ABSTRACT
The aim of this study was to evaluate the effect of indigenous yeasts on the aroma of fermented Chenin Blanc grape must by determining the volatile compounds, the odor activity value (OAV) and carrying out sensory analyses. The must, fermented by Hanseniaspora opuntiae/Saccharomyces cerevisiae combinations, presented higher concentrations of compounds such as ethyl acetate, ethyl hexanoate, 2-methyl-1-propanol and 2-phenylethanol, and a lower production of acids and acetaldehyde. This fermented must presented higher OAV values for compounds such as 2-methyl-1-propanol and ethyl acetate, 687.06 and 1264.16, respectively. 2-phenylethanol was produced by H. opuntiae and Hanseniaspora guilliermondii in combination with S. cerevisiae in amounts that resulted in OAV values of 5.63 and 4.62, respectively. In appropriate concentrations, these volatile compounds contribute positively to the aromatic quality of the fermented must. The highest mean acceptability and purchasing intention scores were obtained by the must fermented by H. opuntiae/S. cerevisiae. In the must fermented by H. guilliermondii/ S. cerevisiae, the absence of ethyl hexanoate and high concentrations of octanoic acid and acetaldehyde probably contributed to its low acceptability. Thus, it was suggested that the yeast genus Hanseniaspora in combination with S. cerevisiae, showed the potential to positively impact the wine aroma.
Key words: Non-Saccharomyces yeasts, volatile compounds, odor activity value, acceptability test, Hanseniaspora, “São Francisco Valley region”.
Grape juice in natural or spontaneous alcoholic fermentation, is routinely dominated by strains of the yeast
Saccharomyces cerevisiae, but
non-Saccharomyces yeasts such as Candida,
Hanseniaspora,
Pichia,
Torulaspora,
Hansenula,
Issatchenkia,
Metschnikowia,
Kluyveromyces and
Zygosaccharomyces initiate the process (
Fleet, 2003; González et al., 2006; Lopandic et al., 2008). Non-
Saccharomyces yeasts develop in the early stages, but do not survive to the end of fermentation. Nevertheless, they produce sufficient biomass to impact the chemical composition of the wine, and the contribution of these yeasts to the overall wine character is important (Swiegers and Pretorius, 2005)
.
Some studies have suggested that different yeasts (Torrens et al., 2008; King et al., 2011; Robinson et al., 2011) due to their genetic differences (Bisson and Karpel, 2010) can influence the volatile composition, and consequently the aroma and type of the wine. For example, some
Hanseniaspora/
Kloeckera species may produce more appealing mixtures of flavor volatiles than
Saccharomyces species (
Mendoza et al., 2009; Moreira et al., 2008; Assis et al., 2014),
Hanseniaspora osmophila produces more 2-phenylethyl acetate than a pure culture of
S. cerevisiae (Viana et al., 2009). Thus carrying out wine fermentations by co-inoculating with mixtures of different starter cultures, is a strategy which could harness the unique activity of such yeasts (Fleet, 2008).
The influence of the volatile compounds on the final aroma depends on their concentration in the wine, and on the perception threshold of the specific compound. A volatile compound contributes to the aroma when its concentration in the wine is above the perception threshold, therefore odorants with odor activity value (OAV) > 1 can be perceived (Guth, 1997; Jiang and Zhang, 2010; Juan et al., 2012; Welke et al., 2014). Capone et al. (2013) identified 51 volatile compounds in Negroamaro red wines, and of these, only 18 components were perceived as active odorants (OAV > 1).
A combination of analytical and sensory methodologies has been particularly important in resolving the effects of interactions between aroma compounds and the nonvolatile matrix, as well as with other volatile compounds (Mamede et al., 2005; Pineau et al., 2009; Aleixandre-Tudo et al., 2015). According to Robinson et al. (2014) these interactions may result in variations in the sensory character of the mixture, due to perceptual enhancement and suppression effects as well as to physicochemical effects on the volatility and release of aroma compounds.
The São Francisco Valley is the second most important wine production region in Brazil. This region stands out for the volume of grapes produced and the geochemical characteristics that favor the production of white wine and sparkling wine. Wine making demands innovation, so the aim of this study was to investigate the contribution of using a combined inoculation of indigenous non-Saccharomyces yeasts (Hanseniaspora opuntiae, Hanseniaspora guilliermondii, Issatchenkia terricola and Cryptococcus flavescens), obtained from the natural ecosystem in the “São Francisco Valley region”, together with the commercial Saccharomyces cerevisiae var. bayanus, on the aroma of the fermented Chenin Blanc grape must. The results are discussed based on the data obtained in the quantification of the volatile compounds/OAV and a sensory analysis.
Yeast strains
Hanseniaspora opuntiae, Hanseniaspora guilliermondii, Issatchenkia terricola and Cryptococcus flavescens were obtained from grapes cultivated in the municipality of Lagoa Grande (PE-Brazil) at 361 meters, 8°59'47'' south latitude and longitude 40°16'18", and isolated using the method of Assis et al. (2012). The non-Saccharomyces yeasts were identified by sequencing the D1/D2 region of the 26S subunit of the ribosomal RNA (Kurtzman et al., 2011). The pure colonies were maintained on Yeast Malt Agar (YMA) (1% glucose, 2% agar, 0.5% peptone, 0.3% malt extract and 0.3% yeast extract) slopes with the addition of sterile glycerol (Vetec), and kept under refrigeration (4°C).
