ABSTRACT
Zalmati olive oil was aromatized with essential oils obtained from rosemary (Rosmarinus officinalis L.), to improve its quality. Parameters such as fatty acids composition, pigments and quality parameters were characterized for various blends of virgin olive oils and essential oils (0.005, 0.01 and 0.02%). Results show that aromatized oils had an improved composition as compared to that of pure olive oils. An increasing in the oleic acid content and a decreasing in the palmitic and linoleic acids levels was observed with aromatization process. Moreover, this process improved the oxidative stability and the antiradical activity.
Key words: Essential oils, fatty acids, oxidative stability, virgin olive oils, volatile compounds.
Olive oil is a fundamental component of the Mediterranean area diet, and it is widely used as a condiment, cooking medium, and in the storage of vegetable and animal food. Olive oil cultivation originated in Asia Minor and has spread to Greece, Italy, Spain, and North Africa. The Tunisian olive culture constitutes one of the principal economical and agricultural strategic sectors. About, 60 million trees as distributed and spread on 1.6 million hectares, representing a third of the cultivated area. The olive growing area spread from the northern to the southern regions. VOO is extensively consumed due to its nutritional value and its organoleptic characteristics. Besides, mention should also be mad of its use in medicine. It is unique among others vegetable oils due to its high levels of monounsaturated fatty acids (mainly oleic acid) and to the presence of minor component, such as phenol compound. Phenols are an important parameter for the evaluation of VOO quality as they contribute greatly to oil flavor and taste as long as the oil is protected from auto-oxidation. Moreover, it is widely known that the quality of VOO is influenced by various agronomic factors such as olive cultivar, climatic conditions, degree of maturation, and agronomic practices related to irrigation treatment (Morello et al., 2004). EVOO is highly appreciated by consumers due to their health benefits as well as their aromatic characteristics; this is the reason why is important to prevent off-flavours by different contamination processes (
Gamazo-Vázquez et al., 2003). However, other studies have demonstrated that nutritional and organoleptic quality of VOO was ameliorated by some technological procedures as blending and malaxing (Ouni et al., 2011; Reboredo-Rodríguez et al., 2014a)..Zalmati variety which have similar characteristic with Chemlali variety, mainly cultivated in center and southern area of Tunisian country, is a productive variety, well adapted to severe environmental conditions. However, its oil is characterized by relatively low of oleic acid, high level of palmitic and linoleic acid, presents a high acidity and a low amount of phenols (Baccouri et al., 2007). These disadvantages could considerably limit the possibilities of exporting of the Tunisian OO production, especially in the presence of a very competition international market that demand high quality (Issaoui et al., 2009).
Hence, the improvement of oil quality by aromatization could to be a tool to provide a product with balanced fatty acid composition, optimal levels of antioxidants compounds and a good flavor and taste. The main goal of this work is to characterize Zalmati olive oils before and after aromatization with essential oil obtained from rosemary known for its high quality. In order to improve the quality of Zalmati fatty acid composition, phenols, oxidative stability and quality parameters (acidity, PV, K232, K270 extinction coefficient, pigments contents) were attested at different percentage of aromatization with rosemary essential oils.
Essential oil
Extraction
The aerial parts (stems and leaves) of Rosmarinus officinalis (L.) were collected from the South-East of Tunisia on March 2013 (Gabes, bioclimatic zone: lower arid, rainfull (mm/year): 100 to 200, Latitude: 33°27?34?N, longitude: 10°8?17?E, altitude 520 m). The plant material was dried at room temperature in the shadows, for 2 weeks until constant weight. The dried preparation was ground further to obtain a fine powder, and then stored at ambient temperature in a dry and dark place until being used. The dry matter was submitted to hydrodistillationf for 4 h, using a Clevenger-type apparatus. Essential oil was stored in sealed vials protected from light at -20°C until analysis.
