ulva lactuca, and a similar activity in Sargasum spp.( Yangthong et al., 2009).
Antioxidant activity by DPPH suggests phenolic compounds are relevant to the effect. Different authors (Dutra-Rocha et al., 2007) have indicated an association between the phenolic content of seaweeds and DPPH• scavenging. Previous results from our group, regarding Halimeda genus, have indicated an association of antioxidant activity in DPPH scavenging with phenolic content (Vidal et al., 2009; 2011). Indeed, Katsube et al. (2004) found a tendency of higher antioxidant activity in the inhibition of LDL oxidation, and of DPPH• scavenging in plants with an increasing phenolic content. Hydroxyl radicals are a main initiator of lipid peroxidation. Thereafter, hydroxyl radical scavenging is a relevant indicator of the antioxidant activity of a natural compound, and in this area, plant polyphenols are compounds of interest, as they can react with these radicals to avoid oxidative damage (Stocker and Keaney, 2004).
Aqueous seaweed extract inhibited desoxiribose oxidation with a dose-dependent effect both in the presence of EDTA (Figure 2A) (IC50 = 1.91± 0.09 mg/mL), or in absence of EDTA (Figure 2B) (IC50 = 2.95 ± 0.01 mg/mL Fe chelating). Additionally, aqueous extracts had a concentration-dependent antioxidant effect, both with and without, EDTA, which is stronger than the one referred to for various antioxidant extracts (Vidal et al., 2006).
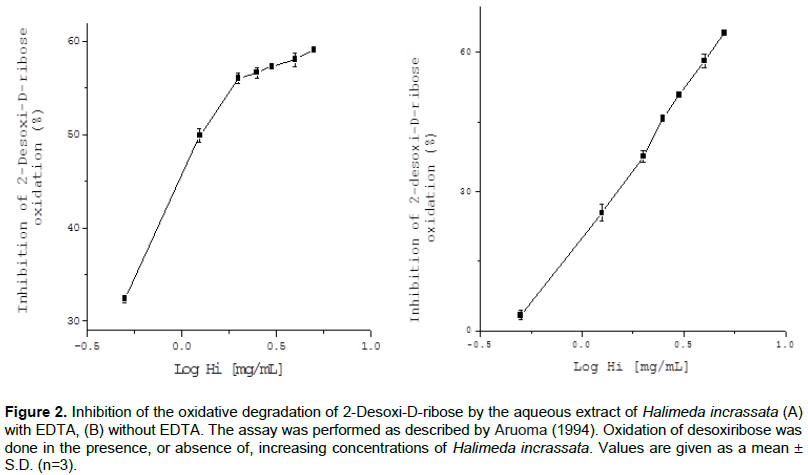
Antioxidants in Halimeda incrassata seaweed might offer protection from damage, by avoiding an attack by OH• radicals, generated by a Fenton reaction in the presence of EDTA. On the other hand, when Fe3+ is added to the reaction milieu (in the absence of EDTA), some ions might join desoxiribose sugar and take part in the Fenton reaction. Antioxidant activity in this assay would then show the Fe3+ chelating capacity of the extracts. Thus, our results also indicated that the extracts have the capacity to interfere with the site specific generation of OH•, catalyzed by Fe 3+ ions, bound to desoxiribose. Indeed, other authors have remarked that the presence of phenolic compounds in seaweeds confers them with a heavy metal chelating capacity, which is also related to OH. radical scavenging (Vidal et al., 2006; Yuan and Walsh, 2006).
Antioxidant activity protecting against liver damage in CCl 4-induced Wistar rats
Antioxidant and hepatoprotective properties of the aqueous extract from H. incrassata were investigated in Wistar rats with a CCl4-induced liver injury. Through the ASAT and ALAT activities (Table 1), it verified partial liver injuries caused by CCl4. We observed that the rats treated with an aqueous extract from H. incrassate, or Ferulic acid, proved to be capable of attenuating the toxic effect produced by CCl4. As will be appreciated, it was observed that a partial recuperation occurred with an aqueous extract from H. incrassata. In previous work, Mancini et al. (2009) reported similar results when investigating polyphenol fractions from Halimeda monile. These results are also in accordance with de Oliveira et al. (2012) whom investigated the hepatoprotective properties of polyphenolic fractions from H. opuntia under an experimental model CCl4 injury. Tanigushi et al. (Tanigushi et al., 2004) reported that hepatic damage by CCl4 may occur in a range of between 6 to 12 h after CCl4 administration, and that restoration starts after 48 h. It is possible then to suppose that FPA- H. incrassata-treated rats had a faster recovery from liver injury at 24 h than expected, criterion that is concordant with our results.