Physicochemical analyses
The pH was determined using a digital pH meter (pHtek/P100, USA). The total soluble solids were determined using a refractometer (Atago/2313- USA), and the results expressed as °Brix. The SO2, titratable acidity and nitrogen concentrations present in the grape musts were determined according to Brazil (2005). These analyses were carried out on the unfermented must. The alcohol content, residual sugar, volatile acidity and titratable acidity were determined in the fermented grape must, using the method described in Brazil (2005). The alcohol content was determined by hydrostatic balance Densi -Mat (Gibertini ®, Italia) at 20°C, after a previous distillation into Automatic distiller of drinks Super D.E.E.R (Gibertini ®, Italia) and the dried extract using the AlcoMat-2 reading module of the same hydrostatic balance. The total residual sugars of musts fermented was determined by the Lane-Eynon method in which the products of residual sugars, in alkaline medium, are oxidized by copper of the Fehling's, in which it is forming a complex cupro-tartaric-sodium-Potassium. The total acidity was analyzed by the neutralization of titratable acid with 0.1 N NaOH solution. The titratable acidity was determined with the aid of the distiller Super D.E.E.R, and the distillate titrated with 0.1N NaOH, using phenolphthalein as the indicator, through the titrator Quick Analyzer (Gibertini ®, Italia). All analyses were carried out in triplicate.
Fermentation conditions
Chenin Blanc grape must, donated by Vinibrasil S/A, located in the state of Pernambuco (Brazil), was used for fermentation. The four non-Saccharomyces yeasts were inoculated individually and in combination with Saccharomyces cerevisiae var. bayanus, according to the method described by Assis et al. (2014). The inoculums of S. cerevisiae var. bayanus, H. opuntiae, H. guilliermondii, I. terricola and C. flavescens were previously cultured in YMA (1% glucose, 2% agar, 0.5% peptone, 0.3 malt extract e 0.3 yeast extract) medium, and incubated in a BOD incubator (Alfakit) for 48 h at 28°C. A suspension of each yeast was obtained in Milli-Q water. They were then standardized at 1 x 106 yeast cells.mL-1 according to the McFarland scale, and readings made in a spectrophotometer (Femto 800 XI) with an optical density range of 0.08 to 0.1, and wavelength of 625 nm. An aliquot of 1 ml of the standardized suspension was removed and inoculated into 250 ml conical flasks containing 83.3 ml of Chenin Blanc grape must. The samples were placed in a model TE-424 temperature controlled orbital shaker (Tecnal) at 100 rpm for 168 hours (7 days) in triplicate, at a temperature of 15ËšC. After fermentation, the musts were filtered through a Millipore membrane (0.22 μM pore) to carry out the analyses. The commercial yeast S. cerevisiae var. bayanus (Maurivin) was used as the control in the fermentation process.
Volatile compound analysis
The determinations of higher alcohols, esters and carboxylic acids were carried out according to Webber et al. (2014), as reported in the Reference Laboratory of Enology – LAREN (Caxias do Sul-RS, Brazil). A HP 6890 Agilent Technologies gas chromatograph (Palo Alto, USA) was used with a flame ionization detector (GC-FID). The compounds were identified by comparison with authentic standards from Sigma–Aldrich under the same chromatographic conditions.
Determination of the esters, alcohols and volatile acids
The samples of the fermented cell free must were subjected to three liquid/liquid extractions (4:2:2) with a mixture of diethyl ether/n-hexane (1:1). Aliquots of 2 ml of 3-octanol (40 mg.L-1) and 2 ml of heptanoic acid (50 mg L-1) were added to 50 ml of fermented Chenin Blanc grape must as internal standards, plus 0.3 ml of phosphoric acid (1:3). After decantation in a separation funnel, the organic phases were pooled, constituting the extract. A CP Inowax (30 m, 250 µm and 0.25 µm) capillary column was used and the sample injected (2.0 µL) in the splitless mode at 60 ml min-1 and 240°C. The carrier gas was hydrogen 5.0 at 2.0 ml min-1, and the nitrogen as auxiliary gas at 37 ml min1. The oven temperature was 40°C for 5 min; followed by 40 to 230°C at 3°C min.-1; and finally 230°C for 20 min. Combustion maintained with a synthetic air was used at an airflow of 350 mL/min and nitrogen at 35 mL.min-1. The detector temperature was 230°C.
Determination of higher alcohols, acetaldehyde, ethyl acetate and methanol
After a simple distillation, 70 µL of a 4-methyl-2-pentanol solution (5 g L-1, internal standard) were added to 5 ml samples of the fermented cell free must. A CPWax 57CB capillary column (60 m, 250 µm and 0.25 µm) was used, and the injection (1.0 µL) done in the split mode at 60 ml min-1 and 220°C. The carrier gas was hydrogen 5.0 at 2.0 ml min-1 and the nitrogen as auxiliary gas at 37 ml min1. The oven temperature was 40°C for 5 min, followed by 40 to 90°C at 3°C min-1, 90 to 200°C at 10°C min-1, and finally 200°C for 5 min. Combustion maintained with synthetic air was used at an airflow of 350 mL/min and nitrogen at 35 mL min-1 and the detector temperature was 230°C.
Odor activity value
The odor activity value (OAV) was calculated by dividing the mean concentration (n=3) of a compound by its odor threshold value (Guth, 1997; Peinado et al., 2004; Escudero et al., 2007; Li et al., 2008; King et al., 2008).
Sensory analyses
The typical aroma of the Chenin Blanc wine was evaluated, after fermentation using an acceptability test and purchasing intention. A total of 50 wine consumers were recruited based on their consumption frequency (at least once a week). The evaluations were carried out in individual booths under artificial daylight, a temperature between 22 and 24°C and air circulation. The samples were evaluated in a monadic way. Each consumer evaluated the nine beverage samples (A, B, C, D, E, F, G, H and I) in three sessions, according to an experimental design of complete balanced and randomized blocks. The same consumer panel took part in the three sessions.
The samples (50 ml) were served in tulip-shaped glasses covered with watch glasses, coded with random 3-digit numbers. A 9-cm non-structured hedonic scale was used in the acceptability test with extremes of “(1) disliked extremely” and “(9) liked extremely” (Stone and Sidel, 2004). The consumers also registered their purchasing intentions for each sample on the same score sheet, using a five-point attitude scale, with extremes of “(1) would definitely not buy” and “(5) would definitely buy” (Meilgaard et al., 2007).
