ABSTRACT
Lippia alba essential oils were obtained by hydrodistillation (HD) and supercritical fluid (SFE) extraction methods. These were analyzed by gas chromatography-mass spectrometry-flame ionization detector (GC-FID-MS). Antioxidant activity was tested by DPPH and ABTS methods, and total soluble phenolics (TSP) were also determined. While in the SFE extract 14 compounds were identified (mostly: myrcenone 3.4%, α-terpineol 1.0% and β-caryophyllene 2.3%), in the HD extract 17 compounds were identified (mostly: eucalyptol 15.6%, myrcenone 9.3% and Z-ocimenone 5.8%). The results showed that L. alba essential oils obtained by SFE was IC50=17.35 mg/mL and by HD was IC50 = 12.45 mg/mL in DPPH assay. L. alba has an excellent eucalyptol content.
Key words: Antioxidants, bioactive compounds, supercritical fluid, green technologies.
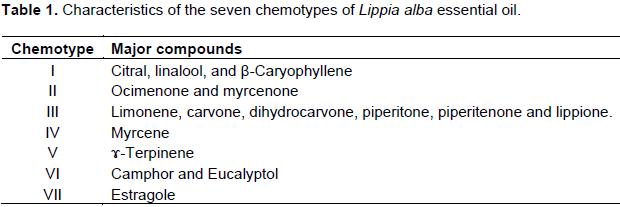
The Verbenaceae family consists of 76 genera with over 2000 species widely distributed across almost all the planet. The genus Lippia includes about 200 species that are abundantly present in Central America, South America and Africa. L. alba produces aromatic leaves and flowers and aerial portions of the plant are routinely used in native medicines. Some of the medicinal properties of L. alba or other Lippia species have been attributed to the presence of biologically active volatile components found in the essential oil of the plant (Hennebelle et al., 2006). The composition of the essential oil is variable and depends on the characteristics of the geographic location, climate and soil. Based on the identity of the major constituents found in essential oil samples from around the world, it is proposed that at least seven chemotypes exist (Table 1) (Hennebelle et al., 2006). Although the essential oil composition of L. alba samples from many regions has been reported, there are presently no reports for the analysis of plants harvested in Mexico (García-Abarrio et al., 2014; Linde et al., 2016; Braga et al., 2005). In Mexico, L. alba is an aromatic shrub or tree, up to 2 m tall; this plant is popularly known by the name of mints leaves (Willman et al., 2000). The leaves and flowers of this plant are commonly prepared as an infusion or decoction that is employed as folk remedies for the treatment of diarrhea and stomach ache (Pascual et al., 2001).
Aromatic and medicinal plants have been extensively studied for their antioxidant activity among other functional properties. This is mainly due to an obvious consumer preference for natural ingredients combined with concerns about toxic effects of synthetic antioxidants (Agnaniet et al., 2005; Wojdylo et al., 2007). Essential oils are composed of many chemical compounds; therefore some of them might be regarded as valuable components and might exhibit antioxidant potency (Kamaliroosta et al., 2012).
With the exception of the work by Stashenko et al. (2004), L. alba essential oil extracts have been principally obtained by a singular method, either hydrodistillation or microwave radiation-assisted hydrodistillation (Bahl et al., 2000; Lorenzo et al., 2001; Fischer et al., 2004; Mesa-Arango et al., 2009; Escobar et al., 2010). The Supercritical Fluid Extraction (SFE) systems extract chemical compounds normally using supercritical carbon dioxide instead of an organic solvent, as compared to the hydrodistillation method. The supercritical fluid state occurs when a fluid is above its critical temperature (Tc) and critical pressure (Pc), when it is between the typical gas and liquid state (Oliveira et al., 2016; Zermane et al., 2014; Bagheri et al., 2014).
Stashenko et al. (2004) compared different methods of extraction in terms of composition. Extracts were obtained through hydrodistillation, microwave radiation-assisted hydrodistillation, distillation solvent extraction and supercritical fluid extraction and their results showed that the method of extraction affected quantitatively the essential oil composition. In other work, Duran et al. (2007) found that extraction time directly impacted the composition of L. alba essential oil extracts prepared by microwave radiation-assisted hydrodistillation. The influence of extraction method on the resulting essential oil composition has also been reported for clove buds, patchouli, and Mirtus communis L. (Wenqiang et al., 2007; Donelian et al., 2009; Ghasem et al., 2011). Thus, it appears essential to use more than a single extraction technique when characterizing a plant’s volatile secondary metabolite composition (Stashenko et al., 2004).
Given the above, this work aimed to obtain fractions of essential oil of L. alba by supercritical CO2 and hydrodistillation, and to evaluate their chemical composition and antioxidant activity.
Plant material
L. alba leaves were collected in Sinaloa, Mexico (24°45´56.8´´N, 107°39´03.6´´W), and plants were confirmed and authenticated by the botanical researcher Rito Vega-Aviña from the herbarium “Jesus Gonzalez-Ortega” located at the Agricultural School from the Sinaloa State University [Lippia alba (Mill.) N. E. Br. ex Britton & Wilson, ACJA 85 (UAS), AEJA 43 (UAS); PTJL 86 (UAS); VAR 7248 (UAS)]. The leaves (old and young) were separated from the plant manually and combined into a single lot. L. alba leaves were dried in an oven at 40°C for 12 h. The dried leaves were ground with a grinder to produce a fine powder that was stored at room temperature in polyethylene bags.
