ABSTRACT
The objective of the current study was to evaluate and compare the effectiveness of cranberry extracts and bone marrow cells against chlorambucil (CHB) effect on rats' fertility. Forty adult male albino rats were divided randomly into eight equal groups as the following; normal control, rats injected orally with 0.2 mg/kg of CHB for 14 days, rats injected orally with 100 mg/kg of cranberry extract (CB) for ten days, rats intravenously injected with bone marrow cells (BMC) through tail vain, rats protected with both CB and BMC, rats treated with CHB+CB, rats treated with CHB+BMC and rats treated with CHB+BMC+CB. Genotoxicity were evaluated by counting and comparing the value of sperm abnormalities and normal sperm count. Results show that rats injected with CHB had remarkable increase in sperm head abnormalities as without hook, banana shape and hummer shape. Admission of cranberry extract and bone marrow cells after chemotherapy improved the frequency of the sperm abnormalities.
Key words: Chlorambucil, cranberry, bone marrow, sperm.
Chlorambucil (CHB) is a potent chemotherapy bifunctional alkylation agent which is commonly known as Leukeran and known chemically by IUPAC system as 4-[p-[bis (2chloroethyl) amino] phenyl] butyric acid (Evert et al., 1953). CHB is used as treatment for chronic lymphatic leukemia and other clinical applications such as Hodking's and non-Hodking's lymphoma (Wohrer et al., 2005). Although it has a high therapeutic activity, CHB is a potential human carcinogen with increasing risk towards the development of secondary cancer in patients (Neugut and Ulpric, 2008). The cytotoxicity of CHB is caused by its ability to form a strong covalent bond with proteins, RNA and DNA double strand which leads to structural and functional DNA damage that is considered one of the leading causes of mutagenic and carcinogenic effects (Tew, 2008). CHB also has damaging effects on gonads by causing temporary azospermia and damage on the Leydig cells of male testis. Hence, it causes a malfunction in the reproductive system as well as permanent azospermia when the drug has been taken for long periods or in high doses (De Vita et al., 2001). Bone marrow originated from hematopoietic stem cell and mesenchymal stem cells which give the bone marrow its importance and function; where it has the replacement ability for various types of damaged cells (Teitelbaum, 2000). Bone marrow transplantation is considered as an effective method in the treatment of different diseases like leukemia (Gratwohl et al., 2006), accelerate wound healing in diabetic rats (Mcfarlin et al., 2006), and it is used after a high dose of radiation or chemotherapy to help the body recover from the damaging effects of chemotherapy.
Cranberry (Vaccinium macrocarpon) is a small, dark red fruit that is known as a very rich source of phytochemicals (Cunningham et al., 2003). It contains many active components such as flavonols, sugar, organic acid and flavonoids as anthocyanins and proanthocyanidis (Ariga, 2004). Nowadays, using cranberry has been expanded from using it as a source of healthy food into medical one. Cranberry components exhibit various health benefits including prevention of adhesion of microbes in the urinary tract (Reid et al., 1992) and cholesterol reduction (Reed, 2001). Moreover, it has a very strong antioxidant activity (Yan et al., 2002) and anticancer effects because its activity prevents tumor cells from proliferating (Sun et al., 2002). The mammalian sperm is a terminally differentiated cell which appears deceptively simple (Ramalho-Santos et al., 2002). It has a limited function in the vast majority of creatures which is delivering the haploid genome to the oocyte during fertilization and this function is associated with many physiological, cytological and molecular biology changes that is able to alter animal production, infertility and toxicology. Sperm morphology is used to study the medical and chemical effects on an experimental animal body before it can be implemented into human body (Hu and Yan, 2002). Hence, numerous drug discoveries are tested in rats before approval for human clinical trials (Alias et al., 2011). The purpose of the present study was to evaluate the protective effect of the cranberry extract and bone marrow cells on sperm abnormalities induced by CHB on Swiss albino rats (Rattus norvigicus).
Chemicals
CHB (leukeran) was purchased from (Excella pharma GmbH, Germany) as 2 mg tablet which is dissolved in distilled water and given to animals through gastric tubes. Each animal received (0.2 mg/kg/day) for body weight for fourteen days daily according to Olyinka et al. (2014). Cranberry was purchased from EMA pharma found in capsule form that contains 270 mg of cranberry extraction. Each rat was given cranberry through gastric tube as dose (100 mg/kg/day) for body for ten days daily according to Elberry et al. (2010).