The study plan was evaluated by the Ethics in Research Committee of the Federal University of Bahia-UFBA, resolution number 01 of 13/06/1988-CNS, presenting a favorable opinion for the development of the study (number 094/2009 and CEP record 102/2009).
Data analysis
The acceptance data were evaluated using a main effects ANOVA procedure, and the physicochemical data evaluated by a one-way ANOVA and Tukey test (5% significance level) using the STATISTICA software (version 8.0, StatSoft, Inc., Tulsa, OK, USA). The variability of the samples in relation to the volatile compounds was analyzed by Principal Component Analysis (PCA), carried out using the Pirouette 4.0 software (Infometrix, Washington, USA). The results for purchasing intention were presented as a percentage.
Physicochemical characterization of the grape must and fermented grape musts
The Chenin Blanc grape must presented concentrations of SO2, N2, pH value, titratable acidity and °Brix of 60.0 mg L-1, 150 mg L-1, 3.1, 71.64 mEq L-1 (5,4 g L-1) and 19.8, respectively. According to Ribéreua-Gayon et al. (2006) the Chenin Blanc grape must was in appropriate conditions to start fermentation.
The ethanol content of the fermented musts reached an average of 11.53% for S. cerevisiae, 10.56% for the yeast combination of H. opuntiae/S. cerevisiae, 7.30% for H. guilliermondii/S. cerevisiae, 8.53% for I. terricola/S. cerevisiae and 8.04% for C. flavescens/S. cerevisiae. No ethanol was produced in the grape musts fermented by the non-Saccharomyces yeast cultures (Table 1). The sugar consumed may have been used in a different metabolic routes giving rise to volatile compounds such as alcohols and acetaldehyde. In mixed culture fermentations, Moreira et al. (2008) observed antagonistic competition between the inoculated microorganisms, making the production of ethanol less efficient.
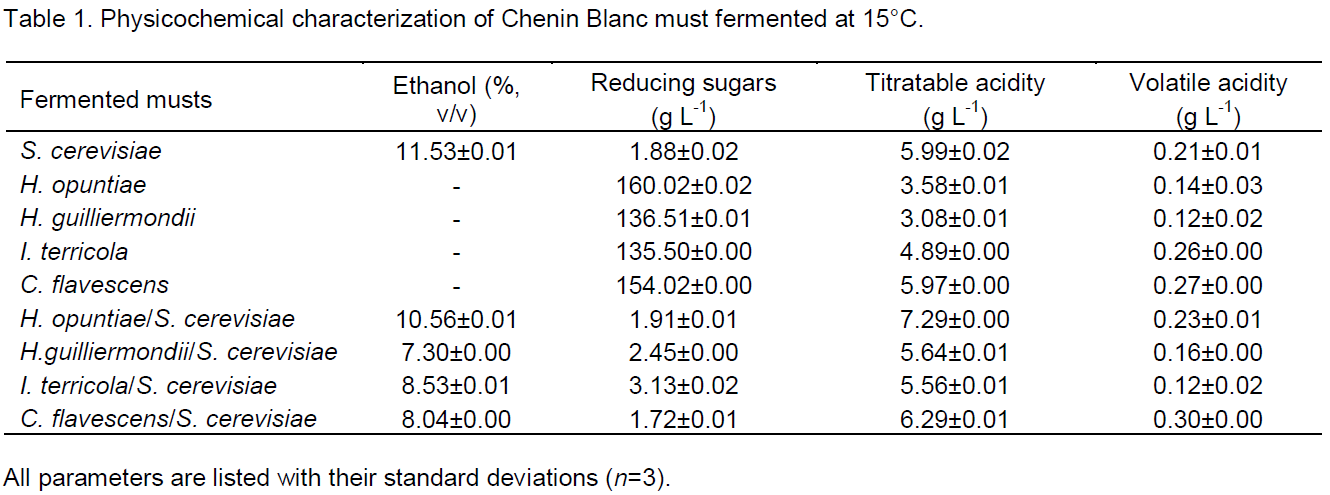
The sugar concentrations were derived from the sugars present in the grapes, which were not metabolized during fermentation of the wine. As expected, the sugar was not completely consumed in the samples fermented by pure cultures of non-Saccharomyces yeasts, resulting in elevated residual sugar values of 160.02 g L-1 for fermentation by H. opuntiae, 136.51 g L-1 by H. guilliermondii, 135.50 g L-1 by I. terricola and 154.02 g L-1 by C. flavescens (Table 1). This demonstrates the higher efficiency in the use of the sugar present in the must in the fermentations carried out by mixed yeast cultures.
In relation to the titratable acidity, only the values found in the grape musts fermented by H. opuntiae and H. guilliermondii individually were below the minimum value (55 mEq L1) set by Ribéreua-Gayon et al. (2006). Thus one can deduce that these yeasts (H. opuntiae and H. guilliermondii) were not good acid producers. In the present study the volatile acidity levels were between 0.12 g L-1 and 0.30 g L-1, all conforming with Brazilian legislation (Brazil, 2005) which permits a maximum of 20 mEq L-1 volatile acidity (corrected) or 1.2 g L-1 acetic acid.
Analysis of volatile compounds
Table 2 shows the results obtained for the volatile components, expressed in mg L-1, present in the Chenin Blanc grape musts fermented by different non-Saccharomyces yeasts, inoculated alone and in combination with S. cerevisiae.
The odor activity value (OAV) can be used to establish which compounds contributed to the aroma, since the OAV calculation depends on both the concentration determined and the odor threshold of a specific compound in a determined matrix. In order to be perceived by the human nose, the compound must have an odor activity value > 1 (Welke et al., 2014). The values obtained in this study are presented in Table 4.
