ABSTRACT
The objective of this study was to determine the elicitor potential of plant extracts from Caatinga biome by enzyme activity and epidemiological components of Alternaria brown spot (Alternaria alternate f. spp. citri). These were prepared ethanolic, dichloromethanic, and aqueous extracts from 14 plants (Anadenanthera macrocarpa, Schinopsis brasiliensis, Maytenus rigida, Caesalpinia pyramidalis, C. ferrea, Peltophorum dubium, Capariscyn ophallophora, Ziziphus joazeiro, Mimosa hostilis, Momordica charantia, Erythrina velutina, Cleome hassleriana, Sideroxylum obtusifolium Spondias tuberosa) after dilution, (1mg/mL) they were sprayed on Citrus reticulate ‘Ponkan’seedlings and after 0, 24, 48, 72 and 96 h the leaves were collected for determination of polyphenol oxidase and peroxidase activity. For epidemiological disease components, leaves were collected 72 h after spraying, deposited on Petri dishes with moist paper, inoculated with a spore suspension of A. alternata f. spp. citri (2x105 conidia mL-1) and evaluated for 12 days initial and final severity, area below the progress curve of disease and protection. The extracts of A. macrocarpa and M. rigida potentiated the polyphenol oxidase and peroxidase activity which had great potential in the management of the disease, reducing the final severity and area under the disease progress curve, providing high levels of control.
Key words: Citrus reticulata, alternaria brown spot, elicitor.
socioeconomically for the country (Struiving et al., 2013). Despite having sweet orange [Citrus sinensis (L.) Osbeck] as the main spp produced, several spp of tangerines and some of their hybrids such as tangelos, willow leaf and tangors stands out in Brazilian orchards when the target is the National fruit market in natura (Azevedo et al., 2010). However, these orchards are constantly targets of Alternaria brown spot (ABS) infections, initiated by A. alternate f. spp. citri. which considered the main fungal disease of this specie (Peres et al., 2003), and caused serious damage in the Brazilian orchards resulting in the reduction of some susceptible varieties planted, mainly in the state of Sao Paulo, where many growers are eradicating their orchards due to the drastic increase on production costs, as a consequence of the high number of fungicide applications, discouraging planting in new areas (Azevedo et al., 2010).
The search for alternative strategies to control pests and diseases in agriculture has been the strategy used to reduce the use of pesticides with high toxicity, reconciling the safe food production, environmental preservation (Farooq et al., 2011) and the economic viability of commercial plantations. In other to do that, the use of medicinal plants represent a rich source of natural compounds to be explored for the identification of new defense principles (Belting, 2009). Plant extracts have secondary metabolites which represent biologically active substances. Several authors have demonstrated the potential of medicinal plants in the control of plant pathogens, both by its direct fungi toxic action, and the ability to stimulate the accumulation of molecules with elicitor features capable of inducing defense responses (Bonaldo et al., 2004; Celoto et al, 2008; Bulhões et al., 2012). These resistance mechanisms may include the accumulation of phenolic compounds, phytoalexins and pathogenesis-related proteins such as β-1,3glucanase, chitinase, peroxidase, phenylalanine ammonia lyase and polyphenol oxidase (Barros et al., 2010). Hence, this study aimed to evaluate the potential of native plant extracts from Caatinga biome as elicitors of defense response by enzymatic quantification in ponkan mandarin seedlings and its efficiency to reduce the severity of infection by Alternaria alternate f .spp. citri in detached leaves.
