ABSTRACT
A thermotolerant strain of Rhizopus oryzae was grown in three agro-industrial by-products: brewers’ rice, corn grits and wheat bran. Different substrates, cultivation time, moisture content, additional nitrogen sources, pH and temperature of incubation were evaluated aiming to optimize growing conditions. The highest enzymatic activity was observed after 24 h of cultivation using wheat bran as substrate with the following salt solutions: NH4NO3, MgSO4.7H2O and (NH4)2SO4 0.1% at temperature of 35°C. It was observed that changes in the pH range 4.0-6.0 did not significantly affect α-amylase activity. The optimum operation conditions were 75°C and pH 4.5. The enzymes remained stable at 75°C in the absence of substrate for 25 min.
Key words: Starch hydrolysis, fermentation parameters, amylolytic enzyme.
Amylases are a group of enzymes commonly used in the food, textile, pharmaceutical and detergent industries and in the sugar-energy sector. Several microorganisms have been investigated for potential production of this enzyme. However, only a few strains of fungi and bacteria meet the criteria for production of this commercial enzyme (Souza and Magalhães, 2010).
Submerged fermentation is commonly used for amylase production, but solid-state fermentation (SSF) is emerging as a promising technology with the use of several agro-industrial by-products as substrates (Balkan and Figen, 2010). Most substrates used in SSF are agro-industrial by-products such as soy bran, rice bran, cassava bagasse, wheat bran and sugarcane bagasse among others (Bhargav, 2008). These by-products usually have several substances of high nutritional value, essential for the cultivation of microorganisms, especially filamentous fungi, which have the ability to convert these materials into other biomolecules or materials that can be used in different processes (Couto and Sanromán, 2006).
In addition to the type of substrate, enzyme production by microorganisms is affected by several factors, such as fermentation time, and by physicochemical factors such as moisture, pH, temperature and nitrogen sources (Pandey et al., 2000). This study aimed to evaluate the type of substrate, cultivation time, nutrient supplemen-tation, moisture, pH, and temperature, parameters that can affect the activity of α-amylase using a Rhizopus oryzae strain.
Media, cultivation of microorganism and enzyme production
Some agro-industrial by-products of rice, corn and wheat were used as substrates for the strain cultivation because, in addition to their high availability and low cost, they have great potential for production of amylases. All substrates were obtained from pet food and supply stores in the municipality of Frutal, MG, Brazil and were sieved through a 9-mesh sieve. Sugarcane bagasse (10%, total amount of the culture medium) was used to support the growth of the amylolytic strain.
The fungus was grown in 500 mL-Erlenmeyer flasks containing Sabouraud agar medium supplemented with 1% starch, and it was incubated at 35°C for 48 h. In order to obtain mycelial suspension, 100 mL of distilled sterile water were added to each vial containing the fungus. The direct spore count was performed by optical microscopy using a Neubauer chamber.
Cultivation of R. oryzae in the agro-industrial by-products was performed on 250 mL Erlenmeyer flasks containing 5 g of each substrate, which were autoclaved for 40 min at 120°C. Initially, the fermentation media were inoculated with the mycelial suspension in saline solution composed of 1% (p v-1) of (NH4)2SO4, MgSO4.7H2O and (NH4NO3), with 105 mL-1 spores and incubated for 168 h. The initial moisture content was 70%; samples were taken every 24 h to determine enzyme activity.
To obtain the enzyme extract, 40 mL of distilled water were added to each flask; the flasks were shaken on a horizontal shaker at 100 rpm for 30 min; the extract was filtered in a funnel lined with gauze, and centrifuged at 1800 g for 20 min; the supernatant containing the crude enzyme extract was obtained.
Two additional nitrogen sources were evaluated: an inorganic source, 1% (p v-1) of (NH4)2SO4, MgSO4.7H2O and (NH4NO3), and an organic source composed of 1% (p v-1) soy bran; both were sterilized at 121°C for 30 min. Distilled water was used as the control. After defining the best nutrient supplemental source (or the additional nitrogen source), three moisture content values (50, 60 and 70%) were evaluated. Enzymatic activity was evaluated every 24 h of cultivation.