In Table 1, it may be appreciated that TBARS levels in the liver tissues of CCl4-treated rats increased, confirming the successful induction of oxidative damage, while pre-treatment with H. incrassata (300 mg/kg) led to an approximate 50% reduction in liver TBARS levels. A previous study from our laboratory showed that an aqueous extract from H. incrassata was effective in significantly reducing serum and brain TBARS levels and other parameters in rats with oxidative stress induced by methyl-mercury (2004). According to de Oliveira e Silva et al. (2012), a pre-treatment with polyphenol rich fractions from Halimeda opuntia led to reductions in serum and liver TBARS. Kim et al. (2011) also observed a comparable reduction in hydroperoxide levels in the liver, relative to the CCl4-treated group, in a study of rats fed on Saengshik, a non-cooked food containing vegetables and seaweeds.

Animals with liver injuries caused by CCl4 had GSH levels increased statistically in respect of all groups, while in an aqueous extract from the H. incrassata group was observed with only minor values. According to Chan et al (Chan et al., 2001), these increased levels may be explained as an adaptive response of the rats reacting against the oxidative stress introduced by CCl4.
The CAT and SOD enzymes are considered to be as a fundamental antioxidant defense system in mammals, and it was demonstrated that CCl4 treatment significantly reduced the activities of these enzymes. In this study, we observed the ability of CCl4 to diminish the antioxidant enzyme activities.
As may be appreciated in Figure 3, treatment with the seaweed led to a significant increase in the activity of the CAT enzyme, which in turn resulted in an enhanced antioxidant defense. These results suggest antioxidant and hepatoprotective activities of the phenolic fraction of H. incrassata. Ozturk et al. (2003) observed in the CCl4-treated group significant increases in kidney CAT activity. These results are in agreement with Mancini-Filho et al. (2009) that reported a considerable increase in the activity of CAT in rats treated with a polyphenol-rich fraction similar to that from Halimeda monile. High antioxidant enzyme activity has been reported through repeated administration of Sargassum spp. (Phaeophyceae) extracts (Raghavendran et al., 2005). Treatment with Caulerpa prolifera (Chlorophyta) and Laurencia obtusata (Rhodophyta) extracts also led to a rise in enzyme activity (Abdel-Wahhab et al., 2006).

Expression of CAT hepatic enzymes by PCR- RT
When considering results from antioxidant enzyme activities, only the expression of Catalase by PCR-RT technique was studied. As can be seen in Figure 4, the levels of CAT in liver tissues partially increased with a treatment of seaweed and a posterior CCl4 administration, which shows alterations in the expression of catalase genes.
Treatment with H. incrassata aqueous extract (band 3) resulted in a higher catalase gene expression when compared with that observed in the CCl4-treated group (band 2). A review by Stevenson and Hurst (2007) discusses recent evidence that polyphenols also have an indirect antioxidant effect through the induction of endogenous protective enzymes, and that these inductive or signaling effects may occur at concentrations much lower than those required for effective radical scavenging. Vidal et al. (2011) reported that a total phenolic contents of the hydrophilic fractions from H. incrassata were 255 μg of gallic acid equivalents/g of fresh seaweed, which more than half (63%) corresponds to free phenolic acids, and in this fraction, about 32% was identified as salicylic acid, while a small fraction was associated to ferulic acid. Yeh and Yen (2006) suggested that these three phenolic acids, including ferulic acid, modulate the phase II antioxidant enzymes and the phase II sulphate conjugative enzymes; and they seem to selectively induce hepatic mRNA transcripts for CAT, probably through the up-regulation of gene transcription, as well as the Nrf2 transcription factor. In previous results from our group, Mancini-Filho et al. (2009) reported an over-expression of CAT genes by treatment with FPA from Halimeda monile; while de Oliveira e Silva et al. (2012) showed that by using (RT/PCR) analysis increased the catalase (CAT) gene expression in the group treated with free phenolic acid (FPA) fractions from Halimeda opuntia, suggesting inducing effects on the enzyme.
Effect of Halimeda incrassata hydrophilic fractions on the inhibition of LDL oxidation mediated by Cu2+ and HRP/H2O2
The atheroprotective potential of Halimeda incrassata was determined through its effect on LDL oxidation. The inhibition of LDL oxidation by Cu2+, or HRP/H2O2, was dose dependent, with a higher concentration needed for the aqueous extract than for the FPA fraction (Table 2). It was compared with the two oxidation systems relevant to LDL oxidation in the artery wall: by Cu2+, or HRP/H2O2; and by studying the antioxidant effect in the mediated transition metal and independent LDL oxidation (Stocker and Keaney, 2004). As shown, seaweed hydrophilic fractions had an inhibitory effect on both models of LDL oxidation, indicating its potential in atheroprotection.