14 leaves of native plant species (Table 1) of the Brazilian semi-arid region in Boa Vista-PB (7º 15'28''S, 36°14'7''W) were collected and taken to the Phytopathology Laboratory of the Universidade Federal da Paraíba, Campus II, Areia-PB and to the Phytochemicals Laboratory of the Universidade deTrásdos Montes e Alto Douro - UTAD, in Vila Real, Portugal. The leaves were collected, washed and dried at 25+2°C and then placed in a forced air circulation chamber at a temperature of 65°C until constant weight, grounded in a blade-grinder to obtain powder. The material was properly identified and stored until further use. For determination of enzyme activity and efficiency in the control of ABS, an extraction was performed from plant material with water, ethanol and dichloromethane to determine solvent with increasingly polarity, more suitable for better extraction of bioactive compounds. The ethanolic extract and dichloromethane were made from dried plant suspension material (powder) in ethanol (90%) and dichloromethane in a ratio of 1: 3 for 24 h with periodic agitation.
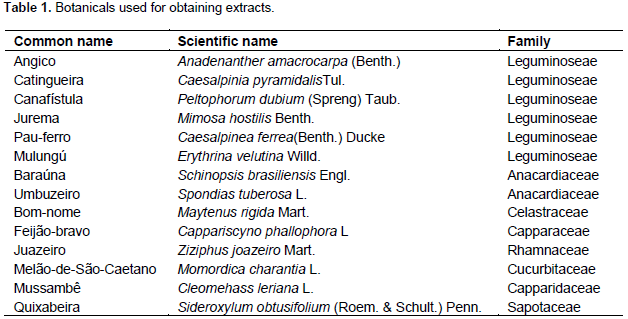
The material was filtered through filter paper and the liquid obtained was subjected to evaporation in a rotary evaporator held under constant agitation of 60°C until complete evaporation, yielding crude extract, which was placed in test tubes, identified and stored at -20°C until further analysis. For aqueous extract, the vegetable powder was suspended in distilled water for 24 h and kept under constant agitation at 35°C for further filtering, concentrate obtaining was then freeze-dried. In a preliminary test, dichloromethane caused death of pathogen, even without the plant extract which caused damage in the leaves of the sprayed plants, masking the results, and for this reason, they were not tested for enzymatic activity and protection. Experiment was carried out in a greenhouse at the Department of Plant and Environmental Sciences and the enzymatic assays were performed at the Laboratory of Biomass of the Department of Biological Sciences, at Center for Agrarian Sciences of the Federal University of Paraiba, Areia - PB. A randomized block design was used with three blocks by 28 treatments distributed in factorial arrangement (14 x 2 x 5) + (2 x 5), 14 herbal extracts (Table 1) with two extraction solutions (water and ethanol) in five periods (0, 24, 48, 72 and 96 h after spraying) and two controls (water and acibenzolar-S-methyl - ASM [0.2 mg / ml]) in five periods (0, 24, 48, 72 and 96 h after spray). The experimental unit consisted of three seedlings of Citrus reticulata, Ponkan variety grafted onto Citrus aurantifolia. Each seedling received 20 mL of solution at 1.0 mg.mL-1. For enzymatic activity two leaves of each seedling were collected at 0, 24, 48, 72 and 96 h after the application of the extracts. These samples were stored individually in aluminum foil, frozen in liquid nitrogen (N2) and stored in an Ultra freezer at -20°C for experimental analysis, performed in triplicate.
0.25 g of citrus leaves were weighed which were mechanically homogenized in a mortar with 4.0 mL of 100 mM sodium acetate buffer (pH 5.0). The homogenate was centrifuged at 12,000 g for 20 min at 4°C and the supernatant obtained was considered as an enzymatic extract for determination of the peroxidase and polyphenol activity. Peroxidase activity was determined by direct spectrophotometric method with modifications by the measurement of guiacol conversion in tetraguaiacol at 470 nm (Lusso, 1999). The mixture for the reaction consisted of, 0.25 mL protein extract, 0.25 mL guaiacol and 0.25 mL hydrogen peroxide in 0.75 mL 0.1 M phosphate buffer (pH 6.0). The peroxidase activity was expressed as specific activity (absorbance units’ min-1 mg-1 protein). The polyphenol oxidase activity was determined by Duangmal (1999) methodology with modifications. The assay consisted in measuring the oxidation of catechol converted into quinone, being this reaction mediated by enzyme under study. The substrate was prepared with catechol in concentration of 20 mM and dissolved in 100 mM sodium phosphate buffer (pH 6.8). Reaction was developed from the mixture of 0.25 mL substrate, 0.5 mL enzyme extract and 0.75 mL reaction buffer in a temperature of 30°C for 15 min, being interrupted by the addition of 0.05 mL of 5 N HCl after this period. A spectrophotometric was made at 420 nm and the results were expressed in min-1 mg-1 protein absorbance.