The incubation temperatures of 30, 35, 40, and 45°C and the pH values of 4.0, 4.5, 5.0, 5.5 and 6.0 were evaluated under optimum conditions of substrate, supplemental nutrient source, moisture, and cultivation time.
Enzyme activity measurements
The α-amylase activity was determined by measuring the decrease in iodine-binding capacity, between starch and iodine, of a starch solution treated with a crude enzyme solution, according to the method described by Fuwa (1954). One enzyme unit (U) was defined as the amount of enzyme needed to hydrolyze 10 mg of starch in 10 min under the assay conditions.
Enzyme characterization
To determine the optimum pH of enzymatic activity, the crude extract was incubated at the pH range 3.0-10.5 and 60°C using the following buffer systems: acetate (pH 3.0-5.5), citrate/NaOH (pH 5.5-7.0), Tris-HCl (pH 7.0-8.5) and glycine/NaOH (pH 8.5-10.5).
To determine the optimum temperature of enzyme activity, the enzyme extract was incubated at the previously determined optimum pH and at temperatures ranging from 30 to 90°C with variation range of 5°C. The enzymatic activity was measured using the dextrinizing method. The stability of the enzyme was assessed by incubating the crude enzyme solution in screw-cap tubes at the previously determined pH and temperature values until it exhibited no activity. Samples were taken every 2 min, and residual activity was measured by the dextrinizing method.
Statistical analysis
The data obtained were analyzed using the Microcal Origin 6.1 software. Analysis of variance and comparison of means by the Tukey test at 5% probability were also conducted. All experiments were performed in triplicate.
Under the initial conditions evaluated, the highest α-amylase activity in R. oryzae was achieved after 24 h of fermentation with the production of 6.350 U mL-1 on wheat bran (Figure 1). On the brewers’ rice substrate, the highest activities were achieved after 120 h of fermentation, with values ??of 2.906 and 2.963 U mL-¹, respectively; on corn grits, it was achieved after 120 h of fermentation and exhibited lower enzymatic activity (1.835 U mL-¹) (Figure 1).
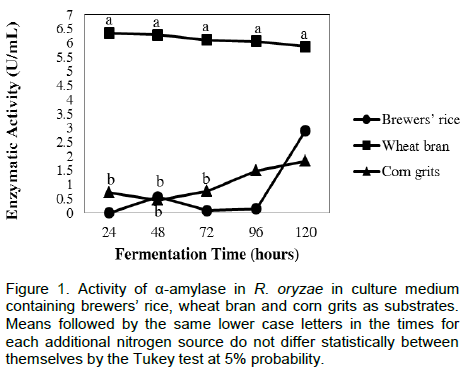
The supplementation with the salts suspension increased the synthesis of α-amylase by R. oryzae on wheat bran up to 96 h of fermentation. However, there was a decrease in the enzyme activity when corn grits and rice bran were used as substrates (Table 1).
The absence of salts increased the enzyme activity (5.295 U mL-¹) in the substrate containing rice after 48 h of fermentation; the highest activity value was achieved after 72 h of fermentation (6.100 U mL-¹). The lowest enzyme activity was achieved using the corn based sub-strate (Table 1), regardless of supplementation with salts.
There was a significant reduction in enzyme activity by the fungus when soy bran suspension (1%) was used in the rice and corn based substrates. There was a significant reduction in enzyme activity by the fungus when soy bran suspension (1%) was used in the rice and corn based substrates. These results similar to those obtained by Celestino et al. (2014), who found that the use of supplemental organic solution Aspergillus oryzae was less effective in the production of α - amylase (Table 1). In the period of time at which there was greater activity (24 h) on wheat bran, the absence of supplementation delayed the synthesis of α-amylase.