The inhibition of LDL oxidation is considered a key target in atherosclerosis management, since oxidized LDL levels are presently one of the main emerging factors for cardiovascular risk (Kang et al., 2003; Levitan et al., 2010). In vivo is a complex process, and there is no certainty as to the precise mechanism of the initiation of lipoperoxidation in the vascular wall (2004). In the quest for the atheroprotective potential of natural compounds, different researchers have determined the antioxidant properties of seaweed extracts in the inhibition of LDL oxidation (Bocanegra et al., 2009; Jimenez-Escrig et al., 2001; Yang et al., 2011).
The activity of the inhibition of LDL-oxidation found in this work is promissory when compared to other natural extracts. Hseu et al. (2008) found 37% inhibition of TBARS formation in oxidation mediated by AAPH and 74% in peroxidation mediated by Cu2+ ions. In this study, the Toona sinensis extracts had 6.5 μg GAE, a phenolic content in the range of the one needed in our study for the 50% inhibition of TBARS formation by Halimeda incrassata extracts.

LDL oxidation studies were done with LDL that had interacted with heparin as a model of glycosaminoglycan since it has been reported that lipoproteins that have interacted with glycosaminoglycans are more susceptible to oxidation (Upritchard and Sutherland, 1999). In the case of peroxidase, this is associated with a change in the lipoprotein structure, giving an increased access of peroxidase to apo B, and forming free radicals from apo B and vitamin E that would mediate the oxidation of lipids by mieloperoxidase and HRP. The mechanism is different from Cu2+ mediated oxidation, where free radical formation and peroxidation takes place directly in the lipid phase.
Other authors have evaluated the effect of natural extracts in the inhibition of LDL oxidation by peroxidases. Wang et al. (2003) found a significant decrement in TBARS formation in extracts from C. mukul on LDL oxidation mediated by lypoxigenases. The extracts also had an antiatherogenic action in the inhibition of cholesterol uptake by macrophages and LDL oxidation mediated by Cu2+ ions.
Few studies approach the effect of antioxidants in LDL oxidation mediated by HRP. It has been shown that in this area, vitamin E acts by transferring radicals, from the aqueous to the lipid phase, and does not protect it from oxidation. However, in the presence of vitamin C, oxidation mediated by peroxidases is inhibited, as in this case, vitamin C acts as a co-antioxidant avoiding the formation of α-tocoferoxil (Upritchard and Sutherland, 1999). Other antioxidants, like some phenolic hydrophilic compounds, act synergistically in the scavenging of free radicals protecting vitamin E, licopene and β-carotene, contained in LDL from oxidation (Kaliora et al., 2006). The antilipoperoxidative activity of the hydrophilic fractions of Halimeda incrassate, in this model, indicates that the extracts are efficient in the inhibition of oxidation mediated by protein radicals, and add evidence to the antiatherogenic and antioxidant potential.
Other authors have obtained an excellent activity of antilipoperoxidation for Halimeda incrassata in β-carotene linoleate systems, with an inhibition of bleaching of 75% for Halimeda incrassate, while for Bryothamnion triquetrum seaweed , the activity was 20% at a dose of 2 mg (Rivero et al., 2003; Vidal et al., 2011).
Our results are also comparable to the work of Yuan and Walsh (2006) with a significant inhibition of conjugated dienes and TBARS formation, during the oxidation of linoleic acid for an aqueous extract from the Palmaria palmate seaweed, suggesting that the antioxidant activity was due to the complex mixture of antioxidants present in the extract, with the presence of chlorophyll, polyphenols, carotene, and ascorbate.
Association of antioxidant activity in Fe Ions chelation and the inhibition of oxidation of LDL mediated by Cu2+
The association of antioxidant activity and the inhibition of LDL-oxidation, evaluated by different methodologies in this paper, were performed to explain the antioxidant mechanisms of Halimeda incrassata. Figure 5A and B indicate the positive correlation between the inhibition of desoxiribose oxidation, with or without EDTA, and the inhibition of LDL oxidation mediated by Cu2+ (r2= 0.997 and 0.943 respectively).
In this work, a positive correlation was found between the scavenging of OH• radicals in the inhibition of the desoxiribose assay in the presence of EDTA (r2 = 0.997), and the inhibition of LDL oxidation mediated by Cu2+. A similar behavior was obtained for the chelating effect of metal ions in the inhibition of desoxiribose oxidation assay in the absence of EDTA (r2 = 0.943) and the inhibition of LDL oxidation mediated by Cu2+.