Total proteins were determined by Bradford method. 50 μL from the patterns solutions or sample extracts were pipetted separately with 50 μL of distilled water and 1 mL of Bradford reagent being gently shaken and allowed to rest for 10 min. Spectrophotometric were made at a wavelength of 595 nm. On the pH of the reaction (5.0), the interaction between the high molecular weight protein and the reagent dye Coomassie Brilliant Blue (G-250) present on the Bradford dye reagent causes equilibrium shift to the anionic form that strongly absorbs at 595 nm. For evaluation of plant protection efficiency against ABS mediated by plant extracts, Ponkan mandarin seedlings and bare-root of one year old were used, commercially available in Lagoa Seca -PB (07°10'15"S and 35°51'13"W), transplanted to plastic bags with 1L of capacity and acclimated in a greenhouse for 15 days with daily watering. The extracts applications were made by spraying at a concentration of 1mg.mL-1. After 72 h of the first spray, mandarin leaves were collected, identified by treatment, packed in cool boxes and taken to the Phytopathology Laboratory of UFPB. Fifteen leaves from each treatment were immersed in a sodium hypochlorite solution at 0.5% for 5 min and subsequently washed in distilled water. Three leaves were placed in petri dishes containing moist filter paper with sterile distilled water. Subsequently, leaves spore suspension of A. alternate f. sp. citri at a concentration of 2x105 conidios mL-1 were given. After inoculation, leaves were maintained at room temperature (25±5°C) under light for 72 h before the start of the evaluations. The disease severity was evaluated for 15 days, in which the initial severity was related to the first day of assessment, starting from the onset symptoms (3 days after inoculation). Evaluations were made from a proposed grade scale by Martelli (2011). Area under disease progress curve was calculated by Shaner and Finney (1977) using the following equation;
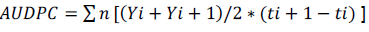
Where Y is the severity of the disease at ith observation, ti is the time in days on ith observation and n is the total number of observations. A randomized experimental design was used with blocks in a factorial arrangement (15 x 2 + 2 witnesses) with 14 extracts and two extracting solutions, two additional witnesses (water and ASM) and five replications. The experimental unit was composed of three leaves.
Data analysis
For the effect of enzyme activity comparison and epidemiological variables with initial and final severity, area under the disease and protecting progress curve, were made contrasts between extracts and additional treatments (Water and ASM), using the Dunnett test (α = 0.05) (SAS Institute, 2002) and the data normality was evaluated by Cochran.
To determine the content of soluble proteins, the calibration equation y = 0.0237 + 0,0451x obtained from different standard concentrations were used. The polyphenol-oxidase activity was not significantly different among treatments in period zero for two extractant solutions (water and ethanol), since the leaves were collected shortly after application, there was no immediate enzyme activity. Although the acibenzolar-S-methyl is considered as a resistance inducer, but do not promote increase in the polyphenol oxidase activity when compared to control (water) in all periods (Table 2). The use of plant extracts in Ponkan mandarin seedlings provided a highly variable response to the tested species over time. Within 24 h after treatment application, the aqueous extracts of Peltophorum dubim, Capparis cynophallophora, Mimosa hostilis and ethanolic extracts of M. hostilis, Maytenus rigida promote the highest activities of polyphenol oxidase, being superior to the controls with water and ASM. These same extracts had their activity reduced within 48 h and only Z. joazeiro extract, in both extraction, enhanced the enzyme activity in this period, showing a later activity, however, most intense.