Ethyl acetate was detected in higher concentrations, varying between 2.76 and 15.17 mg L-1, in the grape musts fermented by S. cerevisiae and by H. opuntiae/S. cerevisiae, respectively (Table 2). At low levels (<50 mg L-1) this compound may be adequate and add complexity to the flavor, whereas at high concentrations, it tends to produce an unpleasant aroma in the wine (Swiegers and Pretorius, 2005). Remarkably, the OAV value for this compound in grape musts fermented by H. opuntiae/S. cerevisiae was 1264.16 (Table 3).
Esters confer characteristic aromas on wines, even when present in low concentrations. Isoamyl acetate (banana and fruit aroma), ethyl hexanoate (apple aroma) and hexyl acetate (apple, pear, floral) are fairly influential in the aromatic composition of the majority of wines (Peinado et al., 2004). However, the OAV value for ethyl hexanoate was only significant for the aroma of the must fermented by H. opuntiae/S. cerevisiae (Table 4). Hexyl acetate, ethyl octanoate and ethyl decanoate were detected in low concentrations and did not contribute to the aromatic composition of the fermented grape must, with OAV values < 1 (Tables 3 and 4).
2-Phenylethyl acetate, described as an aroma with "rose" and "flowers" nuances in wines, is very important for the wine aroma composition, providing elegance to the beverage (Swiegers and Pretorius, 2005). This compound was not detected in the must fermented by S. cerevisiae alone, but it was detected in the other fermented musts at very low concentrations (Table 3), without influencing the aroma (Table 4). In their studies Viana et al. (2008) found that non-Saccharomyces yeasts showed high potential for the production of 2-phenylethyl acetate, and stressed that the co-inoculation of Saccharomyces/non-Saccharomyces yeast could be of interest.
High levels of volatile acids such as butyric acid and isobutyric acid are undesirable in wines, since they can decrease beverage acceptance (Nikolaou et al., 2006). High levels of these acids were detected in musts fermented by C. flavescens and I. terrícola (Table 3), negatively impacting the aroma. Thus it is understood that the use of these yeasts in wine production brings no benefit or improvement in the aroma of the wine. The methanol levels in the fermented musts were within the limits established by Brazilian law, which establishes a maximum of 300 mg L-1 (Brazil, 2005). The methanol content in the fermented grape musts ranged from 17.47 mg L-1 to 34.56 (Table 3). Higher alcohols such as 1-propanol, 2-methyl-1-propanol, 2-methyl-1-butanol and 3-methyl-1-butanol belong to the group of active aroma compounds which form the aromatic basis of the wine.
The majority of higher alcohols present in wine are secondary products of the alcoholic fermentation, formed from the fermentation of sugars, or via the Ehrlich reaction or from amino acids present in the grapes (Ribéreua-Gayon et al., 2006).
1-Propanol occurred in all the musts, but in higher concentrations in the co-inoculated musts,where the concentrations were 37.31, 23.91 and 23.04 mg L-1 respectively for musts fermented by H. opuntiae/S. cerevisiae, H. guilliermondii/ S. cerevisiae and I. terrícola, respectively. The OAV values calculated were > 1 for all the fermented grape musts. According to Ribéreau-Gayon et al. (2006), the concentration of this compound should be from 10 to 50 mg L-1, and values above this amount may contribute negatively to the aroma. This compound is described as having a ripe fruit aroma (Peinado et al., 2004). The must fermented by C. flavescens/S. cerevisiae presented a concentration slightly higher than the others, negatively influencing the aroma.
The production of 2-methyl-1-propanol and 3-methyl-1-butanol was high enough to positively increase the aroma in all the fermented grape musts, with OAV values > 1, especially in the co-inoculated musts, but the concentration of 2-methyl-1-butanol was not high enough, and thus its impact on the aroma was limited (Tables 3 and 4). Notably the must fermented by H. guilliermondii/S. cerevisiae showed the highest concentrations and OAV values for 2-methyl-1-propanol and 3-methyl-1-butanol. Total higher alcohols below 300 mg L-1indicate quality and positively contribute to the wine aroma, since at higher concentrations they can mask the aroma and compromise all the sensory properties of the beverage (Escudero et al., 2007).
The musts fermented by S. cerevisiae in combination with non-Saccharomyces yeasts showed the highest total higher alcohols, and the musts fermented by H. opuntiae, H. guilliermondii, I. terricola and C. flavescens produced more total higher alcohols than S. cerevisiae. Thus it can be considered that the high levels found in the co-fermented musts referred specifically to the ability of the non-Saccharomyces yeasts to produce this class of alcohols (Table 3).
2-Phenylethyl alcohol plays an important role in the aroma of white wines when present in concentrations above the threshold of 0.200 mg L-1 (Peinado et al., 2004). In all the fermentations, the values found for 2-phenylethanol were above the threshold, and the odor activity values were > 1 (Table 4). Thus it effectively contributed to the aromatic composition of the musts analyzed.
The highest acetaldehyde concentrations (60.56 mg L-1, Table 3) were found in the musts fermented by S. cerevisiae. In co-inoculation the production of acetaldehyde decreased, the metabolic pathway producing this compound possibly being diverted to balance the aroma of the fermented must. When present in high concentrations, acetaldehyde results in a "pungent" aroma, negatively affecting the wine aroma (Gurbuz et al., 2006).