Chemicals
Cinnamon essential oil, butylated hydroxytoluene (BHT), carvacrol, limonene, carvone, geraniol, camphor, citral, p-cymene, thymol eucalyptol, β-caryophyllene, cinnamaldehyde, benzyl alcohol, estragole, myrcene, α-terpineol, caryophyllene oxide, linalool, eugenol and ɤ-terpinene were purchased from Sigma Aldrich (St Louis, Mo, USA). Origanum oil was purchased from LKT Laboratories. Oregano, lemongrass and chinese cinnamon oils were purchased from Lhasa Karnak Herb company (Berkeley, Ca, USA). Piperitone was purchased from TCI America (Portland, Or, USA). Dichloromethane (HPLC grade) was purchased from Acros (New Jersey, USA) and methanol (HPLC grade) was purchased from Fisher Scientific (New Jersey, USA). Kovats standard (C7-C30) was purchased from Supelco (Bellefonte, PA, USA). 2,2-diphenyl-1-picrylhydrazyl (DPPH) was purchased from MP Biomedicals (Aurora, Ohio,USA).
Hydrodistillation
Dried leaves (10 g of powder) were submitted to hydrodistillation for 2 h, using a distilling flask, a vigrex column, a condenser and a receiving vessel. The essential oil layer and aqueous layers were separated and the oil collected. The oil was weighed using an analytical balance (Mettler-Toledo, Columbus, OH, USA) and stored in a glass vial at 4°C protected from light.
Supercritical fluid extraction
Extraction of L. alba essential oil was prepared using a supercritical fluid equipment (Model SFT-150, Supercritical Fluid Technologies, Inc., Newark, DE, USA). The flow rate of supercritical CO2 was 5 mL/min. The extraction vessel (100 mL) was charged with 10 g of plant powder. The conditions of extraction were 47°C and 3800 Psi. The extract was collected in a glass vial. In order to improve the collection efficiency, the collected vial was placed in an ice bath during the dynamic extraction. The extract weight was measured using an analytical balance and stored in a glass vial at 4°C protected from the light.
GC and GC/MS analyses
GC analyses were performed using a Hewlett-Packard 6890 series gas chromatograph (Wilmington, DE, USA) equipped with a flame ionization detector (FID) and a DB-1 column (60 m × 0.2 mm i.d. x 0.25 µm of film thickness) (Agilent Technologies, Wilmington, DE, USA). Oven temperature was programmed at 45°C (2 min), and then increased to 270°C (20 min) at a rate of 4.5°C/min. Injector temperature was 250°C and detector temperature was 270°C. Helium was used as carrier gas with a linear velocity of 32 mL/min. The samples (1 µL) were injected using the splitless mode.
The GC/MS analyses were carried out on two separate systems. The first system consisted of a HP-589066 with a DB-Wax column (60 mx 0.25 mm i.d. × 0.25 µm of film thickness) coupled to a HP-5972 MSD. The GC oven temperature was programmed from 35°C hold for 5 min to 230°C hold for 40 min at 4.5°C/min. Injector temperature was 250°C. The temperature of the ionization chamber was 285°C. The second system was a HP-6890 series (Wilmington, DE, USA) with a DB-1 column (60 m × 0.2 mm i.d. x0.25 µm of film thickness) coupled to a HP-5972 MSD (Agilent Technologies, Wilmington, DE, USA). The GC oven temperature was programmed from 45°C hold for 5 min to 270°C hold for 20 min at 4.5°C/min. Injector temperature was 250°C. The temperature of the ionization chamber was 285°C. The samples were diluted in dichloromethane and 1 µL of the solution were injected using the splitless mode. The compounds of the extracts were identified by Kovats retention indices and comparing their mass spectra with those in the NIST 2011 mass spectra library and with authentic standards.
Antioxidant activity
DPPH radical assay
The radical scavenging capacity was determined using 2,2-diphenyl-1-picrylhydrazyl (DPPH) following the Agnaniet et al. (2005) method with some modifications. Cinnamon, oregano, origanum, Chinese cinnamon and L. alba (HD) extracts and standard samples were dissolved in methanol at different concentrations. Supercritical fluid extraction of L. alba extracts was dissolved in methanol, centrifugated (1300 rpm, 5 min at 5°C) and then filter (13 mm and 0.22 µm porosity, PVDF. Millex-GV, Millipore). DPPH was dissolved in methanol to give a 100 µM solution. To 1.2 mL of the methanolic solution of DPPH were added 100 µL of a methanolic solution of the antioxidant compounds (standards and essential oils) at different concentrations. The control is represented by the DPPH methanolic solution containing 100 µL of methanol. After a 30 min incubation period at room temperature the decrease in absorption at 517 nm was measured with a SpectraMax Plus 384 spectrophotometer (Molecular Device, USA). Antioxidant activity was reported as IC50 (the amount of antioxidant necessary to decrease the initial DPPH concentration by 50%) and was calculated using SoftMax® Pro software Version 5.0.1 (Molecular Devices Corp., USA). BHT was used as a positive control. The assay was carried out in triplicate and results are reported as averages.