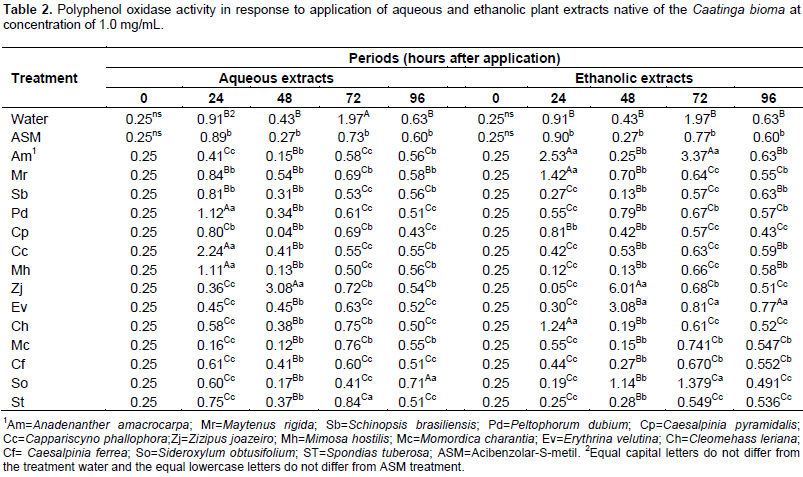
For aqueous extract within 72 h, the seedlings treated with water obtained the highest average for the polyphenol oxidase activity, differing from the other treatments, while for the ethanol extracts, Anadenanthera macrocarpa promoted a higher activity of this enzyme. In the last assessment period (96 h) the Sideroxylum obtusifolium aqueous extract and ethanolic of Erythrina velutina provided the highest average (Table 2). Within 96 h, the plants treated with different extracts had low peroxidase activity, however, the aqueous extract of Caesalpinia pyramidalis and ethanolic of C. cynophallophora provided the highest average, differentiating from the controls. The peak of peroxidase activity was checked after 48 h in plants treated with the aqueous extract of M. rigida, averaging 7, 86 UA per mg of protein per min, and the ethanolic extract of M. hostilis (2.74 AU/mg protein/min). The Schinopsis brasiliensins aqueous extracts, Spondias tuberosa and ethanolic of A. macrocarpa, M. rigida and C. cynophallophora presented higher peroxidase activity than plants treated with water and ASM already at 24 days after application. The enzymatic activity for the aqueous S. brasiliensis extract and ethanolic of A. macrocarpa decreased after 48 h, however, returned to high activity, differing from the control treatments (water and ASM) within 72 h (Table 3).
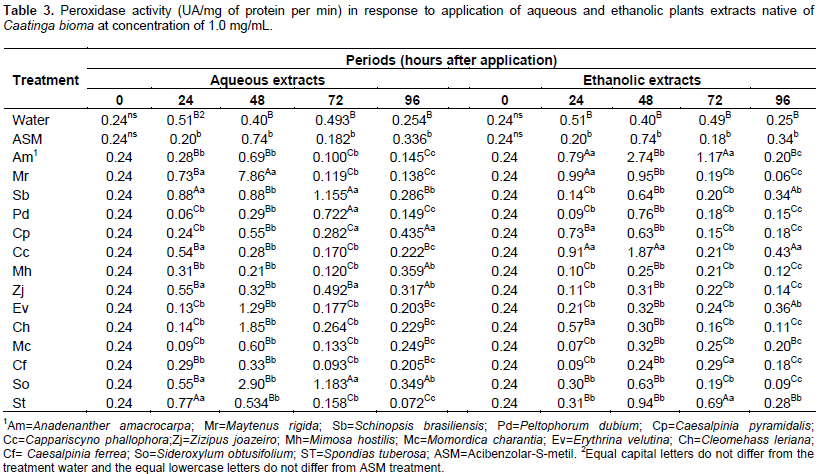
Within 96 h, the plants treated with different extracts had low peroxidase activity, however, the aqueous extract of C. pyramidalis and ethanolic of C. cynophallophora provided the highest average, differentiating from the controls. The peak of peroxidase activity was checked after 48 h in plants treated with aqueous extract of M. rigida, averaging 7,86UA per mg of protein per min, and the ethanolic extract of M. hostilis (2.74 AU/mg protein/min). The application of plant extracts influenced the epidemiological variables evaluated in detached leaves of Ponkan mandarin seedlings positively reducing infection and in some cases negatively, as occurred with the aqueous extract of C. pyramidalis which caused an increase in the initial disease severity, higher than the control with water and