Bone marrow preparation
The bone marrow was taken from young male Swiss albino rats, approximately six weeks old. Recipients and donors were chosen from the same inherited strain (brother to brother) which were sacrificed. Both femurs ends were cut off with sharp bone cutter and then by applying air pressure gently the bone marrow was forced out into small sterile test tube. It was possible to obtain 80:100 mg bone marrow (wet weight) from both femurs, then the weighted bone marrow was suspended in appropriate volume of cold M/15 phosphate buffer (pH=7.2). Rats were injected with 1 ml of bone marrow suspension via tail vein by using a 26 gauge needle (Zowail et al., 2012).
Experimental design
Forty male Swiss albino rats (R. norvigicus) (180 to 200 g) were used in this study. Animals were obtained from the National Research Center in Dokki, Cairo, Egypt. The experimental study was performed as international followed by ethical standards according to the guide for the care and use of laboratory animals of the National Institutes of Health. Animals had free access to water and complete food supplied ad libitum during the whole experimental period and kept under suitable conditions.
The experimental animals were categorized in 8 equal groups, 5 animals each. The animal received the various treatments for different periods as shown in Table 1.
Sperm head morphology assay
The sperm suspension was obtained from rats by excised both caudal epidedymis then they were minced together in isotonic solution and filtered to exclude the large tissue fragments. The sperm suspension was stained with 5% Eosin Y stained (aqueous), then spread on clean glass slides (Mukherjee et al., 1988). One thousand were examined directly under light microscope (100X) to detect the morphological abnormalities in sperm according to the criteria of Wyrobek and Bruce (1975). Any overlay or contact sperms or heads without tails were ignored.
Statistical analysis
Statistical analysis was performed on SPSS software (version18) using one-way analysis of variance (ANOVA). Significance was considered when P values are less than 0.05. Mean standard error (SE) was expressed to all values where 5 animals were evaluated in each group.
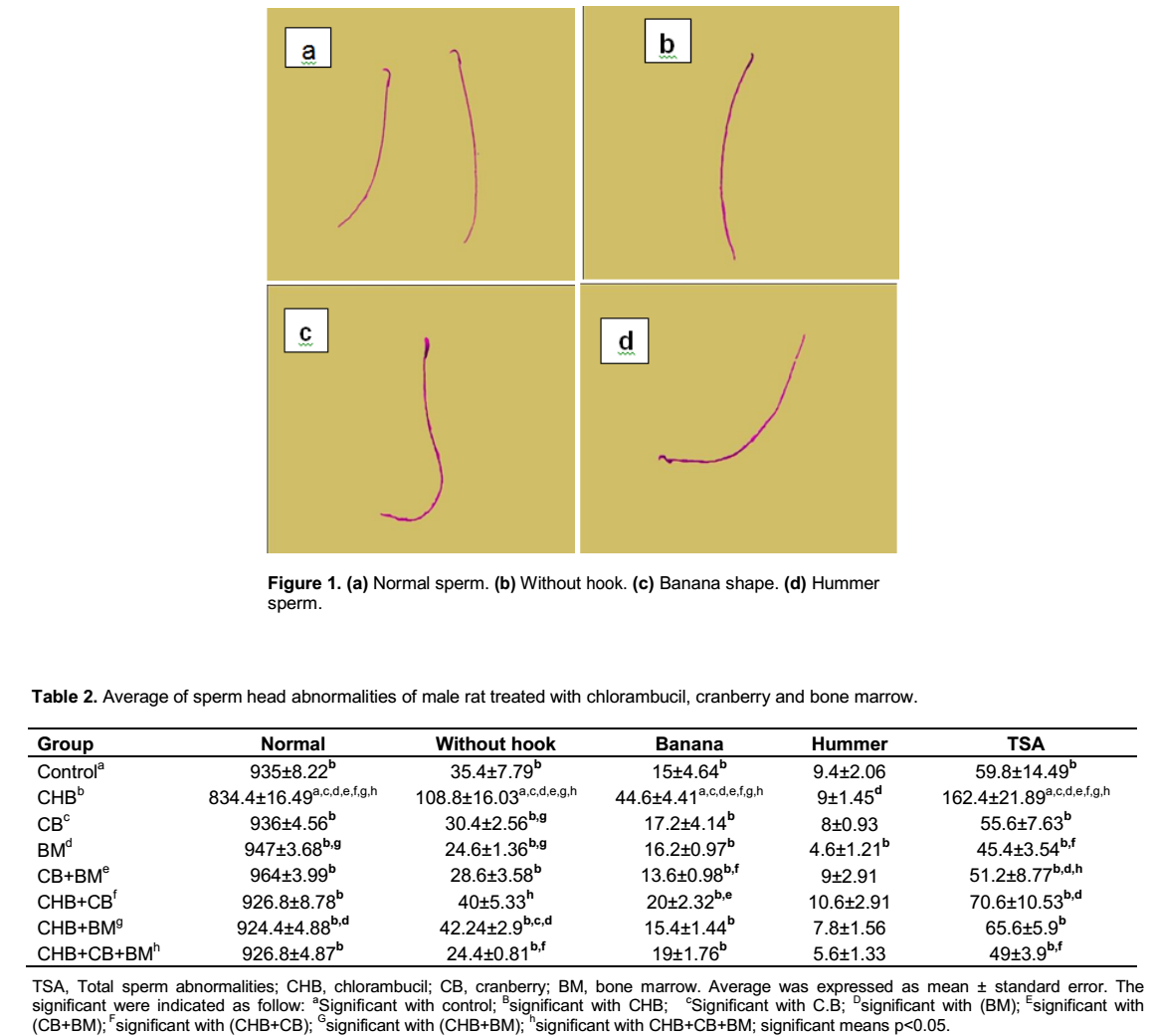
Various types of sperm head abnormalities appeared in Swiss albino rat injected with CHB. These were identified and quantified relatively to control group. The sperm head abnormalities represented as without hook (Figure 1b), banana shape (Figure 1c), and hummer sperm (Figure 1d).