ABTS radical cation decolorization assay (TEAC)
The antioxidant activities of essential oils were determined by the method of Re et al. (1999) with some modifications. Cinnamon, oregano, origanum, Chinese cinnamon and L. alba (HD) oils were dissolved in methanol. Supercritical fluid extraction of L. alba essential oil was dissolved in methanol, centrifuged (1300 rpm, 5 min at 5°C) and then filtered (13 mm and 0.22 µm porosity, PVDF. Millex-GV, Millipore). ABTS was dissolved in water (HPLC grade) to a 7 mM concentration. ABTS radical cation was produced by reacting ABTS stock solution with 2.45 mM potassium persulfate and allowing the mixture to stand in the dark at room temperature for 16 h before use. For the determination of antioxidant activity the ABTS radical solution was diluted with methanol to an absorbance of 0.70 ± 0.01 at 734 nm. To 20 µL of the sample was added 400 µL of diluted ABTS radical solution. After incubation for 6 min at 30°C, the absorbance was measured with a SpectraMax Plus 384 spectrophotometer at 734 nm. Ascorbic acid (0.05 mg/mL) and BHT (0.125 mg/mL) were used as a positive control and cinnamic acid (1 mg/mL) as a negative control. The activities of essential oils were estimated within the range of Trolox calibration curve and results were expressed as µmol Trolox equivalent per gram (Tr eq µmol/g). The assay was carried out in triplicate and results are reported as averages.
Total soluble phenolics
The Folin-Ciocalteau method for the colorimetric estimation of total polyphenols was adapted to a 96-well plate format according to Breksa et al. (2010). Cinnamon, oregano, origanum, chinese cinnamon and L. alba (HD) oils were dissolved in methanol. Supercritical fluid extraction of L. alba essential oil was dissolved in methanol, centrifuged (1300 rpm, 5 min at 5°C) and then filtered (13 mm and 0.22 µm porosity, PVDF. Millex-GV, Millipore). Standards (100 µL) were mixed with water (1500 µL) in a 2 mL polypropylene plate. Trolox (250 µg/mL) and BHT (250 µg/mL) were used as positive controls and cinnamic acid (1.0 mg/mL) was used as a negative control. Samples were mixed with water 100:1500 (sample: H2O). Controls were diluted with water in the same way as samples. All dilutions were mixed with Folin Ciocalteu’s phenol reagent (1 N, 100 µL). After a brief incubation at room temperature (5 min), saturated sodium carbonate (300 µL, 75 g/L) was added. Solutions were mixed and incubation continued at room temperature. After 2 h, 300 µL were transferred to a well of a 96-well plate and the absorbance measured at 765 nm with a molecular Devices Spectromax 384-Plus plate reader (Sunnyvale, CA). Quantification was based on the standard curve generated with 50, 100, 200, 300, 400 and 500 mg/L of gallic acid. Samples with absorbance values greater than the 500 mg/L standard were diluted and reanalyzed. Values were reported as µmol gallic acid equivalents (GAE) per gram material ± SD and represent the average of three independent analyses.
Chemical composition
Table 2 shows the relative proportion of the compounds identified in both samples (SFE and HD) using a DB-1 column. Also in order to confirm the identity of the compounds a DB-wax column was used (data not show) but it is important to highlight that the retention index value for the ketone: myrcenone was 1588, based on the fact that no previous reports were found using this type of column.
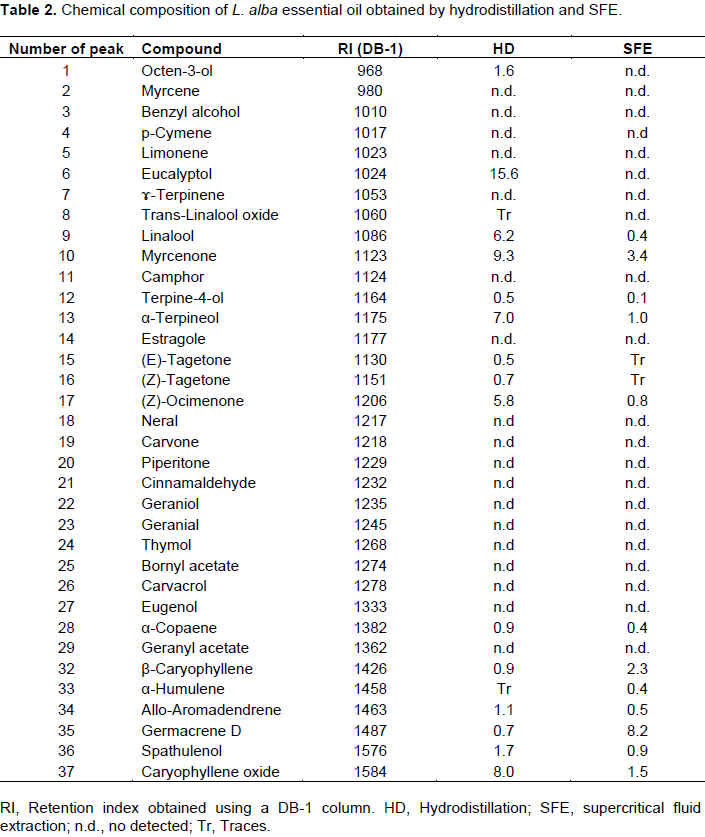
L. alba is probably the most studied species in the Lippia genus however, in spite of the diversity and medicinal properties of this plant, in Mexico no previous reports have reported about the chemical compositions of L. alba essential oil (Hennebelle et al., 2008). As show in Table 2 the major compounds in L. alba essential oil obtained by SFE were myrcenone (3.4%), α-terpineol (1.0%), β-caryophyllene (2.3%), germacrene-D (8.2%) and caryophyllene oxide (1.5%), while by hydrodistillation the major compounds were eucalyptol (15.6%), myrcenone (9.3%) and (Z)-ocimenone (5.8%) (Table 2). Based on these results and in accordance with Henebelle et al. (2006) chemotypes classification (Table 1), L. alba essential oil could correspond to chemotype II (ocimenone and myrcenone) since both compounds are present in the sample composition of L. alba extract obtained by hydrodistillation.
