The family Cactaceae encompasses 100 genera and 1500 to 2000 species distributed in the American continent, with the exception of genus Rhipsalis, which occurs also in tropical Africa, Madagascar, islands in the Indian Ocean and Sri Lanka (Rojas-Aréchiga and Vázquez-Yanes, 2000; Pérez-Molphe-Balch et al., 2015). Its species possess great ecological and economic importance, and are under several threats of anthropic origin such as habitat destruction, predatory collection and illegal trading (Oldfield, 1997; Machado, 1999; Taylor and Zappi, 2004; Pérez-Molphe-Balch et al., 2015).
The two target species of this study, Micranthocereus flaviflorus subsp. densiflorus (Buining and Brederoo) P.J.Braun and Esteves and M. polyanthus subsp. alvinii M. Machado and Hofacker, are endemic to the state of Bahia, Brazil, and have ornamental potential (Charles, 2009). M. flaviflorus subsp. densiflorus has shrub bearing with ramifications from the base, dense yellowish spines and reddish tubular flowers with yellow center, which appear in great quantity in lateral pseudocephalia, composed of wool and hairs, and their fruits are also reddish (Machado, 1999). M. polyanthus subsp. alvinii also possesses shrub-like bearing with branching from the base, but its spines are whitish. Its small and numerous tubular flowers are white in the center and rosy around. They are organized into lateral pseudocephalia, composed of wool and hairs, and have rosy fruits.
Both species are threatened and listed in Appendix II of CITES (Convention on International Trade in Endangered Species of Wild Fauna and Flora) and IUCN (International Union for Conservation of Nature) Red List of Threatened Species (Machado et al., 2013; Machado and Braun, 2013). Given their precarious in situ conservation, endeavors to achieve ex situ preservation were made, consisting of seed storage and cryopreservation studies (Veiga-Barbosa et al., 2010; Civatti et al., 2015a,b).
In vitro germination, for some species can provide higher germination rates than ex vitro germination, as was the case for Mammillaria mathildae Kraehenb and Krainz (García-Rubio and Malda-Barrera, 2010). This approach can also be used to supply in vitro germinated plants for plant tissue culture enterprises such as micropropagation and somatic embryogenesis protocols, and maintenance of in vitro germplasm banks (Dias et al., 2008).
With the advent of plant tissue culture, the problems of traditional cultivation like low germination rates or difficulty in obtaining seeds, can be evaded and cacti can be multiplied in large scale and/or conserved in vitro (Lema-RumiÅ„ska and Kulus, 2014; Pérez-Molphe-Balch et al., 2015). One of the approaches provided by tissue culture is the in vitro multiplication of plants previously established in laboratory, meeting the demand of the ornamental market and indirectly protecting the integrity of natural populations (Malda et al., 1999; Pence, 2010).
Clonal propagation from tissues of ex vitro adult plants may not represent the real variability of species, besides often resulting in loss of material due to fungal or bacterial contamination during plant in vitro establishment. This can be avoided by applying propagation techniques on plants introduced in the in vitro environment through their seeds, as was done with Pelecyphora aselliformis Ehrenb. (Santos-Díaz et al., 2003) and eight species of Turbinicarpus (Dávila-Figueroa et al., 2005). The micropropagation of cacti, however, has demonstrated that protocols developed so far are species-specific, and that even for closely related species requirements for in vitro multiplication may vary (Lema-RumiÅ„ska and Kulus, 2014).
This work aimed to establish both species in vitro, evaluating ideal media and temperature conditions for in vitro germination of M. flaviflorus, as well as to multiply in vitro M. polyanthus and M. flaviflorus, subsequently acclimatizing the shoots obtained in this manner.
Plant materials
Ripe fruits of Micranthocereus flaviflorus subsp. densiflorus and Micranthocereus polyanthus subsp. alvinii were collected in Morro do Chapéu, Bahia, Brazil. The seeds obtained were processed, dried and stored in paper bags at room temperature for 2 weeks until the setup of experiments. Seed disinfestation was performed in laminar flow cabinet immersing seeds in absolute alcohol for 1 min followed by soaking in sodium hypochlorite at 2.5% for 15 min, and then rinsing 3 times with sterile water before sowing.