Gas chromatography (GC)
An Agilent Technologies 6890N GC equipped with HP-5MS capillary column (30 m × 0.25 mm i.d., film thickness 0.25 μm; Hewlett-Packard) and connected to a FID was used. The column temperature was programmed at 50?C for 1 min, then 7?C/min to 250?C, and finally left at 250?C for 5 min. The injection port temperature was 240?C; while that of the detector was 250?C (split ratio: 1/60). The carrier gas was helium with a flow rate of 1.2 ml/min. The analyzed essential oil volume was 2 μl. Percentages of the constituents were calculated by electronic integration of FID peak areas, without the use of response factor correction. Mean per-centage of R. officenalis L. volatiles compounds represented the average calculated on three individuals. Retention indices (RI) were calculated for separate compounds relative to C9-C16 n-alkanes mixture (Aldrich Library of Chemicals Standards) (Kovàts, 1958).
Gas chromatography/mass spectrometry (GC/MS)
The volatile compounds isolated by HD were analysed by GC/MS, using an Agilent Technologies 6890N GC. The fused HP-5MS capillary column (the same as that used in the GC/FID analysis) was coupled to an Agilent Technologies 5973B MS (Hewlett-Packard, Palo Alto,CA, USA). The oven temperature was programmed as previously (50°C for 1 min, then 7°C/min to 250°C, andthen left at 250°C for 5 min). The injection port temperature was 250°C and that of the detector was 280°C (split ratio: 1/100). The carrier gas was helium (99.995%purity) with a flow rate of 1.2 ml/min. The MS conditions were as follow: ionization voltage, 70 eV; ion source temperature, 150°C; electron ionization mass spectra were acquired over the mass range 50 to 550 m/z.
Volatile compounds identification
The volatile compounds of R. officinalis L. leaves were identified by comparing the mass spectra data with spectra available from the Wiley 275 mass spectra libraries (software, D.03.00). Further identification confirmations were made referring to RI data generated from a series of known standards of n-alkanes mixture (C8 to C26) and to those previously reported in the literature (Adams, 2001).
Olive oil
Extraction
Olive oil samples were obtained from fruits of Zalmati olive cultivar, which were picked by hand at the same stage of maturity from three trees during the crop season 2012/ 2013 (October) in an olive orchard located in Bengardene south of Tunisia. The olives were washed and deleafed and crushed; the same laboratory mill was used to prepare the olive oil samples. Only healthy fruits, without any kind of infection or physical damage, were processed. After harvesting, fresh olives (1.5-2.0 kg) were washed and deleafed, crushed with a hammer crusher, and the paste mixed at 25°C for 30 min, centrifuged without addition of warm water (oil produced from each extraction was 200-250 ml/kg). Samples were prepared by blending olive oils with essential oil in different pre-established proportions (0.005, 0.01 and 0.02%) and then transferred into dark glass bottles, and stored in the dark at 4°C until analysis.
Determination of oil quality parameters
Free acidity, expressed as percent of oleic acid (%18:1); peroxide value, given as milliequivalents of active oxygen per kilogram of oil (meqO2/kg); and UV absorption characteristics (K232 and K270) were determined according to the analytical methods described in the European Union Commission Regulations EEC/2568/91 and EEC/1429/92.
Determination of chlorophyll and carotenoid contents
Chlorophyll and carotenoid contents were determined colorimetrically as previously described (Minguez-Mosquera et al., 1991).The maximum absorption at 670 nm is related to the chlorophyll fraction, while the maximum absorption at 470 nm is related to the carotenoid fraction. The values of the coefficients of specific extinction applied were E0 = 613 for pheophytin, a major component in the chlorophyll fraction, and E0 = 2,000 for lutein, a major component in the carotenoid fraction. Thus, the pigment contents were calculated as follows:
Chlorophyll (mg/ kg) = (A670× 106 )/ (613 × 100 × d),
Carotenoid (mg/ kg) = (A470 × 106)/ (2,000 × 100 × d)
Where A is the absorbance and d is the spectrophotometer cell thickness (1 cm).