In the SFE prepared sample the sesquiterpenes including β-caryophyllene (2.3%), α-humulene (0.4%) and germacrene-D (8.2%), were at a greater proportion than found in the hydrodistillation sample. A similar result was observed by Stashenko et al. (2004) who found more sesquiterpenes in the supercritical CO2 extract of L. alba than in extracts obtained with other extraction techniques. Eucalyptol was reported in Lippia alba essential oil as the main compound in samples obtained from Uruguay while was differed from that reported from Colombia where the main compounds were carvone (51%) and limonene (32.60%) carvone (31.8-52.6%) and geraniol (15-21.5%) (Mesa-Arango et al., 2009; Dellacassa et al., 1990). In Brazil L. alba essential oil was rich in geranial (12.9%) and myrcene (15%) (Oliveira et al., 2006). While in Guatemala, Senatore and Rigano (2001) reported as the main compounds limonene (44%) and piperitone (31%). Fischer et al. (2004) analyzed 16 L. alba populations from all over Guatemala. They reported myrcenone (37.8-58%) as the main compound in 14 samples and neral (17.6-18.9%) and geranial (24.7-27%) as the main compounds in two samples collected from two different geographic locations. The variability in the chemical composition of L. alba essential oil was attributed to different factors such as season of harvest, collection site, soil composition, water hydric stress, extreme temperatures and sunlight (Nogueira et al., 2007; Olivero-Verbel et al., 2010; Blanco et al., 2007). However, other works have suggested that the composition of the essential oil of L. alba may not only be due to climatic or environmental conditions. Pandelo et al. (2012) analyzed three chemotypes of L. alba cultivated at the same conditions and were collected at the same time. No changes were observed in the main compounds of the samples. The authors concluded that these results are a consequence of genetic variation among the chemotypes. Also, it was observed that developmental stage such as flowering and vegetative growth affect the total oil production but not the quality of oil in leaves of L. alba, reinforcing the suggestion of genetic control.
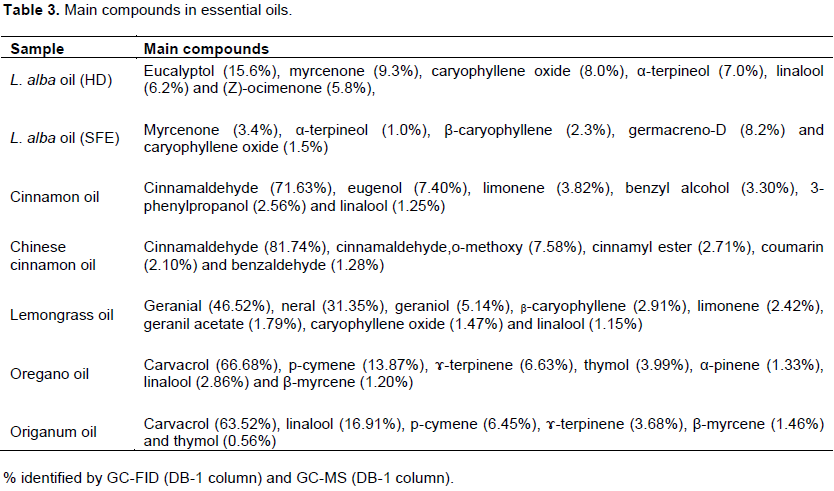
In order to compare the chemical composition of L. alba, essential oil commercial oils were tested in this work. As show in Table 3 six main compounds were identified in cinnamon oil, representing 89.96% of the total oil. The most abundant components were cinnamaldehyde (71.63%) and eugenol (7.4%). Cinnamaldehyde is one of the main aroma-active compounds in cinnamon essential oil (Schmidt et al., 2006). The results found in this work are in accordance with those of Tomaino et al. (2005) who reported cinnamaldehyde (45.84%) and eugenol (49.09%) as the main compounds in cinnamon oil. The major compounds in Chinese cinnamon oil were cinnamaldehyde (81.74%) and cinnamaldehyde,o-methoxy (7.58%) but eugenol was not present in the sample analyzed. The composition of the essential oil of Chinese cinnamon oil (Cinnamomum cassia) have been reported that trans-cinnamaldehyde and cinnamaldehyde,o-methoxy as the main compounds and eugenol content was no present in the sample evaluated (Geng et al., 2011). The major compounds in lemongrass were geranial (46.52%) and neral (31.35%), representing already of 80% of the total oil. These results are similar with previous reports. Sacchetti et al. (2005) reported geranial (41.30%), neral (32.30%) and geraniol (3.35%) as the main compounds. Katsukawa et al. (2010) reported citral (mixture of geranial (31.43%) and neral (26.03%) as the main compound in lemongrass essential oil. The most abundant components in oregano were carvacrol (66.68%) and p-cymene (13.87%) and for origanum, carvacrol (63.52%) and linalool (16.91%) (Table 1). In both essential oils, carvacrol was the main compound identified. It is well known that in Origanum species carvacrol is the main constituents (Hussain et al., 2011). However, thymol concentrations found in this work were lower than that reported previously (Puertas-Mejia et al., 2002).