ASM, while there was no interference of ethanolic extracts for this variable (Table 4). The aqueous extracts of M. rigida, S. brasiliensis, M. hostilis, Ziziphus joazeiro, Momordica charantia and S. tuberosas significantly reduced the final severity and AUDPC compared to control (water) and did not differ from ASM treatment, providing therefore, the higher control rates (Table 4). Plants treated with the ethanolic extract of M. hostilis behaved differently from the one treated with aqueous extract, with less protective potential, initial severity and AUDPC equal to control (water) and also, the same happened with Peltophorum dubium, Cleome hassleriana and Sideroxylum obtusifolium extracts.
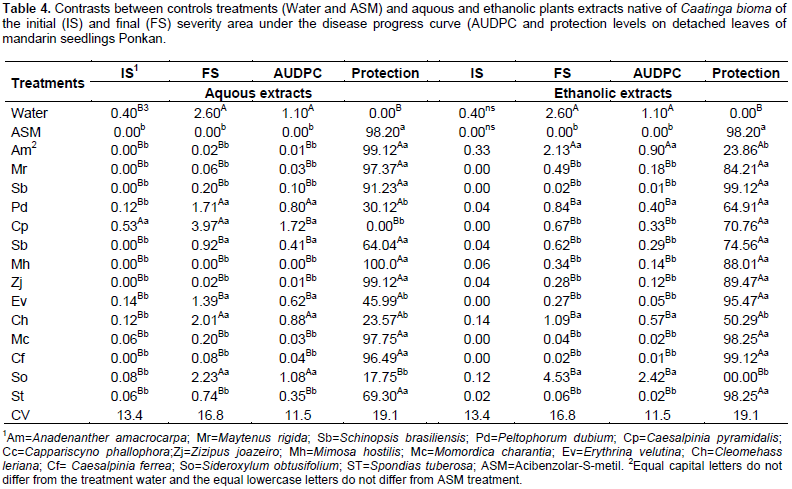
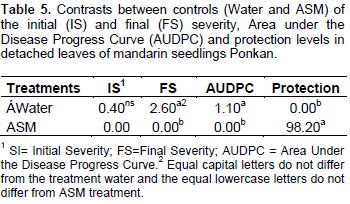
The remaining ethanolic extracts significantly reduced the final severity and AUDPC, representing high rates of protection with values up to 99.12%, not differing from the control provided by the treatment with ASM. The difference between treatments with water and ASM were significant (Table 5), indicating the elicitor efficiency in the protection against A. alternate f. spp. citri. Many studies shows the potentiating effect of Acibenzolar-S-methyl enzymes involved in plant defense such as peroxidase and polyphenol oxidase (Baysal et al., 2003; Cavalcanti et al., 2006; Andrade et al., 2013), however, the majority are performed with infected plants. In this case, it is difficult to define if treatment with ASM or if the infective process caused the activation of these enzymes. It is considered that, the polyphenol oxidase is associated with infected tissues, thus playing an important role in plant defense mechanism or senescence (Agrios, 2005). Lucas (2012) found an increased polyphenol oxidase activity in tomato (Solanum licopersicum L.), just six days after application of ASM and essential oil of lemon grass (Citrus aurantifolia) in non-inoculated plants which observed lower activity in these treated plants inoculated with A. alternata. Song et al. (2011) has shown that the increased polyphenoloxidase, peroxidase and phenylalanine ammonia lyase activity in plant tissues are an important factor in the induction of resistance against pathogens.