Fatty acid composition
The fatty acids were converted to fatty acid methyl esters before analysis by shaking a solution of 0.2 g oil and 3 ml of hexane with 0.4 ml of 2-N methanolic potassium hydroxide, and analyzed using a Hewlett-Packard (HP 4890D; Hewlett-Packar Company,Wilmington, DE) chromatograph equipped with a capillary column (Supelcowax: 30 m × 0.53 mm; 0.25 mm), a split/splitless injector and an flame ionization detection (FID) detector. The carrier gas was nitrogen, with a flow rate of 1 ml/min. The temperatures of the injector, the detector and the oven were held at 220, 250 and 210°C, respectively. The injection volume was 1 μl.
Antiradical activity
The olive oil samples were examined for their capacity to scavenge the stable 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) (Kalantzakis et al., 2006). Oil solution 1 ml in ethyl acetate (10%, w/v) was added to 4 ml of a freshly prepared DPPH solution (10-4 M in ethyl acetate) in a screw-capped 10 ml test tube. The reaction mixture was then vigorously shaken for 10 s in a vortex apparatus and the tube was maintained in the dark for 30 min, until a steady state was reached. The absorbance of the mixture was measured at 515 nm against a blank solution. A control sample (no oil) was prepared and daily measured. A refined olive oil (Minerva S.A. edible Oils, Shimatari, Viotia, Greece), devoid of pro-oxidants/antioxidants, was used for comparison. The radical scavenging activity (RSA) toward [DPPH] was expressed as the percent reduction in DPPH concentration by the constituents of the oils:
% [DPPH] red = 100-(1 [DPPH]30/[DPPH]0)
Where, [DPPH]0 and [DPPH]30 were the concentrations of DPPH in the control sample (t = 0) and in the test mixture after the 30 min reaction, respectively.
Total phenol contents were quantified colorimetrically (Ranalli et al., 1999). Phenolic compounds were isolated by triple extraction of a solution of oil (10 g) in hexane (20 ml) with 30 ml of a methanol water mixture (60:40, v/v). The Folin–Ciocalteau reagent (Merck Schuchardt OHG, Hohenbrunn, Germany) was added to a suitable aliquot of the combined extracts, and the absorption of the solution at 725 nm was measured. Values are given as milligrams of caffeic acid per kilogram of oil (Gutfinger, 1981).
Oil stability
Oxidative stability was evaluated by the Rancimat method (Gutiérrez, 1989). Stability was expressed as the oxidation induction time (h), measured with the Rancimat 743 apparatus (Metrohm, Herisau Switzerland), using an oil sample of 3.6 g. The oil temperature was 101.6°C and the air flow was 10 L/h.
Statistical Analysis
Significant differences between means were determined by an analysis of variance, which applied a Duncan’s test. Differences were considered statistically significant when the probability was greater than 99% (P < 0.01). The statistical analysis was performed using SPSS 13.0 for Windows (SPSS Inc., 2004).
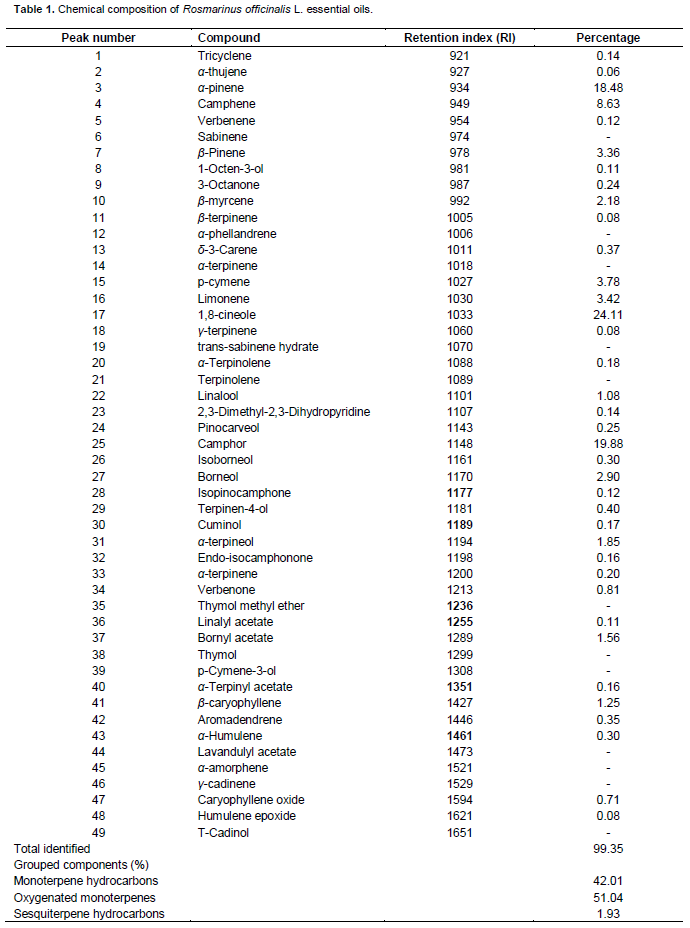
Chemical essential oil composition