Antioxidant activity
The chemical complexity of essential oils, often a mixture of dozens of compounds with different functional groups, polarity and chemical behaviour, could lead to scattered results depending on the test employed (Sacchetti et al., 2005). DPPH and ABTS (TEAC) assays are useful for determining the activity of both hydrophilic and lipophilic species (Sacchetti et al., 2005; Katsukawa et al., 2010; Hussain et al., 2011; Puertas-Mejia et al., 2002). The radical scavenging activity of essential oils, assessed by the antioxidant concentration required for 50% reduction in DPPH radical concentration in 30 min (IC50), decreased in the following order: cinnamon oil>oregano oil>origanum oil>L. alba oil (obtained by hydrodistillation)>L. alba (obtained by SFE)>lemongrass oil; chinese cinnamon oil showed no antioxidant activity (Table 1). It was found that the essential oils analyzed showed very different antioxidant activity (Table 4). The radical scavenging activity (IC50) of standard compounds decreased in the following order: eugenol> carvacrol> thymol. The other standards tested showed low radical scavenging activity (IC50 = >20 mg/mL) (Table 5). Table 4 also shows the antioxidant activities of essential oils tested by ABTS assay. The highest antioxidant activity
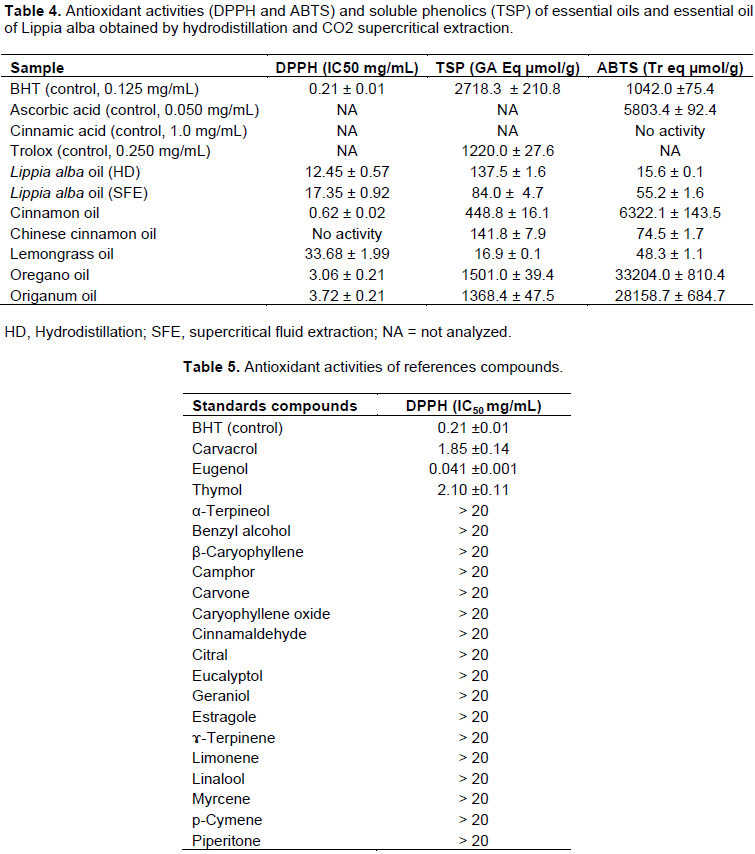
decreased in the following order: oregano > origanum > cinnamon > Chinese cinnamon > L. alba (SFE) > lemongrass > L. alba (HD). The antioxidant activity tested was different between both methods. Antioxidant activities of essential oils from aromatic plants are mainly attributed to their chemical structure (Saleh et al., 2010). Politeo et al. (2006) suggested that the antioxidant activity may be attributed either to high percentage of the main constituents but also to the presence of other constituents in small quantities or to synergy among them. Respectively the antiradical scavenging activity of L. alba (HD) was modest in comparison to the others oils with an IC50= 12.45 mg/mL and 15.6 Trolox eq µmol/g in DPPH and TEAC assay. In this case it was difficult to attributed the antioxidant activity to the main compounds since eucalyptol showed a low antiradical activity, whereas can be attributed to high percentage of the others main constituents but also to the presence of other compounds in small quantities or to synergy among them (Politeo et al., 2006). Puertas-Mejia et al. (2002) reported that L. alba essential oil samples from Colombia showed a low IC50=0.28 kg oil/mmol DPPH and also displayed lower activity against the ABTS radical (14.4 mmol Trolox/kg oil).