The results of the correlation of antioxidant activity in Fe ion chelation and the inhibition of oxidation of LDL mediated by Cu2+ suggest that the mechanism of action in the inhibition of LDL oxidation by hydrophilic fractions could be associated with the antioxidant properties of the seaweed in metal ion chelating and free radical scavenging.
Antioxidant activity of hydrophilic extracts of Halimeda incrassata in macrophages
As shown in Figure 6A, the degree of cell lipoperoxidation was decreased by about two-fold in the presence of Halimeda incrassata seaweed extract. The FPA fraction also inhibited TBARS production, indicating the contribution of phenolic compounds to the effect.
The improvement of antioxidant activity was also shown by the 1.2 fold decrement in ROS levels by the treatment with Halimeda incrassata aqueous extract, when aqueous extracts were added immediately before stimulation (Figure 6A), or by a nearly 50% decrement in ROS levels, when aqueous extracts were added 24 h before stimulation of the cells (Figure 6B).
The macrophages associated with oxidative stress are directly involved in the atherosclerosis progression, so when evaluating compounds of interest for atheroprotection antioxidant activity, it is frequently sought at the macrophage level (Kaliora et al., 2006).
A decreased peroxide basal content was found in the cell supernatant as a result of the preincubation with seaweed hydrophilic extracts. About a 3.3 fold higher concentration was required from the FPA fraction for it to reach a similar inhibition of MDA formation as to that of1.5 µg GAE/mL (0.5 mg/mL) for the aqueous extract. To evoke ROS production in macrophages, opsonized zymosan was added to the cells after the addition of aqueous seaweed extracts. About a 20% decrement in ROS production was found with the highest concentrations of seaweed used in the assay. Seaweed preincubation with the cells improved the antioxidant capacity with a 50% decrement in ROS production with the highest concentration tested (1 mg/mL).
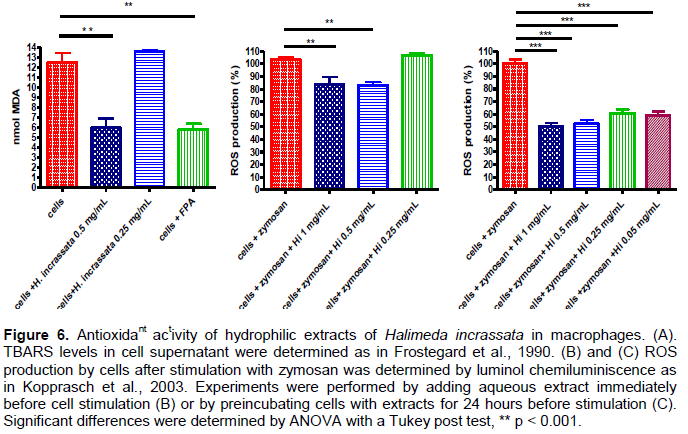
These results are in agreement with previous studies by our group for the aqueous extracts of Halimeda incrassata (Chlorophyta) and Bryothamnion triquetrum (Rhodophyta) seaweeds, and where basal and H2O2 elicited peroxides were decreased in the GT1-7 hypothalamic cells in the presence of the seaweeds (Fallarero et al., 2003).
Indeed, the antioxidant activity of natural extracts in macrophages has also been assessed by other authors that have found a decreased macrophage peroxidation and ROS production by incubation with phenolic rich extracts. Yang et al. (2011) found a 1.5% decrement in the peroxide levels in oxLDL stimulated macrophages, after preincubation with 1mg/mL mulberry leaf extracts (where quercetin, gallocatechin gallate and naringenin were the main polyphenolic constituents identified). The atheroprotective effect was related to an elevation of antioxidant enzymes GPx and SOD by the extracts.
Likewise, a significant inhibition of peroxidation was found in cells stimulated with oxLDL after preincubation with anthocyanin rich purple sweet potato extracts (0.5-0.6 mg/mL); that also inhibited the LDL uptake by macrophages and had antioxidant activity in DPPH• scavenging (Park et al., 2010).
The antioxidant activity in macrophages has been studied by other groups that have found improved in vitro and in vivo antioxidant capacity, as a result of different phenolic rich extract supplementation (Aviram et al., 2008). Targeting oxidative stress as a cause of atherosclerosis progression, they have correlated phenolic content, in vitro antioxidant activity in DPPH• radical scavenging, and the atheroprotective effect in macrophages (evaluated as the inhibition of LDL uptake, decreased macrophage peroxidation, and increased antioxidant enzyme activity) to be in vivo atheroprotection in apo E-/- mice.