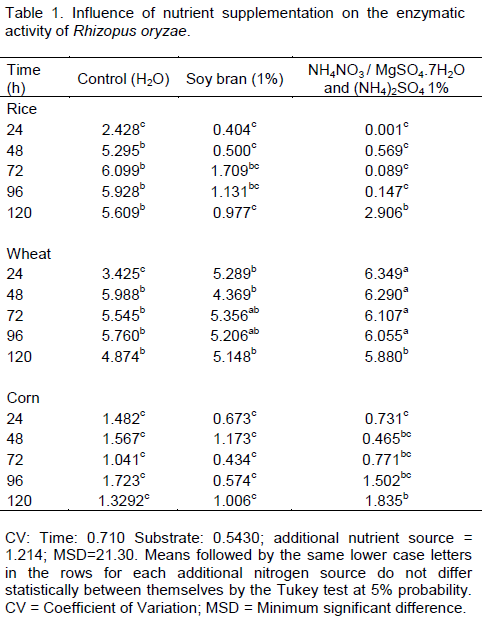
According to Bakir et al. (2001), R. oryzae showed higher affinity for inorganic nitrogen sources for xylanase production. This behavior was also observed in the present study for the synthesis of α-amylase when wheat bran was supplemented with soy bran and inorganic salt solution. The time for higher production of enzyme was reduced from 72 to 24 h, respectively (Table 1).
In the culture medium containing wheat bran as substrate, the moisture contents did not significantly affect the synthesis of α-amylase by R. oryzae. This was the best substrate for enzyme production under the three moisture conditions evaluated (Figure 1).
As for the substrate containing brewers’ rice, the reduced moisture favored α-amylase activity and there was significant difference between the moisture contents of 50 and 60%; for moisture content of 50%, the highest values ??were achieved after 24 and 48 h (3.769 U mL-¹ and 2.812 U mL-¹, respectively) and 120 h (3.542 U mL-¹) of fermentation, and for moisture content of 60%, the highest values ??were observed after 72 h (3.205 U mL-¹)
and 96 h (3,861 U mL-¹) of fermentation (Figure 2).
Moisture content of 60% was favored with the use of the substrate containing corn grits, since after 120 h of fermentation, the highest activity (6.279 U mL-¹) was achieved, which is similar to that obtained with the substrate containing wheat bran (Figure 2). The results obtained for brewers’ rice and corn corroborate those found by Ramachandran et al. (2004), who used A. niger
and reported that the best moisture content to obtain amylase was 64%. Tunga and Tunga (2003) who used A. oryzae strains in solid state fermentation of sugarcane bagasse and reported the need for higher moisture content for amylase production, achieving highest production of this enzyme after 72 h of fermentation with approximately 80% moisture.
Guandalini (2007), studying Metarhizium anisopliae and using starch waste as a substrate for amylase production, obtained results similar to those found in the present study in brewers’ rice and corn grit substrates, in which the highest values ??of enzyme activity decreased with increasing moisture.
After determining the best substrate and the parame-ters: cultivation time (24 h), nutrient supplementation with inorganic salt solution, and initial substrate moisture content of 70%, the evaluation of the optimum tempera-ture of incubation of the fungus and the initial pH of the best substrate (wheat bran) was performed. There was no significant difference in the parameters pH and temperature, except for the temperature of 45°C, at pH 4.0 and 4.5 (Table 2).
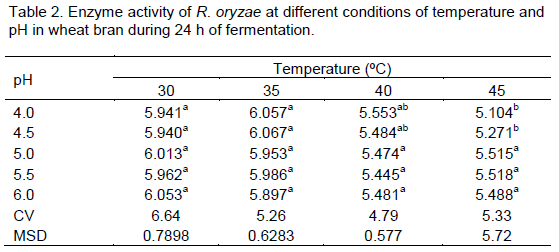
Means followed by the same lower case letters in the rows for each additional temperature do not differ statistically between themselves by the Tukey test at 5% probability. CV = Coefficient of Variation; MSD = minimum significant difference.