Given the restricted distribution and threatened status of M. polyanthus, not enough seeds were obtained for germination tests. The in vitro germination protocol established for M. flaviflorus was later applied to M. polyanthus seeds, and allowed for the generation of plants, used in the micropropagation tests of the latter. Germinated plants were kept in vitro on half-strength Murashige and Skoog (1962) media (MS/2) until the multiplication experiment was assembled at the Biology Institute of the Federal University of Bahia (Salvador, BA) in 2015, with periodical transfers, to allow plant growth and sustainment.
Effect of nutrient media and temperature over in vitro germination
M. flaviflorus seeds were sowed in agar (distilled water with Agar at 6 gL-1), Murashige and Skoog (1962) nutrient media (MS) supplemented with 30 g L-1 of sucrose or MS/2 supplemented with 15 g L-1 of sucrose. Seeds were submitted to constant temperatures of 30±1 and 25±3°C, and to temperatures alternating between 15±1 and 25 ±1°C, under white fluorescent light (60 μmol m–2 s–1). The design was completely randomized using a factorial arrangement of 3x3 (media x temperature) with 4 replicates of 20 seeds in each treatment.
Germination was evaluated daily for 21 days after the first seed germinated, considering the emission of radicle as evidence of germination (Brasil, 2009). The variables used in the analysis were germination (%G), mean germination time (MGT = (∑ni ti)/∑ni, where ni = number of seeds that germinated in day “i”, and ti = day “i” in evaluated time), germination speed index (GSI = ∑(ni/ti), where ni = number of seeds that germinated in day “i”, and ti = day “i” in evaluated time),germination uniformity coefficient (GUC= (∑ni)/∑(MGT-ti)2ni, where ni = number of seeds that germinated in day “i”) (Santana and Ranal, 2000) and absolute frequency of germination over time. As germination (%G) was expressed in %, it was arcsine-transformed.
Seed viability testing
The viability of seeds that did not germinate until the end the experiments was tested using tetrazolium (2,3,5 triphenyl tetrazolium chloride) (ISTA, 2007). In order to do so, 25 non-germinated seeds had their opercula removed and were placed in Petri dishes lined with Germ test paper moistened with distilled water and wrapped in aluminum foil (adapted from Veiga-Barbosa et al., 2010). After 24 h of soaking, seeds were immersed in tetrazolium solution at 0.6% and remained in the dark at room temperature (25±3°C). Followed 24 h, the seed coats were broken and embryos analyzed based on their staining using a magnifying glass.
In vitro multiplication of Micranthocereus flaviflorus subsp. densiflorus and Micranthocereus polyanthus subsp. alvinii
In vitro germinated plants of M. flaviflorus and M. polyanthus, 18 and 15 months old, respectively, were used as explant source to test the influence of plant growth regulators on their multiplication. The nutrient media used for such test was MS/2 supplemented with sucrose at 15 g L-1 and gelled with 6 g L-1 of agar. This nutrient media was chemically sterilized using sodium hypochlorite containing 2.5% of active chloride (adapted from Teixeira et al., 2006). The design was completely randomized with a factorial arrangement of 2x4 plant growth regulator concentrations: α-naphthaleneacetic acid (NAA, synthetic plant hormone of the auxin family) (0 and 1.34 μmol L-1) and Kinetin (KIN, a type of cytokinin) (0, 6.74, 20.22 and 40.44 μmol L-1), totaling 8 treatments for each studied species. These regulators were added to the nutrient media during its preparation, and each test tube was inoculated with only one horizontal explant. The explants were cut from the in vitro germinated plants of M. flaviflorus and M. polyanthus, disregarding apexes and roots of the mother plants, through transversal cross-sections of the cladode to obtain segments with 30 to 40 areoles, approximately.