The polyphenol oxidases are enzymes which oxidize mono- and o-diphenols to o-diquinones (Vaughn et al., 1988), which have antimicrobial activity (Mohammadi, 2002) and participates in the lignification process during tissue invasion by pathogens (Li, 2002), justifying their enhanced activity during the infectious process. Further, peroxidases are also reported in association with the formation of reactive oxygen intermediates (Skelly, 2013) and the oxidative burst is a plant defense response after pathogen recognition, leading to hypersensitivity reaction, promoting the gradual establishment of systemic acquired resistance (SAR) (Resende et al., 2003; Alvarez et al., 1998). However, this enzyme is also synthesized by mechanical stress such injury (Rodrigues et al., 2011) or environmental such water and salt stress (Debouba et al., 2006) can be variable depending on the species, cultivar, age and part of the plant (Amiot et al., 1995) which explains the mutability action of this enzyme compared to spraying with plant extracts. Peroxidases are related to the processes of growth and cell differentiation, and morphogenetic changes in response to physical, chemical and biological stress. Increased activity of this enzyme in plants under these conditions can be a determinant factor for the adaptive capacity of these plants; this activity can be identified as a biochemical marker of stress (Piza et al., 2003).
In a practical way, peroxidases are related to increased hydrogen peroxide production (Hameed et al., 2008) and are central in the plant antioxidant defense responses (Hameed et al., 2009). Carvalho et al. (2013) in experiments with plant extracts on the alternative control of A. alternataf. spp. citri in Tangor Murcot fruits found significant decrease of up to 62% in the rate of spots development with aqueous extract of A. columbrina, which belongs to the same genus A. macrocarpa, showed the greatest potential for protection (99.12%) in detached leaves, being superior to the treatments with commercial fungicides chlorothalonil by copper oxychloride and Azoxystrobin, which provided protection of 37 to 39% respectively. However, these same authors also reported a direct effect of the extract on the pathogen development, not configuring, therefore, a protection only by induced-resistance. The results presented here shows the importance of plant extracts and derivatives as sources of efficient bioactive compounds in the management of plant diseases through direct effect on pathogens or as potential sources of a new defense response elicitors and as an alternative to chemical control, which have been indiscriminately used. According to Ootani (2013) exploration of the biological activity of secondary compounds present in the crude extract or essential oil of medicinal plants can be, with resistant induction, another potential alternative form of disease control in cultivated plants.
The extracts of A. macrocarpa and M. rigida potentiated the polyphenol oxidase and peroxidase activity and had great potential in the management of the disease, reducing the final severity and area under the disease progress curve and providing high levels of control.
The authors have not declared any conflict of interests.
REFERENCES
|
Agrianual (2012). Anuário da Agricultura Brasileira. São Paulo: FNP Consultoria e AgroInformativos.
|
|
|
|
Agrios G (2005). Plant pathology.5ª Ed. Amsterdão, Países Baixos: Elsevier, Academic Press 907p.
|
|
|
|
Alvarez M, Pennell R, Meijer P, Ishikawa A, Dixon R, Lamb C (1998). Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92:1-20.
Crossref
|
|
|
|
Amiot MJ, Tacchini M, Aubert SY, Oleszek W (1995). Influence of cultivar, maturity stage and storage conditions on phenolic composition and enzymatic browning of pear fruits. J. Agric. Food Chem. 43(5):1132-1137.
Crossref
|
|
|
|
Andrade CCL, Resende RS, Rodrigues FA, Silveira PR, Rios JA, Oliveira JR, Mariano RLR (2013). Indutores de resistência no controle da pinta bacteriana do tomateiro e na atividade de enzimas de defesa. Trop. Plant Pathol. 38:28-34.