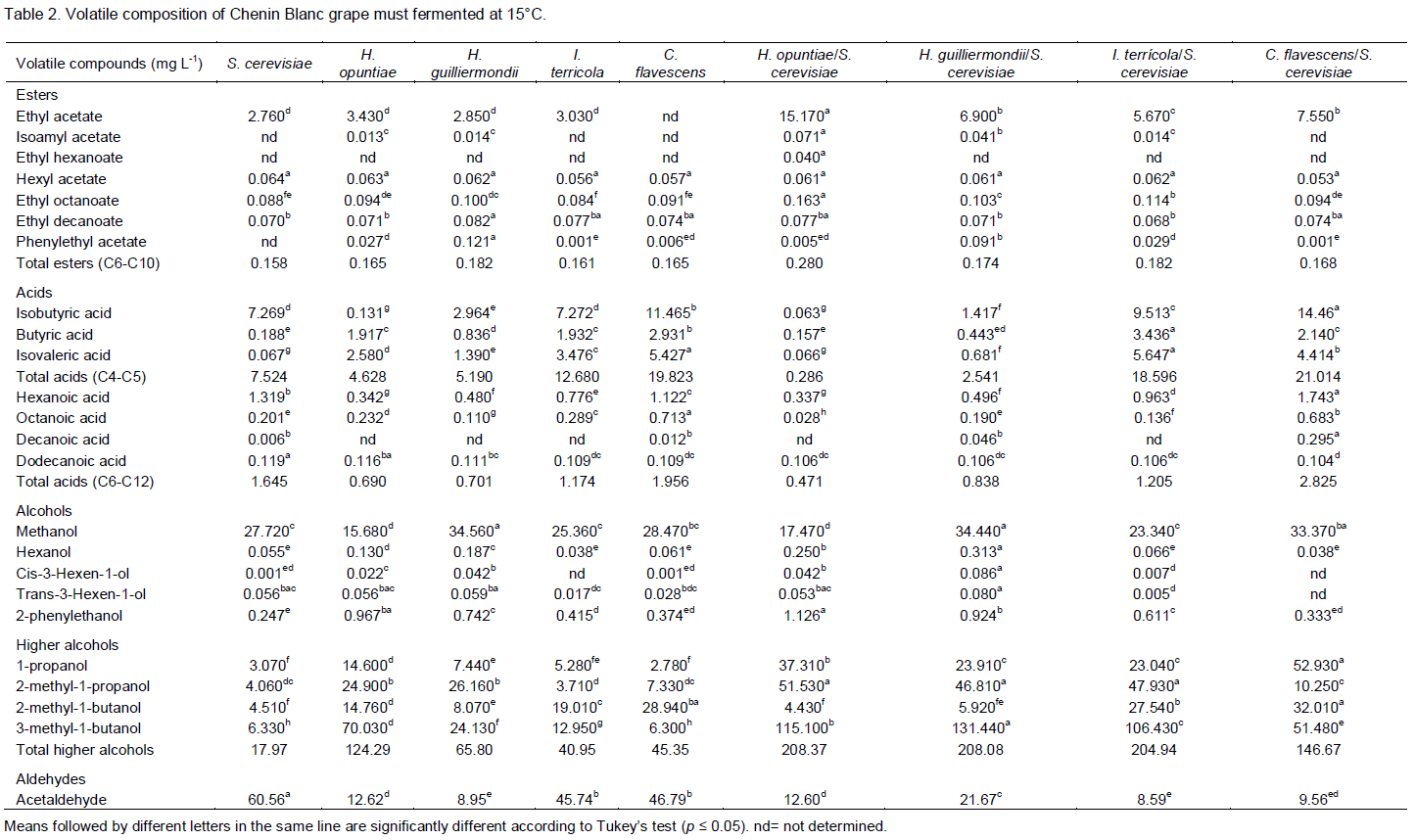
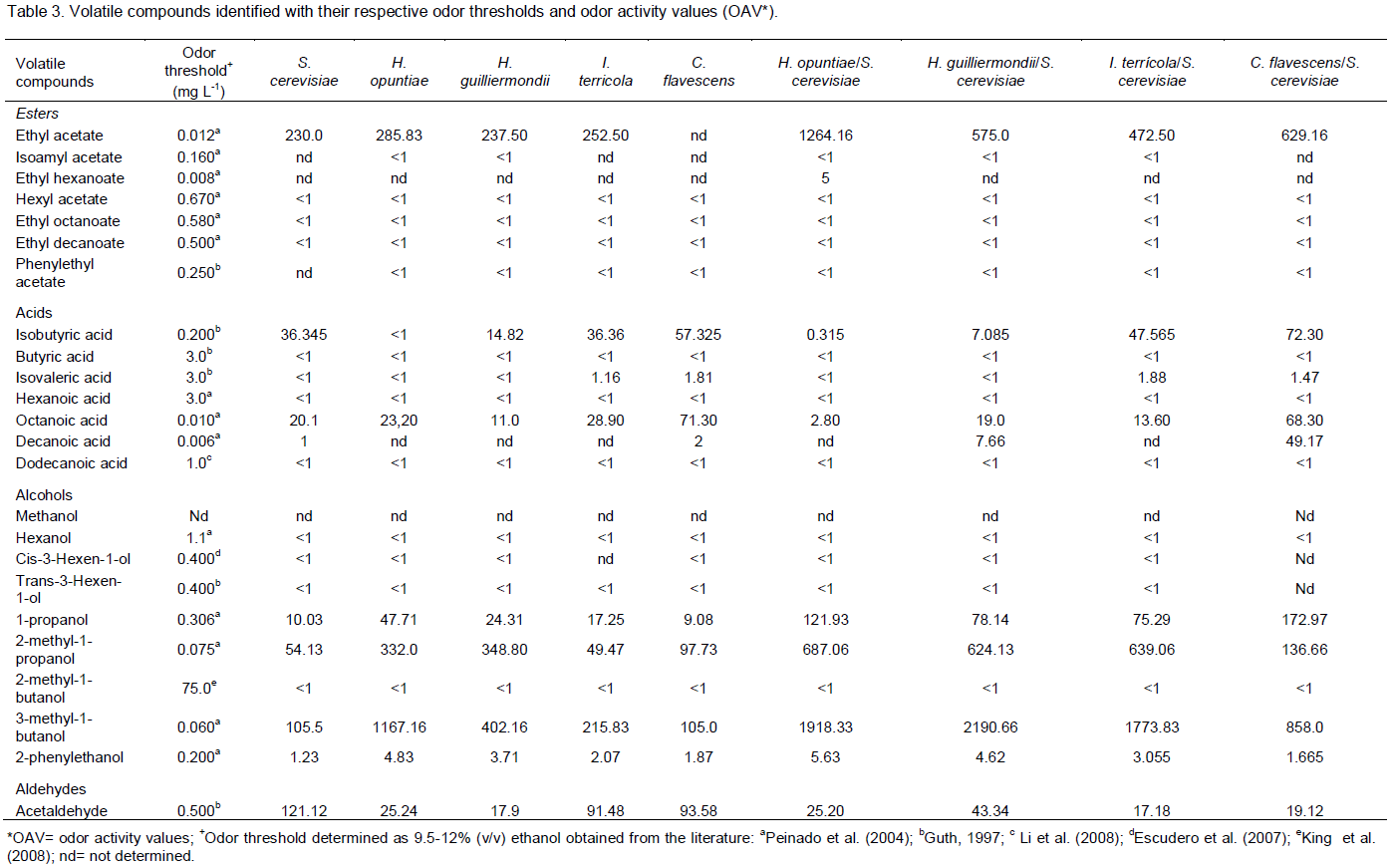
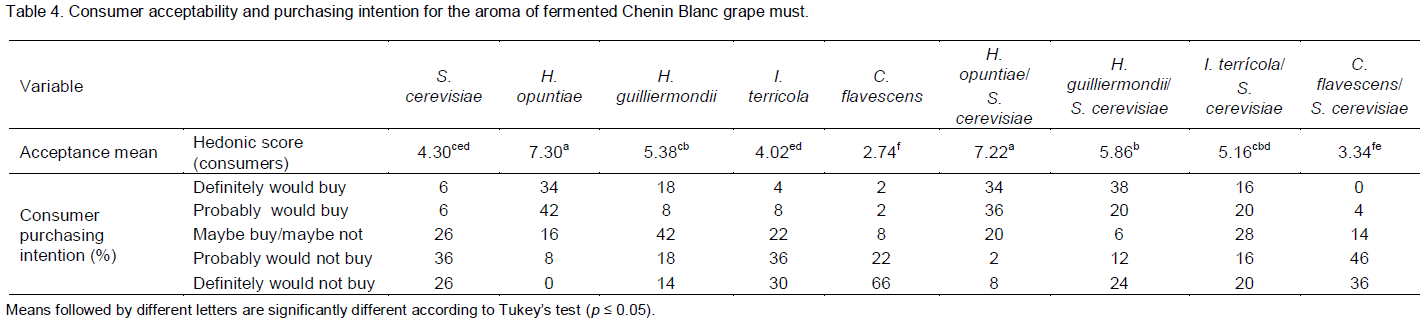
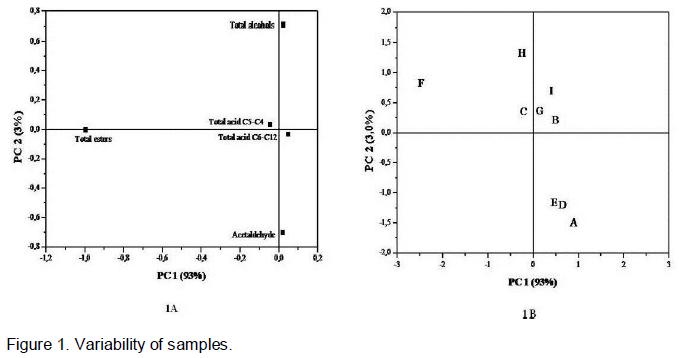
The variability of the samples with respect to the total esters, total acids (C4-C5), total acids (C6-C12), total alcohols and acetaldehyde (Figure 1A and 1B) was determined. The first two PC accounted for 96% of the variability of the experimental data. As shown in Figure 1A, the total esters and total acids (C4-C5) were located high to the left side of PC1 and the total acids (C6-C12) to the right side of the same PC. The total alcohols and acetaldehyde projected into PC2, on the positive and negative sides, respectively. In Figure 1B, the samples F, H and C were located to the left of PC1 and the samples I, G and B to the right side. The samples E, D and A were located to the right of PC1, but also on the negative side of PC2. The total esters were the most important variable of PC1 with respect to explaining the variability of the samples. The total alcohols and acetaldehyde were important in discriminating the samples in groups H, I, G, B and C and the samples in groups E, D and A, respectively. Sample F was on the same side of the PC as the total esters, indicating that this volatile group wasimportant in distinguishing this sample over the others.
Consumer acceptance and purchasing intention
The Chenin Blanc grape musts fermented by H. opuntiae and combined with S. cerevisiae presented the highest acceptability means of 7.30 and 7.22, respectively, statistically different from the other fermented musts. The must fermented by H. guilliermondii/S. cerevisiae presented an acceptability mean of 5.86 and did not differ statistically from the must fermented by H. guilliermondii (5.38) (Table 4). The musts fermented by H. opuntiae and by the H. opuntiae/S. cerevisiae combination showed the highest percentages of purchasing intention, corresponding to the concepts “definitely would buy” and “probably would buy”, with values of 76 and 70%, respectively.
The must fermented by S. cerevisiae presented a lower acceptability than those fermented by H. opuntiae/S. cerevisiae and H. guilliermondii/S. cerevisiae, and also lower percentages for the concepts of “definitely would buy” and “probably would buy” in the purchasing intention test (12%). Similar results were found by Mamede et al. (2005) when evaluating the acceptability and purchasing intention of the aroma of Pinot noir and Chardonnay musts fermented at 15°C by Pichia membranifaciens, Kloeckera apiculata, Candida valida and S. cerevisiae. They observed that the Chardonnay must fermented by S. cerevisiae showed the lowest purchasing intention.