Five replicates of 9 explants were used for each treatment, summing 45 explants per treatment per species. The variables analyzed were, the number of shoots per explant, shooting explants (%), rooting explants (%), oxidizing explants (%), and explants that formed callus (%). The multiplication experiment lasted 120 days in a 16 h photoperiod, under fluorescent light (60 μmol m–2 s–1) at room temperature (25 ± 3°C).
Acclimatization of micropropagated shoots
Shoots of different lengths, derived from in vitro multiplication with NAA at 1.34 μmol L-1 were detached from their explants and directly planted in plastic cups containing earth and sand (1:1).
The shoot length classes tested were ± 0.2 to 3.0 cm, ± 0.4 to 5.0 cm and ± 0.6 to 0.7 cm for M. flaviflorus, while for M. polyanthus they were intervals of 0.3 to 0.5 cm, 0.6 to 0.9 cm and 1.0 to 1.5 cm, with 3 replicates of 6 shoots per class for each species. Culture conditions were established at greenhouse with daily watering for 60 days, at the end of which the variables rooting (%) and survival of shoots (%) were calculated for both species
Statistical analysis
For all experiments, ANOVA was performed and means were compared by the Tukey test at 5% probability. The statistics program used was Sisvar 5.3 (Ferreira, 2008).
In vitro germination of Micranthocereus flaviflorus
M. flaviflorus seeds took 12 days average to germinate (Figure 1), the first germination being observed 3 days after the assembly of the experiment. The different nutrient media did not interfere in germination for any of the analyzed variables (Table 1), and there was no interaction between nutrient media and temperature regime in the evaluated variables: %G (p=0.7436), GSI (p=0.9103), MGT (p=0.3632) and GUC (p=0.3493). Under the constant temperature of 25°C, M. flaviflorus seeds achieved higher %G when compared to the other temperature regimes, while the GSI of said temperature was significantly higher than that of the alternating temperatures (Table 1). The average %G found, considering all treatments, was 20.4%. The low %G of M. flaviflorus (20.4%) suggests that this species may present some kind of dormancy or need a post-maturation period to achieve higher %G, which is consistent with the germination behavior of species that form seed banks on their natural environments and has been documented for other cacti (Rojas-Arechiga and Vazquez-Yanes, 2000; Montiel and Montaña, 2003; Benítez-Rodríguez et al., 2004; Mandujano et al., 2005; Ortega-Baes and Rojas-Aréchiga, 2007).
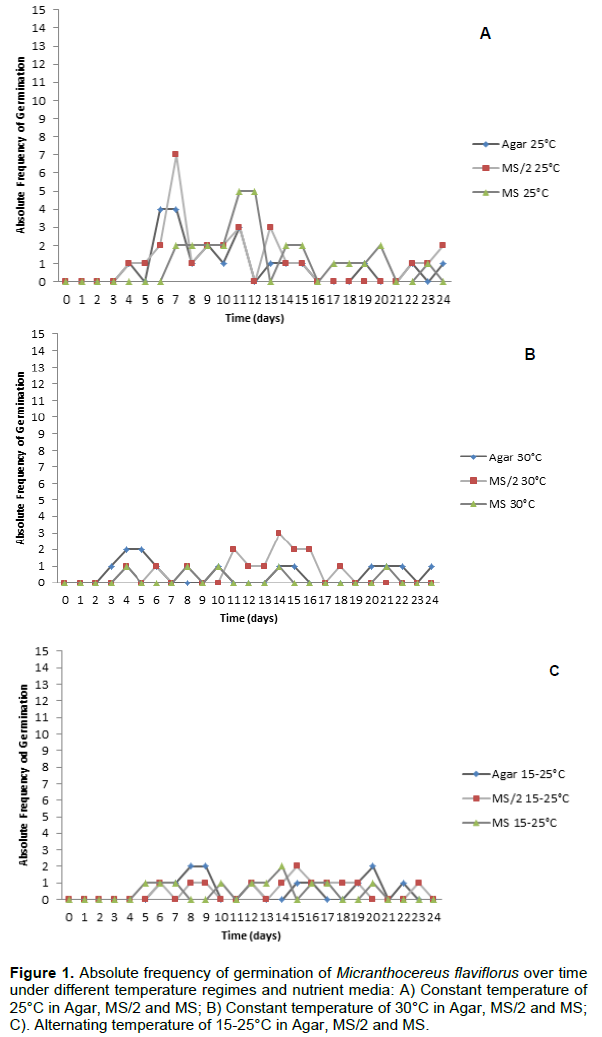
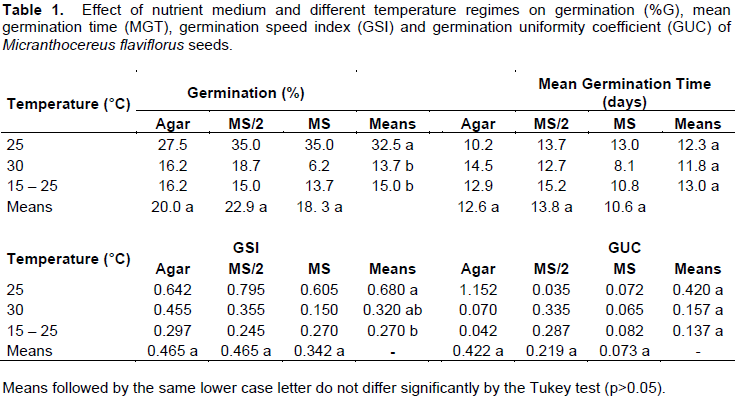