The identified compounds of the volatile constituents of the essential oils are shown in Table 1. Thirty seven different components were identified by GC-MS analyses representing 99.35% of the total oil. In the literature, several methods were used for the comparison between the essentials oils composition of many species (López Mahia et al., 1993). The analyzed oil contained a complex mixture consisting of mainly oxygenated mono and sesqueterpenes, and mono-and sesqueterpene hydrocarbons. The rosemary oil used in this study mostly consisted of monoterpenes: 1,8-cineole, camphor, and α-pinene, constituting 24.1, 19.88 and 18.48% of the essential oil, respectively. Flamini et al. (2002) classified rosemary oil into two chemotypes: The α-pinene chemotype with the main compounds being α-pinene (20.6%) and 1,8 cineole (6.6%) and the 1,8-cineole chemotype with the major components being 1,8 cineole (40.2%) and α-pinene (13.2%). The monotepenes hydrocarbons (42.01%), represented mainly by 1,8-cineole, α-pinene, camphene, formed the major group. Camphor was the major compound of Ketones class (Table 1). These volatile compounds should improve the aroma characteristics of the studied virgin olive oils. Recent study showed that sedimentation of oil samples plus racking for a minimum of 2 months was found to promote the formation of C6 alcohols in most samples (Reboredo-Rodríguez et al., 2013a). Different methods of analysis for many additives in olive oil; to control its aroma fingerprint and for the determination of phenolic compounds were also reported (Quinto-fernández et al., 2003; Reboredo-Rodríguez et al., 2012, 2014b). Others studies showed variation in the aroma profile of virgin olive for different cultivars and in the biogenesis and in the distribution between olive pulps and seeds (Reboredo-Rodríguez et al., 2013b,c).

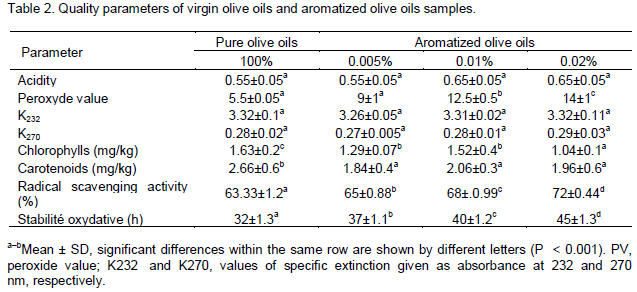
Quality parameters
For all oil samples, values of the analytical parameters fell within the ranges established for the highest quality category ‘extra virgin olive oil’. As shown in Table 2, these parameters are actually affected by the aromatization process. In all samples the free fatty acid content was much lower than the established upper limit of 0.8% for the best commercial quality olive oil, designated extra virgin (Regulation EEC, 2003) (Table 2). Peroxide value evaluates the hydroperoxides content and offers a measure of the degree of lipid oxidation. In all samples, this value was below the limit of 20 meq of oxygen kg-1 of oil, which is accepted as the limit for the extra quality virgin olive oils. To evaluate the oxidation level of the oil, the parameter K232 has been used (Table 2). Also the values of these parameters (K232 and K270) were below the limits established for extra virgin olive oils (2.50 and 0.22, respectively). All samples exhibited very low values in the regulated physico-chemical indices assessed. The lower these values are, the higher is the quality one can expect from oil. These values are consistent with other studies (Reboredo-Rodríguez et al., 2014c). Summarising, all values of the analytical parameters fell within the ranges established for the highest quality category ‘extra virgin’ olive oil. As shown in Table 2, aromatization had no significant influence on these analytical parameters, which are basically affected by factors causing damage to the fruits, e.g., olive fly attacks or improper systems of harvesting, carriage, storage and processing of olives (Ranalli and Angerosa, 1996).