Crossref
|
|
|
|
Azevedo FA, Polydoro DA, Bastianel M, Kupper KC, Stuart RMA, Costa FP, Pio RM (2010). Resposta de diferentes genótipos de tangerinas e seus híbridos Á inoculação in vitro e in vivo de Alternaria alternata. Rev. Bras. de Fruticultura 32:944-951.
Crossref
|
|
|
|
Barros FC, Sagata E, Ferreira LCC, Juliatti FC (2010). Indução de resistência em plantas contra fitopatógenos. Biosci. J. 26(2):231-239.
|
|
|
|
Baysal O, Soylu EM, Soylu S (2003). Induction of defence-related enzymes and resistance by the plant activator acibenzolar-S-methyl in tomato seedlings against bacteril canker caused by Clavibacter michiganensis ssp. michiganensis. Plant Pathol. 52(6):747-753.
Crossref
|
|
|
|
Belting M, Wittrup A (2009). Macromolecular drug delivery: basic principles and therapeutic applications. Mol. Biotechnol. 43:89-94.
Crossref
|
|
|
|
Bonaldo SM, Schwan-Estrada KRF, Stangarlin JR, Tessmann DJ, Scapim CA (2004). Fungitoxicidade, atividade elicitora de fitoalexinas e proteção de pepino contra Colletotrichum lagenarium, pelo extrato aquoso de Eucalyptus citriodora. Fitopatologia Bras. 29(2):128-134.
Crossref
|
|
|
|
Bulhões CC, Bonaldo SM, Santos BT, Trento RA (2012). Produtos alternativos no controle de Antracnose (Colletotrichumgloeosporioides), Cladosporiose (Cladosporiumherbarum) e bacteriose (Xanthomonas campestres pv. passiflorae) em maracujazeiro no norte de Mato Grosso. Revista Ciências Exatas e da Terra e Ciências Agrárias. 7:12-19.
|
|
|
|
Carvalho DDC, Alves E, Camargos RB, Oliveira DF, Scolforo JRS, Carvalho DA, Batista TRS (2013). Plant extracts to control Alternaria alternata in Murcott tangor fruits. Rev. Iberoamericana de Micología 28:173-178.
Crossref
|
|
|
|
Cavalcanti FR, Resende MLV, Lima JPMS, Silveira JAG, Oliveira JTA (2006). Activities of antioxidante and photosynthetic responses in tomato pre-treated by plant activators and inoculated by Xanthomonas vesicatoria. Physiol. Mol. Plant Pathol. 68:198-208.
Crossref
|
|
|
|
Celoto MIB, Souza LB, Moura FG (2008). Atividade antifúngica de extratos de plantas a Colletotrichum gloeosporioides. Acta Scientiarum. 30:1-5.
|
|
|
|
Debouba M, Gouia H, Suzuki A, Ghoebel MH (2006). NaCl stress effects on enzymes involved in nitrogen assimilation pathway in tomato "Lycopersicon esculentum" seedlings. J. Plant Physiol. 163:1247-1258.
Crossref
|
|
|
|
Duangmal K, Apenten RKO (1999). A comparative study of polyphenoloxidases from taro (Colocasia esculenta) and potato (Solanum tuberosum var. Romano). Food Chem. 64:351-359.
Crossref
|
|
|
|
Farooq M, Jabran ZA, Cheema A, Wahid KHM (2011). The role of allelopathy in agricultural pest management. Pest Manage. Sci. 67:493-506.
Crossref
|
|
|
|
Hameed A, Naseer S, Iqbal T, Syed H, Haq MA (2008). Effects of NaCl salinity on seedling growth, senescence, catalase and protease activities in two wheat genotypes differing in salt tolerance. Pakistan J. Bot. 40:1043-1051.
|
|
|
|
Hameed, A, Iqbal N, Malik SA (2009). Mannose-induced modulations in antioxidants, protease activity, lipid peroxidation, and total phenolics in etiolated wheat leaves. J. Plant Growth Regulation 28:58-65.