Taking into account the values of GUC and GSI, which were average 0.238 and 0.424, respectively, it is possible to assume that the germination of M. flaviflorus was not concentrated, but dispersed throughout the evaluated time in the experiment. The germination frequency obtained for M. flaviflorus (Figure 1) at different temperatures also demonstrated a dispersed pattern in germination over time, in which germination seems to occur at random during the evaluated timeframe regardless of the tested treatments (Ferreira and Borghetti, 2004). Seeds of this species show a lack of synchronism that may be caused by the heterogeneity of the used seed lot, a result that is characteristic of native undomesticated species and species capable of generating lasting seed banks (Ferreira and Borghetti, 2004). Similar results were found for other cacti, such as Pilosocereus gounellei (F.A.C.Weber ex K.Schum.) Byles and G.D.Rowley, Stephanocereus luetzelburgii (Vaupel) N.P.Taylor and Eggli and Discocactus zehntneri Britton and Rose (Marchi, 2012). In other species, however, germination is concentrated in time, as is the case of Melocactus ernestii Vaupel, M. glaucescens Buining and Brederoo and M. xalbicephalus Buining and Brederoo, for which germination peaks appeared on the second day after sowing (Cruz, 2011).
Temperature is a determining factor in the germination of several plant species, altering enzyme activity and, consequently,the velocity of reactions associated with germination, as well as denaturing proteins and causing phase transition in membranes, all of which can limit germination when temperature is unfavorable (Ferreira and Borghetti, 2004). Cacti, as most tropical species, have favorable germination temperatures ranging around 25°C, whilst numerous genera have optimum temperatures of 20°C (Rojas-Arechiga and Vazquez-Yanes, 2000). Thereby, it is necessary to evaluate the germination behavior of a species to determine optimum germination temperature, as it varies among species (Rojas-Arechiga and Vazquez-Yanes, 2000; Hartmann Et Al., 2011; Kerbauy, 2012).
Some plant species have seeds that need an increase in thermal variation in a short period of time (e.g. 24h) in order to germinate (Ferreira and Borghetti, 2004). Certain cactus species exhibit higher germination rates when submitted to alternating temperatures (Rojas-Aréchiga and Vázquez-Yanes, 2000). Even so, the alternating regime of 15-25°C did not promote germination in the present study (Table 1), so other thermal variations may be tested in different time intervals to try promoting germination in future works.