The results illustrated in Table 2 and Figures 2 and 3 show the sperm head abnormalities and the number of normal sperm per five thousand in five rats. The result revealed that CHB when given at the dose of 0.2 mg/kg body weight daily for 14 days induced a high frequency of sperm head abnormalities when compared with control group. Without hook, sperm head shape was the most frequent abnormalities followed by banana and hummer shape. There is a significant increase in the total sperm abnormalities in CHB treated group at P<0.05 over the control group where the mean value of total head abnormalities of CHB compared to the control were (162.4±21.89 vs. 59.8±14.49).


The result in Table 2 shows that when cranberry extract (CB) is injected at given dose (100 mg/kg body weight) daily for ten days and bone marrow (BM) as single dose injection was given separately or together did not induce significant difference of sperm abnormalities or normal sperm count compared to control group. Interestingly, the result indicates that when cranberry and bone marrow were administrated separately with CHB showing significant decrease in the rates of rats sperm head abnormalities changes lower than those treated with CHB only. There is a remarkable significant decrease in the mean value of sperm head abnormalities that appeared in the group treated with CB and BM after CHB compared with CHB group, where the mean value of CHB+CB+BM was 49±3.9 which is lower than CHB group mean value (162.4±21.89).
There are ongoing trials to reduce and eliminate the harmful side effects of chemotherapy on the fertility of patients. Nowadays, the objective of most research is to find out the most effective natural products and strategies to use it for treatment of cancer.
The present study aimed to investigate the protection action of cranberry extract and bone marrow transplantation as the way to reduce the side effects of CHB on sperm abnormalities and total normal sperm count.
Cranberry is considered one of the useful natural products that is used as antibacterial and anticancer drug (Yan et al., 2002) as it has antipoliferative activity to protect body cells from damage (Sun et al., 2002). Cranberry extract has a great ability to inhibit more than 50% of prostate cancer, where polyphenols fraction is the most effective cranberry component against prostate tumor (Seeram et al., 2004). Bone marrow transplantation is considered a useful method that helps in body treatment from malignance tumor as they contain both mesenchymal stem cells that is able to differentiate various types of body cells like hepatic cells (Dai et al., 2009).