Cinnamon essential oil demonstrated the highest inhibitory activity (IC50=0.62 mg/mL) compared to other essential oils tested and this is attributed to the hydrogen-donating capacity of the phenolic component eugenol and this compound was present in significant concentration in the essential oil tested and also presented the highest inhibitory activity with an IC50=0.041 mg/mL (Schmidt et al., 2006; Tomaino et al., 2005) while in ABTS assay, cinnamon oil showed lower activity than oregano and origanum oils. It is of note that Chinese cinnamon oil showed no antioxidant activity by DPPH method and this can be attributed to the absence of eugenol in its composition but showed antioxidant activity by ABTS assay. Mantle et al. (1998) reported that cinnamon oil showed the highest antioxidant activity against ABTS assay but showed no significant evidence of antioxidant activity against hydroxyl and superoxide radicals. Suggesting that the variation in antioxidant capacity of oils extracts depends on the particularly assay method employed to determine antioxidant status. This result can be attributed to the fact that ABTS radicals involve electron transfers process while DPPH radical involve H atoms transfers (Kaviarasan et al., 2007). On the other hand, oregano and origanum essential oil exhibited DPPH radical scavenging activity and this can be related to their chemical compositions since both samples are rich in carvacrol and this compound showed radical scavenging activity with an IC50=1.85 mg/mL. Also, thymol was present in the samples, this compound showed a radical scavenging activity similar than carvacrol with an IC50=2.10 mg/mL. Sahin et al. (2004) reported that DPPH radical scavenging activity of Origanum vulgare spp. essential oil was low and this was related to its chemical composition because the percentage of carvacrol were low (0.057%) in the composition. Oregano and origanum essential oils showed better activity by ABTS assay; 33204 and 28158.7 Trolox eq µmol/g, respectively, than positive control (Table 4). Puertas-Mejia et al. (2002) found that total antioxidant activity of O. vulgare L. essential oil against ABTS radical was 25.1 Trolox eq mmol/kg oil while Karakaya et al. (2011) reported that oregano essential oil had a strong inhibitory effect on ABTS radical cation oxidation with a value of 2.69 Trolox eq µmol/ µL of oil.
The radical scavenging of lemongrass essential oil was lower than the other essential oils. The low antioxidant activity can be attributed to the main compounds composition since the standard compounds present in the essential oil were tested and also these compounds showed low activity (Table 5). Also, the antioxidant activity tested by ABTS was low (48.3 Trolox eq µmol/g). Sacchetti et al. (2005) reported an intermediate inhibition with 60% of radical scavenging activity percentage. These results demonstrate the difficulties in comparing data on antioxidant effectiveness obtained by different assays. Therefore, an approach with multiple assays in screening work is highly advisable (Sacchetti et al., 2005).
Total soluble phenolic
The presence of phenolic compounds in essential oil has been investigated in terms of antioxidant activity. This activity is mainly due to their redox potential, which can play an important role in adsorbing and neutralizing free radicals and quenching reactive oxygen species (Kähkönen et al., 1999). The total soluble phenolic (TSP) were measured by Folin-Ciocalteu reagents in terms of the acid gallic equivalent. TSP content of essential oils decreased in the following order: oregano> origanum> cinnamon>chinese cinnamon>L. alba (HD)>L. alba (SFE)>lemongrass (Table 4). Generally, a positive correlation between the phenolic content and antioxidant capacity is reported. It has been shown that the antioxidant activity of extracts is roughly connected to their phenolic composition and strongly depends upon their phenolic structures (Chaillou and Nazareno, 2006). It is of note that oregano and origanum had the highest TSP content; 1501 and 1368 GA Eqv µmol/g, respectively. In this case both essential oils were rich in carvacrol, a phenolic compound. On the other hand, Chinese cinnamon (141.8 GA eq µmol/g) showed less activity but greater than lemongrass. Lemongrass showed lower phenolic content (16.9 GA eq µmol/g); these results are in contradiction with previous reports. Mirghani et al. (2012) reported a high phenolic concentration of 2100.7 mg/L GA eq. No data was found concerning the total soluble phenolic content of L. alba (SFE and HD) essential oil in previous reports. It does appear that this is the first report. It is of note that the essential oil tested did not seem to depend on TSP since antioxidant activity by DPPH and ABTS assay in all samples were very different between both methods.
The chemical composition and antioxidant activity of the extracts obtained by SFE and hydrodistillation methods were different. SFE extracts was characterized by the presence of sesquiterpenes compounds. L. alba plants from Mexico can be classified in chemotype II (ocimenone and myrcenone). Results provided here show that L. alba essential oil has a good eucalyptol content, which makes it an important natural source for the nutraceutical industry.
The authors have not declared any conflict of interests.
REFERENCES
|
Agnaniet H, Makani T, Akagah A, Menut C, Bessiere JM (2005). Volatile constituents and antioxidant activity of essential oils from Lippia multiflora Mold. growing in Gabon. Flavour Fragr. J. 20:34-38.
Crossref
|
|
|
|
Bagheri H, Manap MYBA, Solati Z (2014). Response surface methodology applied to supercritical carbon dioxide extraction of Piper nigrum L. essential oil. LWT Food Sci. Technol. 57(1):149-155.
Crossref
|
|
|
|
|
Bahl JR, Garg SN, Singh SC, Bansal RP, Naqvi AA, Kumar S (2000). Composition of linalool rich essential oil from Lippia alba grown in Indian plains. Flavour Fragr. J. 15:199-200.
Crossref
|
|
|
|
|
Blanco KM, Agudelo AJ, Martínez JR, Stashenko EE (2007). Estudio comparativo de los aceites esenciales de Lippia alba Mill N.E. Brown cultivada con tres tipos de compostaje. Scientia Techn. 33:231-233.
|
|
|
|
|
Braga MEM, Ehlert PAD, Ming LC, Meireles MAA (2005). Supercritical fluid extraction from Lippia alba: global yields, kinetic data, and extract chemical composition. J. Supercrit. Fluids 34(2):149-156.
Crossref
|
|
|
|
|
Breksa AP, Takeoka GR, Hidalgo MB, Vilches A, Vasse J, Ramming DW (2010). Phenolic content of raisin grape varieties and genotypes. Food Chem. 121:740-745.