In Morro do Chapéu temperatures during the day may reach 27°C, and during the night they may stabilize around 14°C, depending on the time of the year (Hong Kong Observatory, 2012; INMET, 2016). It would be expected that alternating temperatures reproducing the environmental conditions of where M. flaviflorus naturally occurs exerted influence over the germination of this species. In Stenocereus stellatus (Pfeiff.) Riccob., similarly to M. flaviflorus, higher germination rates were observed under constant temperature of 25°C when compared to alternating temperatures of 20-35°C (Rojas-Aréchiga et al., 2001). Melocactus bahiensis (Britton and Rose) Luetzelb. did not germinate in alternating temperatures of 20-30ºC (Lone et al., 2007), although for Melocactus ernestii Vaupel, M. glaucescens and M. xalbicephalus the best germination response was observed under the regime of 25-30ºC (Cruz, 2011). Stenocereus queretaroensis (F.A.C.Weber ex Mathsson) Buxb. achieved a mean germination of 84% under the regimes of 25-15°C, 25-25°C and 35-25°C (De La Barrera and Nobel, 2003). D. zehntneri, on the other hand, did not have its germination significantly altered by constant (25 and 30°C) or alternating (25-30°C) temperatures (Marchi, 2012).
M. flaviflorus, P. gounellei and Cereus jamacaru DC. germinated with rates higher than 80% in constant temperatures of 20°C, 25°C and 30°C, and alternating temperatures of 15-25°C (Veiga-Barbosa et al., 2010), as did Trichocereus terscheckii’s seeds in constant temperatures of 15°C, 20°C and 25°C (Ortega-Baes and Rojas-Arechiga, 2007), while none of these species germinated in the extreme temperature of 10°C (Ortega-Baes and Rojas-Arechiga, 2007; Veiga-Barbosa et al., 2010). Such results disagree with those found for M. flaviflorus (Figure 2) in the present study, which did not achieve the high values of %G shown in those species, aside from displaying significant differences between constant and alternating temperature regimes (Table 1). For the in vitro establishment of this species 25°C is suggested, as it is the standard temperature of growth rooms.

Veiga-Barbosa et al. (2010) obtained high %G rates for M. flaviflorus under the regimes of 20°C (96%), 25°C (87%), 30°C (93%) and 15-25°C (91%) in Petri dishes lined with wet Germ test. The differences between the in vitro and ex vitro environments may have been the cause of the divergence between these results and those of the present work. Since water availability in the substrate is crucial for the hydration of seed tissues, allowing seed metabolism to restart and cells to expand (Kerbauy, 2012), it would be expected that media with higher water potential, e.i. media with lower soluteconcentrations, showed better results for germination (Marchi, 2012). Notwithstanding, in the present study different media saline and sucrose concentrations did not induce differences in germination, which means that the lower water potentials of MS/2 and MS media permitted the germination of M. flaviflorus seeds. The nutrient media also did not interfere in the in vitro germination of Arrojadoa spp. (Dias et al., 2008). The water potential of such media did not prevent in vitro germination of D. zehntineri either, whereas seeds of S. luetzelburgii and P. gounellei achieved higher G% in MS and MS/2 media than in agar (Marchi, 2012). The nutrient media recommended for in vitro germination of M. flaviflorus is, therefore, MS/2, since it is the most economical option while also having nutrients to allow healthy initial development of seedlings.
In the tetrazolium test conducted for M. flaviflorus, embryo viability of non-germinated seeds reached an average of 90.69%, and so an experiment to promote dormancy break in this species is underway.
In vitro multiplication of Micranthocereus flaviflorus and M. polyanthus
M. flaviflorus and M. polyanthus exhibited high averages of explant shooting (89.8% and 96.3%, respectively), rooting (98.0% in both) and survival (99.0% in both), while oxidation rates varied between 20.0% and 82.5% in M. flaviflorus (Table 2) and from 21.1% to 77.8% in M. polyanthus (Table 3). None of the explants of either species presented callogenesis or hyperhydricity after 4 months in culture.

NAA and KIN concentrations interacted significantly in the variables shoots per explant (p=0.0060), shooting (p=0.0000) and oxidation (p=0.0023) for M. flaviflorus. Explant oxidation was more frequent in the two highest concentrations of KIN when combined with NAA (82.5% and 56.3%), two treatments that also showed the lowest percentages of shooting explants (72.5% and 61.4%). Concerning number of shoots obtained per explant, values found for KIN concentrations did not differ from the control in the absence of NAA (Table 2). On the other hand, in the presence of NAA and at the higher concentrations of KIN in the nutrient media, the lowest number of shoots per explant in M. flaviflorus was observed. Explant survival and rooting were not affected by the different combinations of plant growth regulators, as M. flaviflorus achieved high rates in all of them (Table 2).