The fermentation carried out by C. flavescens presented the lowest acceptability mean (2.74), and did not differ statistically from the mean obtained by the combined fermentation with C. flavescens/S. cerevisiae (3.34). When associations are made between the sensory data and the quantified data/OAV for the volatile compounds, it is easier to understand the acceptability scores and determine the contribution of each of the yeasts to the aroma of the fermented must.
It can also be seen that the must fermented by H. opuntiae/S. cerevisiae presented the highest OAV values for compounds such as 2-methyl-1-propanol and ethyl acetate, which, in appropriate concentrations, contribute positively to the aromatic quality of the must.
The concentration of the total higher alcohols was within the limits which contribute positively to the aroma. 2-phenylethanol is an important volatile compound in wine quality, and was produced by H. opuntiae and H. guilliermondii in combination with S. cerevisiae in amounts that resulted in OAV values of 5.63 and 4.62, respectively. Under the same fermentation conditions, a low production of the volatile acids (C4-C5 and C6-C12) and acetaldehyde was observed, but the must fermented by H. opuntiae/S. cerevisiae achieved a greater acceptability score. Probably the absence of ethyl hexanoate in the must fermented by H. guilliermondii/ S. cerevisiae and the high concentrations of octanoic acid and acetaldehyde contributed negatively to the acceptability of this fermented must.
A statistical analysis of pearson’s correlation between the acceptability of the fermented musts and the five classes of volatile compounds was carried out. The results showed a strong negative correlation between the acceptability of the must fermented by C. flavescens/S. cerevisiae and the total acids (C4-C5) (r = - 0.81, p = 0.08) and total acids (C6-C12) (r = - 0.83, p = 0.05). Thus, the low mean for acceptability found in the must fermented by C. flavescens/S. cerevisiae may be associated with the presence of high concentrations of volatile acids negatively affecting the wine aroma.
The combined use of non-Saccharomyces yeasts with Saccharomyces cerevisiae var. bayanus in the fermentation of grape musts showed that not all the combinations resulted in good production of volatiles with a consequent positive contribution to the aroma, with the exception of H. opuntiae and H. guilliermondii. The must fermented by H. opuntiae/Saccharomyces cerevisiae showed greater acceptability and purchasing intention, probably due to the production of volatile compounds in amounts sufficient to positively enhance the wine aroma, highlighting total esters (ethyl acetate, ethyl hexanoate), total alcohols and 2-phenylethanol.
The low acceptability of the must fermented by C. flavescens/S. cerevisiae could have been due to increments of undesirable components that contributed negatively to the wine aroma. It was therefore suggested that the yeast species H. opuntiae and H. guilliermondii in combination with Saccharomyces cerevisiae showed potential to positively impact the wine aroma. A more comprehensive survey of the use of non-Saccharomyces yeasts in wine production is feasible, considering their contribution to the aroma, seeking innovations in winemaking and in the types of wine.
The authors have not declared any conflict of interests.
The authors are grateful to Vinibrasil S/A for their assistance and for donating the Chenin Blanc grape must. This study was financially supported by fellowships received from FAPESB and CNPq.
REFERENCES
|
Aleixandre-Tudo JL, Weightman C, Panzeri V, Nieuwoudt HH, du Toit WJ (2015). Effect of Skin Contact Before and During Alcoholic Fermentation on the Chemical and Sensory Profile of South African Chenin Blanc White Wines. S Afr J Enol Vitic. 36(3):366-377.
|
|
|
|
Assis MO, Mamede MEO, Guimarães AG, Santos LS, Rosa CA (2012). Yeasts isolated from Vitis vinifera L. grapes cultivated in Brazilian Equatorial region. Rev. Inst. Adolfo Lutz 71(4):718-722.
|
|
|
|
|
Assis MO, Santos APC, Rosa CA, Mamede MEO (2014). Impact of a Non-Saccharomyces Yeast Isolated in the Equatorial Region in the Acceptance of Wine Aroma. Food and Nutr. Sci. 5:759-769.
|
|
|
|
|
Bisson LF, Karpel JE (2010). Genetics of yeast impacting wine quality. Annu. Rev. Food Sci. Technol. 1:139-162.
|
|
|
|
|
Brazil (2005). Instrução Normativa N° 24, de 8 de setembro de 2005. Aprova o Manual Operacional de Bebidas e Vinagres.
|
|
|
|
|
Capone S, Tufariello M, Siciliano P (2013). Analytical characterisation of Negroamaro red wines by "Aroma Wheels". Food Chem. 141:2906-2915.
|
|
|
|
|
Escudero A, Campo E, Farina L, Cacho J, Ferreira V (2007). Analytical characterization of the aroma of five premium red wines. Insights into the role of odor families and the concept of fruitiness of wines. J. Agric. Food Chem. 55:4501-4510.
|
|
|
|
|
Fleet GH (2003). Yeast interactions and wine flavor. Int. J. Food Microbiol. 86:11-22.
|
|
|
|
|
Fleet GH (2008). Wine yeasts for the future. FEMS Yeast Res. 8:979-995.
|
|
|
|
|
GonzaÌlez SS, Barrio E, Querol A (2006). Molecular identification and characterization of wine yeasts isolated from Tenerife (Canary Island, Spain). J. Appl. Microbiol. 102:1018-1025.
|
|
|
|
|
Gurbuz O, Rouseff JM, Rouseff RL (2006). Comparison of aroma volatiles in commercial Merlot and Cabernet Sauvignon wines using gas chromatography olfactometry and gas chromatography mass spectrometry. J. Agric. Food Chem. 54:3990-3996.
|
|
|
|
|
Guth H (1997). Identification of character impact odorants of different white wine varieties. J. Agric Food Chem. 45:3022-3026.
|
|
|
|
|
Jiang B, Zhang Z (2010). Volatile compounds of young wines from Cabernet Sauvignon, Cabernet Gernischet and Chardonnay varieties grown in the Loess Plateau Region of China. Molecules 15:9184-9196.