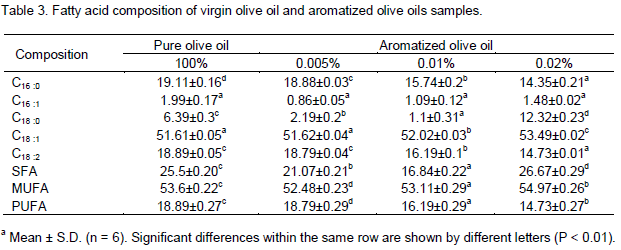
Fatty acid composition
Fatty acids identified in the oils were palmitic (C16:0), palmitoleic (C16:1), stearic (C18:0), oleic (C18:1), linoleic (C18:2), linolenic (C18:3) and arachidic (C20:0) acids (Table 3). Palmitic, stearic, oleic and linoleic acids were the major ones. Oleic acid is always the main abundant compound in olive oils, ranging from 51 to 53% of total fatty acids. As reported Table 3, Zalmati olive oil exhibited a lower oleic acid percentage (51%) and a higher amount of palmitic and linoleic acids (19 and 18%, respectively). It has been observed that aromatization with rosemary essential oils could correct this problem (Table 3). At 0.01% aromatization, palmitic acid decreased from 19.1 to15.7% (Table 3). Using 0.02% essential oils, oleic acid underwent a significant increase to 53.49% and, at the same time, a decrease of palmitic acid to 14.35% was observed. In summary, aromatized oils at 0.005% showed an improved fatty acid composition, characterized by an increase of its oleic acid content and a concurrent reduction of the palmitic and linoleic acids levels with respect to those of pure Zalmati oil. In addition, the fatty acid distribution became within the range expected for high quality olive oils. The monounsaturated fatty acids (MUFA) content is very important because of its effect on nutritional value and oxidative stability of the oils. It has been observed that oils with a high content of saturated fatty acids (SFA) are more viscous and persistent on the mucous of the oral cavity. This gives rise to the defect known as ‘fatty sensation’ (Solinas, 1990). Aromatized oils at 0.005% exhibited a significant increase of MUFA; in contrast, the SFA decreased (Table 3). These results are in agreement with the findings of other authors when blending oils (Issaoui et al., 2009; Ouni et al., 2011).
Pigments
The total pigment content in olive oil is an important
parameter for evaluating olive oil quality. Furthermore, pigments are involved in auto-oxidation and photo-oxidation mechanisms (Gutiérrez, 1989). The interest in the possible beneficial effects of chlorophyll pigments and related compounds has reemerged in the current decade. In this regard, some studies in vivo have appeared which seem to indicate that they may be antioxidants have appeared (Kamat et al., 2000; Lanfer-Marquez et al., 2005). More importantly, some authors have reported that they may also be beneficial in the prevention of cancer (Ferruzzi and Blakeslee, 2007). Zalmati olive oil has very low amounts of pigments (1.6 and 2.66 mg/ kg) of chlorophylls and carotenoids, respectively). At all percentage of aromatization with essential oils, chlorophylls and carotenoids in Zalmati olive oils underwent a slight decrease (Table 2). The production of highly pigmented oils should be of considerable interest for the industry and can influence the consumer usually prefer lighter oils.
Oxidative stability
Stability to oxidation is an important property of olive oil, which is improved by synergistic interactions between the various antioxidants present in the oil itself, and also depends on the lipid composition. As can be noted from Table 2, Zalmati olive oil has a low oxidative stability (32 h). The best influence of the process was clearly observed when aromatization was carried out at 0.02% (from 32 to 45 h) (Table 3).