There was no interaction between NAA concentrations and KIN concentrations in any of the analyzed variables for M. polyanthus, but isolated these regulators interfered significantly in the number of shoots per explant (p=0.0016 and p=0.0000, respectively) and in explant oxidation (p=0.0001 and p=0.0000, respectively). As it was observed in M. flaviflorus, in the presence of NAA the two highest KIN concentrations contributed to explant oxidation (Table 3). In regards to M. polyanthus shoot production, in the absence of KIN or at its lowest concentration (6.74 μmol L-1), together with NAA, shooting was favored. The percentages of explant survival, rooting and shooting in this species were high in all the treatments and were not significantly affected by them (Table 3).
From germination until the propitious moment for multiplication of the targeted cacti species it took 15 or 18 months. Then, from each cactus two cross-cut explants could be obtained. Starting out with 20 plants of each species and taking into account the mean multiplication rates of each species in the media supplemented with NAA, which were 6.4 shoots/explant for M. flaviflorus and 3.6 shoots/explant for M. polyanthus, within 120 days 256 shoots of M. flaviflorus and 144 of M. polyanthus can be produced.
Shoots generated from M. flaviflorus’ explants took longer to be emitted during the experiment of in vitro multiplication than those of M. polyanthus. The latter, thus, reached greater lengths than those of M. flaviflorus (Figure 3), which were, in turn, smaller and more numerous in comparisons at the end of the evaluated period (Figure 4).


Apical dominance break through apex removal is still the most efficient and widely used mode of achieving areolar activation and cactus in vitro multiplication (Rubluo et al., 2002; Pérez-Molphe-Balch et al., 2015). The usage of plant growth regulators, however, must be accommodated to each cactus species, varying according to specific genotypes (Lema-RumiÅ„ska and Kulus, 2014).
Several cacti are propagated in vitro using cytokinins as the sole regulator added to the nutrient media (Dabekaussen et al., 1991; Pérez-Molphe-Balch and Dávila-Figueroa, 2002; Sriskandarajah and Serek, 2004; Ramirez-Malagon et al., 2007; Estrada-Luna et al., 2008; Quiala et al., 2009). The presence of cytokinin increased the in vitro production of shoots in cacti such as Opuntia lanigera Salm–Dyck, Pilosocereus robinii (Lemaire) Byles et Rowley, Pelecyphora aselliformis Eerhenberg and Pelecyphora strobiliformis Werdermann (Pérez-Molphe-Balch and Dávila-Figueroa, 2002; Estrada-Luna et al., 2008; Quiala et al., 2009). These results differ from those obtained with M. flaviflorus and M. polyanthus, for which the addition of KIN singly to the medium did not favor an increased number of shoots per explant. In the case of the cultivars Rhipsalidopsis gaertneri X R. rosea and Schlumbergera russeliana X S. truncata it was necessary to exclude auxins and include BAP (6-benzylaminopurine), TDZ (thidiazuron) and zeatin together (all at 27 μmol L-1) to generate the highest number of shoots per explant (Sriskandarajah and Serek, 2004).