Crossref
|
|
|
|
Li L, Steffens JC (2002). Overexpression of polyphenol oxidase em transgenic tomato plants results in enhanced bacterial disease resistance. Planta 215(2):239-247.
Crossref
|
|
|
|
Lucas GC (2012). Óleos essenciais no controle da pinta preta do tomateiro. 92p. Tese (Doutorado – Área de Concentração em Fitopatologia), Universidade Federal de Lavras, Minas Gerais.
|
|
|
|
Lusso MFG, Pascholatti SF (1999). Activity and isoenzymatic pattern of soluble peroxidases in maize tissues after mechanical injury or fungal inoculation. Summa Phytopatologica 25:244-249.
|
|
|
|
Martelli IB (2011). Manejo da Mancha Marrom de Alternaria em citros: poda de limpeza e correlação com a lagarta minadora. 2011. 41p. Dissertação (Mestrado em Agricultura Tropical e Subtropical), Instituto Agronômico de Campinas, Campinas-SP, Brasil.
|
|
|
|
Mohammadi M, Kazemi H (2002). Changes in peroxidases and polyphenol oxidases activities in susceptible and resistance wheatheads inoculated with Fusarium graminearum and induced resistance. Plant Sci. 162(4): 491-498.
Crossref
|
|
|
|
Ootani MA, Aguiar RW, Ramos ACC, Brito DR, Silva JB, Cajazeiras JP (2013). Utilização de óleos essenciais na agricultura. J. of Biotechnol. Biodiversity. 4(2):162-174.
|
|
|
|
Peres NAR, Agostini JP, Timmer LW (2003). Out breaks of Aternaria brown spot of citrus in Brazil and Argentina. Plant Dis. 87:750.
Crossref
|
|
|
|
Piza IMT, Lima GPP, Brasil OG (2003). Atividade de peroxidase e níveis de proteínas em plantas de abacaxizeiro micropropagadas em meio salino. Rev. Bras. de Agrociência 9 (4):361:366.
|
|
|
|
Resende MLV, Salgado SML, Chaves ZM (2003). Espécies ativas de oxigênio na resposta de defesa de plantas a patógenos. Revista Brasileira de Fitopatologia. 28:123-130.
Crossref
|
|
|
|
Rodrigues LJ, Boas EVBV, Paula NRF, Pinto DM, Piccoli RH (2011). Efeito do tipo de corte e de sanificantes no escurecimento de pequi minimamente processado. Ciênc. Agrotecnológica. 35:560-567.
|
|
|
|
Statistical Analysis System (SAS) institute (2002). (Cary, Estados Unidos). Software andservices: System for Windows, versão 8.0: software. Cary.
|
|
|
|
Shaner G, Finney RE (1997). The effects of nitrogen fertilization on the expression of slow-mildwing in knox wheat. Phytopathology 67:1051-1055.
|
|
|
|
Skelly MJ, Loake G (2013) Synthesis of redox-active molecules and their signalling functions during the expression of plant disease resistance. Antioxidants & Redox Signaling. 19(9):990-997.
Crossref
|
|
|
|
Song W, Ma X, Tan H, Zhou J (2011). Abscisic acid enhances resistance to Alternariasolani in tomato seedlings. Plant Physiol. Biochem. 49:693-700.
Crossref
|
|
|
|
Struiving TB, Machado DLM, Santos D, Siqueira DL, Lucena CC, Matarazzo HM (2013). Qualidade fisiológica de sementes de citros durante o armazenamento em ambiente refrigerado. Ciênc. Rural 43(10):1777-1782.
Crossref
|
|
|
|
Vaughn K, Lax AR, Duke SO (1988). Polyphenol oxidases: the chloroplast oxidase with no established function. Physiologia Plantarum. 72:659-665.
Crossref
|