Antiradical activity
Radical scavenging activity of the Zalmati olive oils varied according to the percentage of aromatization (63.33 to 72%). Zalmati oils produced at 0.02% showed the highest scavenging ability, with an average value of 72%. Conversely, at lower percentage of aromatization, the average value dropped down to 65%. This activity is necessarily linked to the much lower oxidative stability and oleic acid content. Our results show significant variation of the radical scavenging activity of the Zalmati oils due to the effect of the aromatization process. Overall, the correlation showed a positive linear relationship between radical scavenging capacity and oxidative stability measured by Rancimat (r = 0.66) (data not shown). These results are similar to those reported by several authors for other olive oil varieties (Usenik et al., 2008).
The aromatization process using different percentages of essantials oils improved the fatty acid composition by increasing the oleic acid content and decreasing the palmitic and linoleic acids levels, compared to those of pure Zalmati oil. The amount of chlorophylls, carotenoids increased quite slowly at the lower percentages of aromatization. Moreover, aromatization process improved the oxidative stability and the antiradical activity composition. In addition, these results clearly showed that aromatization application can play an important role in the changes of quality of monovarietal VOOs.
The authors have not declared any conflict of interest.
REFERENCES
|
Adams RP (2001). Identification of essential oil components by gas chromatography/quadrupole mass spectrometry. Allured Publishing Corporation, Carol Stream. |
|
|
Baccouri B, Ben Temime S, Taamalli W, Daoud D, M'Sallem M, Zarrouk, M (2007). Analytical characteristics of virgin olive oils from two new varieties obtained by controlled crossing on Meski variety. J Food Lipids, 14:19–34.
Crossref |
|
|
|
EEC (2003). Characteristics of olive and olive pomace oils and their analytical methods. EEC Regulation1989/2003. Offic. J. Eur. Commun. 295:57–66. |
|
|
Ferruzzi MG, Blakeslee J (2007). Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutr Res. 27:1–12.
Crossref |
|
|
Flamini G, Cioni PL, Morelli I, Macchia M, Ceccarini L (2002). Mainagronomic productive characteristic of two ecotypes of two Rosmarinus officinalis L. and chemical composition of their essential oils. J. Agric. Food. Chem. 50:3512-3517.
Crossref |
|
|
Gamazo-Vázquez J, García Falcón MS, Simal-Gándara (2003). Control of contamination of olive oil by sunflower seed oil in bottling plants by GC-MS of fatty acid methyl esters. Food Control 14(7):463-467.
Crossref |
|
|
|
|
|
Gutiérrez F (1989). Determinación de la estabilidad oxidativa de aceites de oliva vírgens. Comparación entre el método del oxígeno activo (AOM) y el método Rancimat (Determinación of the oxidative stability of virgin olive oils. Comparison between AOM and Rancimat methods). Grasas Aceites 40:1–4. |
|
|
Issaoui M, Flamini G, Ben Hassine K, Chehab H, Brahmi, F, Hammami M (2009) Improvement of Chemlali olive oil oxidative stability by blending with Chetoui and Rekhami cultivars. Int. J. Food Sci. Technol. 44:1323–1332.
Crossref |
|
|
|
|
Kamat JP, Boloor KK, Devasagayam TPA (2000). Chlorophyllin as an effective antioxidant against membrane damage in vitro and ex vivo. Biochim Biophys Acta 1487:113–127.
Crossref |
|
|
|
|
Kalantzakis G, Blekas G, Pegklidou K, Boskou D (2006). Stability and radical scavenging activity of heated olive oil and other vegetable oils. . Eur. J. Lipid Sci. Technol. 108:329-335.
Crossref |
|
|
|
|
Kovàts E (1958). Characterization of organic compounds by gas chromatography. Part 1. Retention indices of aliphatic halides, alcohols, aldehydes and ketones. Helvetica Chimica Acta 41:1915-1932.
Crossref |
|
|
Lanfer-Marquez UM, Barros RMC, Sinnecker P (2005). Antioxidant activity of chlorophylls and their derivatives. Food Res Int. 38:885–891.