The application of NAA on the propagation of M. polyanthus and M. flaviflorus, nevertheless, promoted the induction of shoots when applied alone (in M. flavilforus and M. polyanthus) or in combination with low concentrations of KIN (6.74 µmol L-1) (in M. polyanthus). Other cacti did not have their micropropagation enhanced by the presence of auxins in the nutrient media (Dabekaussen et al., 1991; Ramirez-Malagon et al., 2007). This was the case for 10 species of Mammillaria, for which higher concentrations of KIN (27.8 μmol L-1 and 46.5 μmol L-1), in the presence or absence of indole-3-aceticacid (IAA), increased shoot formation (Ramirez- Malagon et al., 2007), and for Sulcorebutia alba Rausch, in which auxin did not cause significant changes in propagation (Dabekaussen et al., 1991). However, M. flaviflorus and M. polyanthus were not the only cacti to have their in vitro multiplication benefited by the presence of auxins in the nutrient media: Rubluo et al. (2002) also observed areole activation in Mammillaria san-angelensis under the influence of exogenous auxins. The use of 1.34 μmol L-1 of NAA potentiated the multiplication of P. gounellei and S. luetzelburgii as well (Marchi, 2012). Furthermore, similarly to M. polyanthus, another 11 rare or threatened species of cacti from different genera [Escobaria missouriensis (Sweet) D.R. Hunt, Mammillaria wrightii Engelm., Sclerocactus spinosior (Engelm.) D Woodruff and L.D.Benson, Pediocactus despainii S.L.Welsh and Goodrich, P. paradinei B.W.Benson, P.
winklerii K.D.Heil., Toumeya papyracantha Britton and Rose, E. robbinsorum (W.H.Earle) D.R.Hunt, S. mesae-verdae (Boissev. and C.Davidson) L.D.Benson, P. bradyi L.D.Benson and P. knowltonii L.D.Benson] achieved higher shooting rates with cytokinin combined with low concentration of auxin in the nutrient medium (Clayton et al., 1990).
For the in vitro multiplication of M. flaviflorus and M. polyanthus, the increase in cytokinin concentration did not increment shooting, since in both species the increase in KIN concentrations in nutrient media in the presence of NAA coincided with a decrease in the number of shoots per explant. Studies suggest, however, that in plant tissue culture a balance in auxin and cytokinin concentrations is favorable to cacti in vitro multiplication (Lema-RumiÅ„ska and Kulus, 2014). Shoot and root differentiation is the outcome of the interaction between cytokinin and auxin hormones, while said interaction depends upon the concentrations produced by the plant tissues themselves and those of exogenous regulators added to the nutrient media (Juárez and Passera, 2002). The highest concentrations of auxins in the propagation of Cereus peruvianus Mill. induced superior rates of adventitious shooting only in the presence of cytokinins (Machado and Prioli, 1996), analogously to the micropropagation of Opuntia ellisiana Griff., in which a higher percentage of activated areoles was obtained with the employment of BAP and indole-3-butyric (IBA) combined (Juárez and Passera, 2002).
Exogenous growth regulators are used aiming to promote areolar activation and induce shooting in explants, but as endogenous levels of plant hormones may vary from one species to the next, a species-specific tissue response is expected to exogenous growth regulators, seeing as concentrations of these regulators affect individual homeostasis and physiological processes (Juárez and Passera, 2002). Therefore, the addition of cytokinins may be unnecessary or even detrimental to shooting in M. flaviflorus and M. polyanthus, which would explain the obtained results for these cacti. Studies indicate that cacti have naturally high levels of endogenous auxins and cytokinins (especially those derived from isopentenyl), which could influence their regenerative potential (Clayton et al., 1990; Sriskandarajah et al., 2006). Adding auxin in low concentration to the nutrient media, with or without KIN at 6.74 μmol L-1, may have been efficient in acting together with the hormones present in explant tissues and stimulating shooting in both M. flaviflorus and M. polyanthus.
Hormonal homeostasis in plants is modulated by enzyme activity which is also species-specific (Sriskandarajah et al. 2006). The measurement of such activity, as well as endogenous concentrations of cytokinins and auxins, in tissues of M. polyanthus and M. flaviflorus may elucidate the questions here arisen, since the balance between these hormones is more propitious to shooting when there is a higher proportion of cytokinins in relation to auxins (Phillps, 2004; Sriskandarajah et al. 2006; Lema-Rumińska and Kulus, 2014). Tests with other auxins are also suggested to investigate if this stimulus can be optimized. Combinations with other cytokinins and auxins could be tested as well, although it
could end up compromising explant metabolism and induce callus formation and hyperhydricity, neither of which occurred in the present work
Micropropagation is, therefore, an alternative to produce plants of M. flaviflorus and M. polyanthus, being recommended 1.34 μmol L-1 of NAA for both species.