The present study results indicate that CHB has a damaging effect on the normal sperm by decreasing its count and increasing the presence of rats sperm head abnormalities as those without hooks, banana shape and hummer head. These results agree with those reported by Viviani et al. (1985) that the combination between alkylating agents as CHB and procarbazine for treating solid Hodgkin's lymphoma showed an excellent high survival rate but the majority of male patients later developed permanent azospermia (Viviani et al., 1985). Wallace et al. (2005) mentioned that cytotoxic chemotherapy such as CHB and melphalan have the ability to cause gonad injury and illustrated that damage nature and extend depends on the dose of the drug given and the period of administration and this injury may be developed to oligospermia or worse condition as azospermia and it indicates that this effect is a result for Leydig cell dysfunction especially after the increasing accumulative dose of gonad toxicity chemotherapy (Wallace et al., 2005). Testes germinal epithelium cells is characterized by rapid divining rate so they are the most sensitive organ to cytotoxic chemotherapeutic agents which lead to permanent damage and disorder in spermatogenesis process (Howell et al., 1999). Haskell study has indicated that chemotherapy targets rapidly dividing cells which causes germinal cell aplasia that quilts the seminiferous tubules and reduces sperm production which may cause oligospermia or azospermia, thereby, affecting the patient’s fertility (Haskell, 2001). The current study result shows a decrease in sperm head abnormalities and an increase in normal sperm count in the group which is protected by cranberry and/or bone marrow separately or in the animals group that received CB and/or BMC after CHB administration. This result agrees with another study was done by Mohammadi who indicated that phytochemical medicines have a potent positive impact on human sperm parameters such as motility, count and viability. Increase in Leydig cell count and seminiferous tubule diameters, improve histopathological recovery of testis, able to decrease abnormal sperm count, enhance sperm motility and increase concentration in ejaculation volume (Mohammadi et al., 2013). Moreover, the study done by Dobrzynska (2013) indicated that resveratol as natural polyphenol (found in cranberry) is considered a promising hope to treat male infertility as it enhances sperm motility and count in rodents. Resveratol was able to inhibit the toxic effect of other agents. Moreover, it has the ability to modulate cell behavior in response to the damaging effects that are induced by radiation. Therefore, using cranberry might be very useful in cancer therapy (Dobrzynska, 2013).
The current study indicates that using cranberry extraction and bone marrow transplantation after CHB administration reduces sperm head abnormality rate and increases the count of normal perm.
The authors have not declared any conflict of interests.
REFERENCES
|
Alias MF, MatIsa NA, Sulaiman SA, Mohamed M (2011). Sprague Dawley Rat Sperm Classification Using Hybrid Multilayered Perceptron Network. J. Wseas Trans. Inf. Sci. Appl. 8(2):109-118.
|
|
|
|
Ariga T (2004). The antioxidative function, preventive action on disease and utilization of proanthocyanidins. Biofactors 21(1-4):197-201.
Crossref
|
|
|
|
Cunningham DG, Vannozzi SA, Turk R, Roderick R, OShea E, Brilliant K (2003). Cranberry phytochemicals and their health benefits Nutraceutical Beverages. Washington, DC, American chemical society: ACS symposium series. Chapter 4. 871:35-51.
|
|
|
|
Dai JL, Ying Li H, XueGuan L, Ritchie G, Zhou J (2009). The therapeutic potential of bone marrow-derived mesenchymal stem cells on hepatic cirrhosis. Stem Cell Res. 2(1):16-25.
Crossref
|
|
|
|
De Vita VT, Hellman S, Rosenberg SA (2001). Cancer Principles & Practice of oncology. 6th Ed. Philadelphia, Pennsylvania: Lippincott Williams & Wilkins. P 2640.
|
|
|
|
Dobrzynska MM (2013). Resveratol as Promising Natural Radio Protector. A Review. J. Rocz. Panstw. Zakl. Hig. 64(4):255-262.
|
|
|
|
Elberry AA, Abdel-Naim AB, Abdel sattar EA, Nagy AA, Mosli HA, Mohamadin AM, Ashour OM (2010). Cranberry (Vaccinium macrocarpon) protects against doxorubicin-induced cardiotoxicity in rats. J. Food Chem. Toxicol. l48(5):1178-1184.
Crossref
|
|
|
|
Evert J, Roberts J, Ross W (1953). Aryl-2-halogenoalkylamines.XII: some carboxylic derivatives of N,N di–2-chloroethylalanine. J. Chem. Soc. pp. 2286-2392.
|
|
|
|
Gratwohl A, Brand R, ApperleyJ,CrawleyC,RuutuT,CorradiniP,Carreras E, Deveragie A, Guglielmi C, Kolb HJ, Niederwieser D (2006). Allogenichematopoietic stem cell transplantation for chronic myeloid leukemia in Europe. J. Haematol. 91(4):513-521.
|
|
|
|
Haskell CM (2001). Cancer treatment: Lymphoid Neoplasm. 4th Edn, WB. U.S.A: Saunders Company, U.S.A. pp. 81-83.
|
|
|
|
Howell SJ, Radford JA, Ryder WD, Shalet SM (1999). Testicular Function after Cytotoxic Chemotherapy: Evidence of Leydig Cell insufficiency. J. Clin Oncol. 17(5):1493-1498.
|
|
|
|
Hu JH, Yan YC (2002). Identificationof Γ1 subunit of gabaa a receptor in rat testis. J. Cell Res. 12(1):33-37.