Studying thermotolerant R. oryzae strains, Kitpreechavanich et al. (2008), found that the fungus had the ability to withstand and grow up to 45°C, and the highest mycelium growth occurred at 34°C. Huang et al. (2005) investigated the impact of temperature on the synthesis of lactic acid in R. oryzae and Rhizopus arrhizus using potato starch waste wastewater as substrate; the authors reported an increase in the hydrolysis of the substrate starch at 40°C. Peixoto et al. (2003) studied a Rhizopus microsporus strain and observed that it was highly tolerant to high temperatures, and that there was a 4-fold increase in the amylase synthesis at 50°C.
The pH of the culture medium is one of the most important factors to be controlled; pH changes during the growth of a microorganisms directly affects the synthesis and stability of excretory-secretory products in the culture media. Liao et al. (2007) investigated the formation of pellet in R. oryzae and observed that there were no significant differences on pellet formation at pH of 3.0-7.0. The strain studied in the present study exhibited similar behavior when evaluated at the pH range between 4.0 and 6.0 without affecting the α-amylase synthesis. This behavior indicates that this strain is probably not very sensitive to changes in pH; a fact that has already been highlighted by other authors in other species of filamentous fungi, such as Aspergillus niger and Penicilium chirysogenun (Galbraith and Smith, 1969) and Syncephalastrum racemosum (Freitas et al. 2014)..
Analyzing the graph of enzyme activity in terms of changes in pH (Table 2, Figure 3), it can be said that there is more than one type of enzyme in the enzyme extract since there is more than one enzyme activity peak; the first peak is between pH 4.5- 6.0 and the second, with lower values, one is near pH 8.5.
The profile of the enzyme produced by R. oryzae is desirable since according to the literature, the optimum pH of activity of most enzymes is between 4.0-5.5. The results of this study highlight the abilities of this strain to synthesize active enzymes at alkaline pH. It is worth mentioning that there are few enzymes with this pH profile, and that they have become highly sought after.
Souza and Magalhães (2010), in a literature review on α-amylase, found that of 18 species of fungi investigated only three displayed activity at pH between 7.0 and 9.0; all others were most active at pH between 4.75 and 6.0. Michelin et al. (2010) reported that the highest α-amylase activity produced by Paecilomyces variotti occurred at acidic pH.
The α-amylase from R. oryzae was found to be thermophilic since it exhibited highest activity at 75°C (Figure 3), which is a desirable result considering that, according to the literature data, most fungi strains exhibit a range of α-amylase activity within a much narrower range of temperature (50-60°C). Michelin et al. (2010) reported that the optimal temperature of an α-amylase produced by Paecilomyces variotti was 60°C. Souza and Magalhães (2010) in a literature review on the application of α-amylase in the industry found that among 10 species of yeasts and fungi filaments investigated, none exhibited optimum temperature of enzyme activity higher than 75°C.
In another literature review on α-amylase, Freitas et al. (2014) also found that among the fungal species investigated, none showed stability above 70°C. Haki et al. (2003), considering industrial development and the application of thermostable enzymes, reported that the majority of α-amylases of fungal origin are thermostable between 50 and 60°C.
The half-life of the fungal α-amylase was approximately 50% in 25 min at 70°C although the literature reports much higher α-amylase thermal stability values. Michellin et al. (2010), studying Paecilomyces variotti, found that this enzyme was stable for 60 min at 55°C; this characteristics allow its use in industrial processes in which no residues of the enzyme is desired, for example in the sugar production from sugarcane.
The R. oryzae strain was proven to be effective for α-amylase production in the substrates studied, showing the feasibility of using agro-industrial by-products as substrates for the production of this enzyme, with optimal operation conditions at 75°C, pH 4.5, and stability at 75°C in the absence of substrate for 25 min.
The authors have not declared any conflict of interests.
The authors are grateful to the postgraduate program in Agropecuaria Microbiology; the financial support provided by the University of Minas Gerais, Frutal and Minas Gerais State Research Foundation (FAPEMIG) the granting of scholarships for the postgraduate program in FCAV / UNESP.