Shoot acclimatization of Micranthocereus flaviflorus and M. polyanthus
The longest shoots from each species achieved the highest survival and rooting rates, whereas the smallest shoots did not survive in either species (Tables 4 and 5). The low rooting percentage of M. flaviflorus shoots did not prevent their survival (Table 4). Concomitantly, M. polyanthus shoots showed similar survival and rooting rates within each size class (Table 5), which indicates that rooting, besides size, could be a determining factor in the acclimatization of these shoots.
Numerous factors can affect acclimatization success of in vitro derived shoots, such as temperature and humidity ex vitro, substrate, previous in vitro rooting of plants, stomata functionality, cuticle presence and amount of water and nutrient reserves in plant tissues (Malda et al., 1999; Cavalcanti and Resende, 2006). In the in vitro environment plants usually do not retain normal control of the opening and closing of stomata, neither do they produce a significant quantity of cuticle, and their photosynthesis does not surpass a basal level (Malda et al., 1999). These factors can interfere in the transition from in vitro to ex vitro. It is generally necessary to perform gradual changes in humidity and luminosity in order for plants to survive (Rout et al., 2006).
Shoots from M. flaviflorus and M. polyanthus did not go through a stage of previous in vitro rooting, something that has be demonstrated as an unnecessary stage for the acclimatization of some cacti such as Melocactus glaucescens (Resende et al., 2010) and Stephanocereus luetzelburgii (Marchi, 2012, 2016). In vitro produced roots do not possess the same quality as those generated ex vitro, which develop in contact with the substrate and tend to form a more complete root system with a larger number of secondary roots (Grattapaglia and Machado, 1998). Nonetheless, various species have had success in acclimatization with shoots previously rooted in vitro, like Turbinicarpus laui Glass et Foster (Rosas et al., 2001), Opuntia ellisiana (Juárez and Passera, 2002), Pelecyphora aselliformis (Pérez-Molphe-Balch and Dávila-Figueroa, 2002), Pelecyphora strobiliformis (Pérez-Molphe-Balch and Dávila-Figueroa, 2002), Ariocarpus kotschoubeyanus (Lem.) K.Schum. (Moebius-Goldammer et al., 2003), Opuntia lanígera Salm–Dyck (Estrada-Luna et al., 2008), Pilosocereus robinii (Quiala et al., 2009), Melocactus glaucescens (Resende et al., 2010) and Pilosocereus gounellei (Marchi, 2012).
Rooting may be directly related to shoot size and survival. Acclimatized shoots of Melocactus glaucescens without any previous rooting were significantly smaller than those in vitro rooted, achieving a significantly lower survival rate (63.33%) than that of previously rooted shoots (86.67% to 90.00%) (Resende et al., 2010). Consequently, the assumption can be made that a previous stage of rooting can increase survival rates in acclimatization. Simultaneously to in vitro root formation, however, happens shoot growth, which causes an uptake in mass and, therefore, in the shoot’s reserve tissues. Thus, it is possible that not only in vitro rooting of cacti may affect their survival during acclimatization, but that in vitro growth may also be a crucial factor in this matter. Seedlings of C. jamacaru with a higher number of lateral buds (i.e. taller plants) developed better in the field, exhibiting higher values for phytomass and dry weight (Cavalcanti and Resende, 2006).
Regarding M. flaviflorus and M. polyanthus, the longer the shoots, the greater their ex vitro rooting and survival rates. The smallest class tested did not survive acclimatization, which was also observed in shoots of S. luetzelburgii ≤ 2 mm long (Marchi, 2012). Such result was attributed to their difficulty in attaching themselves to the substrate and emitting roots (Marchi, 2012). In this same species, however, other size classes tested (8 to 11 mm, 5 to 7 mm and 3 to 4 mm) obtained high survival rates (85% to 100%), which is in accordance with the results of the biggest size classes tested for M. flaviflorus (0.6 to 0.7 mm; 85%) and M. polyanthus (1.0 to 1.5 mm; 67%).