Crossref
|
|
|
|
|
Chaillou LL, Nazareno MA (2006). New method to determine antioxidant activity of polyphenols. J. Agric. Food Chem. 54:8397-8402.
Crossref
|
|
|
|
|
Dellacassa E, Soler E, Menendez P, Moyna P (1990). Essential oils from Lippia alba (Mill.) N. E. Brown and Aloysia chamaedrifolia Cham. (verbenaceae) from Uruguay. Flavour Fragr. J. 5:107-108.
Crossref
|
|
|
|
|
Donelian A, Carlson LH, Lopes TJ, Machado RA (2009). Comparison of extraction of patchouli (Pogostemon cabli) essential oils with supercritical CO2 and by steam distillation. J. Supercrit. Fluids 48:15-20.
Crossref
|
|
|
|
|
Duran GDC, Monsalve LA, Martinez J, Stashenko E (2007). Estudio comparativo de la composición química de aceites esenciales de Lippia alba provenientes de diferentes regiones de Colombia, y efecto del tiempo de destilación sobre la composición del aceite. Scientia Technol. 8:435-438.
|
|
|
|
|
Escobar P, Milena SL, Herrera LV, Martinez JR, Stashenko EE (2010). Chemical composition and antiprotozoal activities of Colombian Lippia spp essential oils and their major components. Mem. Inst. Oswaldo Cruz.105:184-190.
Crossref
|
|
|
|
|
Fischer U, Lopez R, Poll E, Vetter S, Novak J, Franz C (2004). Two chemotypes within Lippia alba populations in Guatemala. Flavour Fragr. J. 19:333-335.
Crossref
|
|
|
|
|
García-Abarrio SM, Martin L, Burillo J, Della Porta G, Mainar AM (2014). Supercritical fluid extraction of volatile oil from Lippia alba (Mill.) cultivated in Aragón (Spain). J. Supercrit. Fluids 94:206-211.
Crossref
|
|
|
|
|
Geng S, Cui Z, Huang X, Chen Y, Xu D, Xiong P (2011). Variations in essential oil yield and composition during Cinnamomum cassia bark growth. Ind. Crops Prod. 33:248-252.
Crossref
|
|
|
|
|
Ghasem E, Raofie F, Mashkouri NN (2011). Application of response surface methodology and central composite design for the optimization of supercritical fluid extraction of essential oils from Myrtus communis L. leaves. Food Chem. 126:1449-1453.
Crossref
|
|
|
|
|
Hennebelle T, Sahpaz S, Dermont C, Bailleul, JH (2006). The essential oil of Lippia alba: analysis of samples from French overseas departments and review of previous works. Chem. Biodivers. 3:1116-1125.
Crossref
|
|
|
|
|
Hennebelle T, Sahpaz S, Joseph H, Bailleul F (2008). Ethnopharmacology of Lippia alba. J. Ethnopharmacol. 116:211-222.
Crossref
|
|
|
|
|
Hussain AL, Anwar F, Rasheed S, Nigam PS, Janneh O, Sarker SD (2011). Composition, antioxidant and chemotherapeutic properties of the essential oils from two Origanum species growing in Pakistan. J. Pharmacogn. 21(6):943-952.
Crossref
|
|
|
|
|
Kähkönen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, Kujala TS, Heinonen M (1999). Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 47(10):3954-3962.
Crossref
|
|
|
|
|
Kamaliroosta L, Gharachorloo M, Kamaliroosta Z, Alimohammad Z (2012). Extraction of cinnamon essential oil and identification of its chemical compounds. J. Med. Plants Res. 6(4):609-614.
Crossref
|
|
|
|
|
Karakaya S, El NS, Karagozlu N, Sahin S (2011). Antioxidant and antimicrobial activities of essential oils obtained from oregano (Origanum vulgare ssp. hirtum) by using different extraction methods. J. Med. Food 14(6):645-652.
Crossref
|
|
|
|
|
Katsukawa M, Nakata R, Takizawa Y, Hori K, Takahashi S, Inoue H (2010). Citral, a component of lemongrass oil, activates PPARα and γ and suppresses COX-2 expression. Biochim. Biophys. Acta 1801:1214-1220.
Crossref
|
|
|
|
|
Kaviarasan S, Naik GH, Gangabhagirathi R, Anuradha CV, Priyadarsinin KI (2007). In vitro studies on antiradical and antioxidant activities of fenugreek (Trigonella foenum graecum) seeds. Food Chem. 103(1):31-37.
Crossref
|
|
|
|
|
Lorenzo D, Paz D, Davies P, Vila R, Canigueral S, Dellacassa E (2001). Composition of a new essential oil type of Lippia alba (Mill.) N.E. Brown from Uruguay. Flavour Fragr. J. 16:356-359.
Crossref
|
|
|
|
|
Mantle D, Anderton JG, Falkous G, Barnes M, Jones P, Perry EK (1998). Comparison of methods for determination of total antioxidant status: application to analysis of medicinal plant essential oils. Comp. Biochem. Physiol. 121:385-391.
Crossref
|
|
|
|
|
Mesa-Arango AC, Montiel-Ramos J, Zapata B, Duran C, Betancur-Galvis L, Stashenko EE (2009). Citral and carvone chemotypes from the essential oils of Colombian Lippia alba (Mill.) N.E. Brown: composition, cytotoxicity and antifungal activity. Mem. Inst. Oswaldo Cruz. 104:878-884.