REFERENCES
|
Bhargav, S, Panda,BP, Ali, M, Javedb, S (2008). Solid-state Fermentation: An Overview, Chem. Biochem. Eng. Q. 22(1):49-70.
|
|
|
|
Bakir U, Yavascaoglu S, Guvenc F, Ersayin A (2001). An endo-β-1,4-xylanase from Rhizopus oryzae: production, partial purification and biochemical characterization. J. Enzyme Microb. Technol. 29:328-334.
Crossref
|
|
|
|
|
Balkan B, Figen E (2010). The production of a new fungal a-amylase degraded the raw starch by means of solid-state fermentation. Prep. Biochem. Biotechnol. 40(3):213-228.
Crossref
|
|
|
|
|
Celestino, JR, Duarte, AC, Silva, CMM, Sena, HH, Ferreira, MPSBC, Mallmann, NH, Lima, NPC, Tavares, CC., Souza, ROS, Souza, ES, Souza, JVB (2014). Aspergillus 6V4, a strain isolated from manipueira, produces high amylases levels by using wheat bran as a substrate. Enzyme Res. 1-4.
Crossref
|
|
|
|
|
Couto SR, Sanromán A (2006). Application of solid-state fermentation to food industry- A review. J. Food Eng. 76:291-302.
Crossref
|
|
|
|
|
Freitas, LS, Martins, ES, Ferreira, OE (2014). Produção e caracterização parcial de α-amilase de Syncephalastrum racemosum. R. bras. Bioci., Porto Alegre 12(4):226-232.
|
|
|
|
|
Fuwa H (1954). A new method for microdetermination of amylase activity by the use of amylose as substrate. J. Biochem. 41:583-603.
|
|
|
|
|
Galbraith JC, Smith JE (1969). Sporulation of Aspergillus niger in submerged liquid culture. J. Gen. Microbiol. 59:31-45.
Crossref
|
|
|
|
|
Guandalini NC (2007). Estudo da produção de enzimas amilolíticas pelo fungo Metarhizium ansiopliae utilizando resíduos amiláceos como substrato. Campinas. 77p. Dissertação (Mestrado). Universidade Estadual de Campinas, Campinas.
|
|
|
|
|
Huang Li P, Jin Bo, Lant P, Zhou J (2005). Simultaneous saccharification and fermentation of potato starch wastewater to lactic acid by Rhizopus oryzae and Rhizopus arrhizus. J. Biochem. Eng. 23:265-276.
Crossref
|
|
|
|
|
Kitpreechavanich V, Maneeboon T, Kayano Y, Sakai K (2008). Comparative Characterization of L-Lactic Acid-Producing Thermotolerant Rhizopus Fungi. J. Biosci. Bioeng. 106(6):541-546.
Crossref
|
|
|
|
|
Liao W, Liu Y, Frear C, Chen S (2007). A new approach of pellet formation of a Wlamentous fungus – Rhizopus oryzae. Bioresour. Technol. 98:3415-3423.
Crossref
|
|
|
|
|
Michelin M, Silva TM, Benassi VM, Peixoto-Nogueira SC, Moraes LAB, Leão JM, Jorge JA, Terenzi HF, Polizeli MLTM (2010). Purification and characterization of a thermostable α-amylase produced by the fungus Paecilomyces variotii. Carbohydr. Res. 345(16): 2348-2353.
Crossref
|
|
|
|
|
Pandey A, Soccol CR, Mitchell D (2000). New developments in solid state fermentation: I-bioprocesses and products. Proc. Biochem. 35:1153-1169.
Crossref
|
|
|
|
|
Peixoto SC, Jorge JA, Terenzi HF, Polizeli MLTM (2003). Rhizopus microsporus var. rhizopodiformis: a thermotolerant fungus with potential for production of thermostable amylases. Int. Microbiol. 6:269-273.
Crossref
|
|
|
|
|
Souza PM, Magalhães PO (2010). Application of microbial amylase in industry – a review. Braz. J. Microbiol. 41(4):850-861.
Pubmed
|
|
|
|
|
Tunga R, Tunga, BS (2003). Extra-cellular Amylase Production by Aspergillus oryzae Under Solid State Fermentation. Japan: International Center for Biotechnology, Osaka University. 12p.
|
|