Crossref
|
|
|
|
McFarlin K, Gao X, Liu Y, Dulchavsky DS, Li Y, Chopp M, Dulchavsky S, Gautam SC (2006). Bone marrowâ€derived mesenchymal stromal cells accelerate wound healing in the rat. Wound Repair and Regeneration. Wound Repair Regen. 14(4):471-478.
Crossref
|
|
|
|
Mohammadi F, Nikzad H, Taherian A, Mahabadi JA, Salehi M (2013). Effects of herbal medicine on male infertility.J. Anat. Sci. 10(4):3-16.
|
|
|
|
Mukherjee A, Giri AK, Sharma A, Talukder G (1988).Relative efficiency of short-term tests in detecting genotoxic effects of cadmium Chloride in mice in vivo. J. Mutat. Res. 206(2):285-295.
Crossref
|
|
|
|
Neugut AI, Ulpric T (2008). Management of drug toxicity.Perry MC.The chemotherapy source book. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, 5th Edition, Chap. 26.
|
|
|
|
Olyinka ET, Ore A, Fashiku KA (2014). Kolaviron and L-Ascorbic acid attenuate Chlorambucil–induced testicular oxidative stress in rats. J. Toxicol. 2014:1-9.
Crossref
|
|
|
|
Ramalho-Santos J, Schatten G, Moreno RD (2002). Control of membrane fusion during spermiogenesis and the acrosome reaction. J. Biol. Reprod. 67(4):1043-1051.
Crossref
|
|
|
|
Reed J (2002). Cranberry flavonoids, atherosclerosis and cardiovascular health. J. Crit. Rev. Food Sci. Nutr. 42(3 Suppl):301-316.
Crossref
|
|
|
|
Reid G, Denstedt JD, Kang YS, Lam D, Nause C (1992). Microbial adhesion and biofilm formation on ureteral stents in vitro and in vivo. J. Urol. 148(5):1592-1594.
|
|
|
|
Seeram NP, Adams LS, Hardy ML, Heber D (2004). Total Cranberry Extract versus Its Phytochemical Constituents: Antiproliferative and Synergistic Effects against Human Tumor Cell Lines. J. Agric. Food Chem. 52(9):2512-2517.
Crossref
|
|
|
|
Sun J, Chu YF, Wu X, Liu RH (2002).Antioxidant and antipoliferative activities of common fruits. J. Agric. Food Chem. 50(25):7449-7454.
Crossref
|
|
|
|
Teitelbaum SL (2000): Bone Resorption by osteoblasts. J. Sci. 289(5484):1504-1508.
Crossref
|
|
|
|
Tew K (2008). Alkylating Agents. In. DeVita V, Lawrence T, Rosenberg S. Cancer: principles and practice of oncology. 8th edn. Lippincott Williams &Wilkins, Philadelphia. pp. 407-419.
|
|
|
|
Viviani S,Santoro A,Ragni G, Bonfante V,Bestetti O,Bonadonna G (1985). Gonadal toxicity after combination chemotherapy for Hodgkin's disease. Comparative Results of MOPP Vs ABVD. Eur. J. Cancer Clin. Oncol. 21(5):601-605.
Crossref
|
|
|
|
Wallace WHB, Anderson RA, Irvine DN (2005).Fertility preservation for young patients with cancer: Who is at risk and what can be offered?. J. Lancet Oncol. 6(4):209-218.
Crossref
|
|
|
|
Wohrer S, Raderer M, Kaufman H, Hejna M, Chott A, Zielinki C, Drach J (2005). Effective treatment of indolent non-hodgkin's lymphomas with mitoxantrone, Chlorambucil and prednisone. Oncol. Res. Treat. 28(2):73-78.
Crossref
|
|
|
|
Wyrobek A, Bruce W (1975).Chemical induction of sperm abnormalities in mice. J. Proc. Natl. Acad. Sci. 72(11):4425-4429.
Crossref
|
|
|
|
Yan X, Murphy BT, Hammond GB, Vinson JA, Neto CC (2002). Antioxidant activities and antitumor screening of extracts from cranberry fruit (Vaccinium macrocarpon). J. Agric. Food Chem. 50(21):5844-5849.
Crossref
|
|
|
|
Zowail MEM, Waer HFM, Eltahawy NA, Khater EHS, Abd El-hady AM (2012). Curative Effect of Bone Marrow Cells Transplantation and/or Low Dose Gamma Irradiation on Liver Injuries Induced by Carbon Tetrachloride. Egypt. J. Hosp. Med. 46:96-114.
|