|
|
|
|
|
Juan SF, Cacho J, Ferreira V, Escudero A (2012). Aroma chemical composition of red wines from different price categories and its relationship to quality. J. Agric. Food Chem. 60:5045–5056.
|
|
|
|
|
King ES, Swiegers JH, Travis B, Francis IL, Bastian SE, Pretorius IS (2008). Co-inoculated fermentations using Saccharomyces yeasts affect the volatile composition and sensory properties of Vitis vinifera L. cv. Sauvignon Blanc wines. J. Agric. Food Chem. 56:10829-10837.
|
|
|
|
|
King ES, Francis IL, Swiegers JH, Curtin C (2011). Yeast strain-derived sensory differences retained in Sauvignon Blanc wines after extended bottle storage. Am. J. Enol. Vitic. 62:366-370.
|
|
|
|
|
Kurtzman CP, Fell JW, Boekhout J (2011). The Yeasts, A Taxonomic Study. 5th ed. Elsevier, San Diego.
|
|
|
|
|
Lopandic K, Tiefenbrunner W, Gangl H, Mandl K, Berger S, Leitner G, Abd-Ellah GA, Querol A, Gardner RC, Sterflinger K, Prillinger H (2008). Molecular profiling of yeasts isolated during spontaneous fermentations of Austrian wines. FEMS Yeast Res. 8:1063-1075.
|
|
|
|
|
Li H, Tao YS, Wang H, Zhang L (2008). Impact odorants of Chardonnay dry white wine from Changli County (China). Eur. Food Res. Technol. 227:287-292.
|
|
|
|
|
Mamede MEO, Cardello HMAB, Pastore GM (2005). Evaluation of an aroma similar to that of sparkling wine: Sensory and gas chromatography analyses of fermented grape musts. Food Chem. 89:63-68.
|
|
|
|
|
Meilgaard MC, Carr BT, Civille GV (2007). Sensory evaluation techniques. 4th ed. CRC Press. Boca Raton, FL.
|
|
|
|
|
Mendoza LM, De Nadra MC, Bru E, Farías ME (2009). Influence of wine-related physicochemical factors on the growth and metabolism of non-Saccharomyces and Saccharomyces yeasts in mixed culture. J. Ind. Microbiol. Biotechnol. 36:229-237.
|
|
|
|
|
Moreira N, Mendes F, Pinho PG, Hogg T, Vasconcelos I (2008). Heavy sulphur compounds, higher alcohols and esters production profile of Hanseniaspora uvarum and Hanseniaspora guilliermondii grown as
|
|
|
|
|
pure and mixed cultures in grape must. Int. J. Food Microbiol. 124:231-238.
|
|
|
|
|
Nikolaou E, Soufleros EH, Bouloumpasi E, Tzanetakis N (2006). Selection of indigenous Saccharomyces cerevisiae strains according to their oenological characteristics and vinification results. Food Microbiol. 23:205-221.
|
|
|
|
|
Peinado RA, Moreno J, Bueno JE, Moreno JA, Mauricio JC (2004). Comparative study of aromatic compounds in two young white wine subjected to pre-fermentative cryomaceration. Food Chem. 84:585-590.
|
|
|
|
|
Pineau B, Barbe JC, Van Leeuwen C, Dubourdieu D (2009). Examples of perceptive interactions involved in specific "red-" and "black-berry" aromas in red wines. J. Agric. Food Chem. 57:3702-3708.
|
|
|
|
|
Ribereau-Gayon P, Glories Y, Maujean A, Bubourdieu D (2006). Handbook of enology. The Chemistry of Wine Stabilization and Treatments. 2nd ed. John Wiley & Sons Ltd, Chichester.
Crossref
|
|
|
|
|
Robinson AL, Boss PK, Heymann H, Solomon PS, Tren¬gove RD (2011). Influence of yeast strain, canopy management, and site on the volatile composition and sensory attributes of Cabernet Sauvignon wines from western Australia. J. Agric. Food Chem. 59:3273-3284.
|
|
|
|
|
Robinson AL, Boss PK, Solomon PS, Trengove RD, Heymann H, Ebeler SE (2014). Origins of Grape and Wine Aroma. Part 1. Chemical Components and Viticultural Impacts. Am. J. Enol. Vitic. 65:1-24.
|
|
|
|
|
Stone H, Sidel JL (2004). Descriptive Analysis. In: Sensory evaluation practices. Stone H, Sidel JL (ed). Elsevier Academic Press, San Diego, USA. pp. 215-235.
|
|
|
|
|
Swiegers JH, Pretorius IS (2005). Yeast modulation of wine flavor. Adv. Appl. Microbiol. 57:131-175.
|
|
|
|
|
Torrens J, Urpí P, Riu-Aumatell M, Vichi S, López-Tamames E, Buxaderas S (2008). Different commercial yeast strains affect¬ing the volatile and sensory profile of cava base wine. Int. J. Food Microbiol. 124:48-57.
|
|
|
|
|
Viana F, Gil JV, Genoves S, Vallés S, Manzanares P (2008). Rational selection of non-Saccharomyces wine yeasts for mixed starters based on ester formation and enological traits. Food Microbiol. 25:778-785.
|
|
|
|
|
Viana F, Gil JV, Vallés S, Manzanares P (2009). Increasing the levels of 2-phenylethyl acetate in wine through the use of a mixed culture of Hanseniaspora osmophila and Saccharomyces cerevisiae. Int. J. Food Microbiol. 135:68-74.
|
|
|
|
|
Webber V, Dutra SV, Spinelli FR, Marcon AR, Carnieli GJ, Vanderlinde R (2014). Effect of glutathione addition in sparkling wine. Food Chem. 159: 391-398.
|
|
|
|
|
Welke LE, Zanus M, Lazzarotto M, Zini CA (2014). Quantitative analysis of headspace volatile compounds using comprehensive two-dimensional gas chromatography and their contribution to the aroma of Chardonnay wine. Food Res Int. 59:85-99.
|
|