Crossref |
|
|
López Mahia P, Sirnal Gándara J, Paseiro Losada P (1993). Infrared spectrophotometry in the citral determination into essential oils from lemons and oranges to correct for limonene interference. Food Chem. 46:193-197.
Crossref |
|
|
|
|
Minguez-Mosquera MI, Rejano-Navarro L, Gandulrojas B, Sanchez Gomez AH, Garrido-Fernandez J (1991). Color-pigment correlation in virgin olive oil. J. Am. Oil Chem. Soc. 86:332-336.
Crossref |
|
|
Morello JR, Motilva MJ, Tovar MJ, Romero MP (2004). Changes in commercial virgin olive oil (cv. Arbequina) during storage, with special emphasis on the phenolic fraction. Food Chem. 85:357-364.
Crossref |
|
|
|
|
Ouni Y, Flamini G, Guerfel M, Ben Youssef N, Douja D, Zarrouk M (2011). The compositional quality and volatile compounds of samples from the blend of monovarietal olive oils cultivated in Tunisia. Int. J. Food Sci. Technol. 46:678–686.
Crossref |
|
|
|
|
Quinto-Fernández EJ, Pérez-Lamela C, Simal-Gándara J (2003). Analytical methods for food contact materials additives in olive oil at the sub-mg/kg level. Food Addit Contam. 20(7):678-683.
Crossref |
|
|
|
|
Ranalli A, Angerosa F (1996). Integral centrifuges for olive oil extraction – The qualitative characteristics of product. J. Am. Oil Chem. Soc. 73:417–422.
Crossref |
|
|
Reboredo-Rodríguez P. González-Barreiro C. Cancho-Grande B. Simal-Gándara J. (2012) Dynamic Headspace / GC-MS method for the characterization and control of the flavour fingerprint of Extra Virgin Olive Oil. Food Control, 25(2): 684-695.
Crossref |
|
|
|
|
Reboredo-Rodríguez P, González-Barreiro C, Cancho-Grande B, Simal-Gándara J (2013a). Effects of sedimentation plus racking process in the extra virgin olive oil aroma fingerprint obtained by DHS–TD/GC–MS. Food Bioprocess Technol. 6(5):1290-1301.
Crossref |
|
|
|
|
|
Reboredo-Rodríguez P, González-Barreiro C, Cancho-Grande B, Simal-Gándara J (2013b). Concentrations of aroma compounds and odor activity values of odorant series in different olive cultivars and their oils. J. Agric. Food. Chem. 61(22), 5252-5259. |
|
|
Reboredo-Rodríguez P, González-Barreiro C, Cancho-Grande B, Simal-Gándara J (2013c) Aroma biogenesis and distribution between olive pulps and seeds with identification of aroma trends among cultivars. Food Chem. 141(1):637-643.
Crossref |
|
|
|
|
Reboredo-Rodríguez P, González-Barreiro C, Cancho-Grande B, Simal-Gándara J (2014a) Improvements in the malaxation process to enhance the aroma quality of extra virgin olive oils. Food Chem. 158:534-545.
Crossref |
|
|
Reboredo-Rodríguez P, Rey-Salgueiro L, Regueiro J, González-Barreiro C, Cancho-Grande B, Simal-Gándara J (2014b). Ultrasound-assisted emulsiï¬cation–microextraction for the determination of phenolic compounds in olive oils. Food Chem. 150:128–136.
Crossref |
|
|
Reboredo-Rodríguez P, González-Barreiro C, Cancho-Grande B. Simal-Gándara J (2014c). Quality of extra virgin olive oils produced in an emerging olive growing area in north-western Spain. Food Chem. 164:418-426.
Crossref |
|
|
|
Solinas M (1990). Olive oil quality and its determinings factors. In: Proceedings of Problems on Olive Oil Quality Congress, Sassari, Italy. pp. 23–55. |
|
|
Usenik V, Fabcic J, Stampar F (2008). Sugars, organic acids, phenolic composition and antioxidant activity of sweet cherry. J. Food Chem. 107:185-192.
Crossref |