Crossref
|
|
|
|
|
Mirghani MES, Liyana Y, Parveen J (2012). Bioactivity analysis of lemongrass (Cymbopogan citratus) essential oil. Int. Food Res. J. 19(2):569-575.
|
|
|
|
|
Nogueira MA, Diaz G, Sakumo L (2007). Caracterização química e atividade biológica do óleo essencial de Lippia alba cultivada no Paraná. Rev. Ciênc. Farm. Básica Apl. 28(3):273-278.
|
|
|
|
|
Oliveira DR, Leitao GG, Santos SS, Bizzo HR, Lopes D, Alviano DS, Alviano CS, Leitao SG (2006). Ethnopharmacological study of two Lippia species from Oriximiná, Brazil. J. Ethnopharmacol. 108:103-108.
Crossref
|
|
|
|
|
Oliveira MS, da Costa WA, Pereira DS, Botelho JRS, de Alencar Menezes TO, de Aguiar Andrade EH, de Carvalho RN (2016). Chemical composition and phytotoxic activity of clove (Syzygium aromaticum) essential oil obtained with supercritical CO2. J. Supercrit. Fluids 118:185-193.
Crossref
|
|
|
|
|
Olivero-Verbel J, Gonzalez-Cervera T, Guette-Fernandez J, Jaramillo-Colorado B, Stashenko EE (2010). Chemical composition and antioxidant activity of essential oils isolated from Colombian plants. Braz. J. Pharmacogn. 20(4):568-574.
Crossref
|
|
|
|
|
Pandelo D, Melo TD, Singulani JL, Guedes AF, Machado MA, Coelho CM, Viccini LF, Santos MO (2012). Oil production at different stages of leaf development in Lippia alba. Rev. Bras. Farmacogn. 22(3):497-501.
Crossref
|
|
|
|
|
Pascual M, Slowing K, Carretero E, Sanchez MD, Villar A (2001). Lippia: traditional uses, chemistry and pharmacology: A review. J. Ethnopharmacol. 76(3):201-214.
Crossref
|
|
|
|
|
Politeo O, Jukic M, Milos M (2006). Chemical composition and antioxidant activity of essential oils of twelve spice plants. Croat. Chem. Acta 79(4):545-552.
|
|
|
|
|
Puertas-Mejia M, Hillebrand S, Stashenko E, Winterhalter P (2002). In vitro radical scavenging activity of essential oils from Columbian plants and fractions from oregano (Origanum vulgare L.) essential oil. Flavour Frag. J. 17:380-384.
|
|
|
|
|
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 26:1231-1237.
Crossref
|
|
|
|
|
Sacchetti G, Maietti S, Muzzoli M, Scaglianti M, Manfredini S, Radice M (2005). Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 91:621-632.
Crossref
|
|
|
|
|
Sahin F, Gulluce M, Daferera D, Sokmen A, Sokmen M, Polissiou M, Agar G, Ozer H (2004). Biological activities of the essential oils and methanol extract of Origanum vulgare ssp. vulgare in the Eastern Anatolia region of Turkey. Food Control 15(7):549-557.
Crossref
|
|
|
|
|
Saleh MA, Clark S, Woodard B, Deolu-Sobogun SA (2010). Antioxidant and free radical scavenging activities of essential oils. Ethn. Dis. 1(20):78-82.
|
|
|
|
|
Schmidt E, Jirovetz L, Buchbauer G, Eller GA, Stoilova I, Krastanov A, Stoyanova A, Geissler M (2006). Composition and antioxidant activities of the essential oil of cinnamon (Cinnamomum zeylanicum Blume) leaves from Sri Lanka. J. Essent. Oil Bear Plants 9(2):170-182.
Crossref
|
|
|
|
|
Senatore F, Rigano D (2001). Essential oil of two Lippia spp. (Verbenaceae) growing wild in Guatemala. Flavour Fragr. J. 16:169-171.
Crossref
|
|
|
|
|
Stashenko EE, Jaramillo BE, Martinez JR (2004). Comparison of different extraction methods for the analysis of volatile secondary metabolites of Lippia alba (Mill.) N.E. Brown, grown in Colombia, and evaluation of its in vitro antioxidant activity. J. Chromatogr. A 1025:93-103.
Crossref
|
|
|
|
|
Tomaino A, Cimino F, Zimbalatti V, Venuti V, Sulfaro V, De Pasquale A, Saija A (2005). Influence on heating on antioxidant capacity and the chemical composition of some spice essential oils. Food Chem. 89:549-554.
Crossref
|
|
|
|
|
Wenqiang G, Shufen L, Ruixiang Y, Shaokun T, Can Q (2007). Comparison of essential oils of clove buds extracted with supercritical carbon dioxide and other three traditional extraction methods. Food Chem. 101:1558-1564.
Crossref
|
|
|
|
|
Willman D, Schmidt EM, Rimpler H (2000). Verbenaceae. Flora del Valle de Tehuacán– Cuicatlán 27 Instituto de Biología, UNAM Press, pp. 1-75.
|
|
|
|
|
Wojdylo A, Oszmianski J, Czemerys R (2007). Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 105:940-949.
Crossref
|
|
|
|
|
Zermane A, Larkeche O, Meniai AH, Crampon C, Badens E (2014). Optimization of essential oil supercritical extraction from Algerian Myrtus communis L. leaves using response surface methodology. J. Supercrit. Fluids 85:89-94.
Crossref
|
|



