ABSTRACT
This study investigates the chemical composition and cytotoxic effects of three essential oils of rosemary, lemon and orange, as well as its effects on breast cancer (MCF-7), human foreskin fibroblasts (HFS) and colorectal carcinoma (HCT116) using gas chromatography-mass spectrometry (GC-MS) methods, leading to identification of a different compound by GC-MS. Eicosapentaenoic acid (13.79%) and heptatriacotanol (13.79%) were the major constituents present in the orange oil. Cytotoxic activity was evaluated through 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) methods. Results revealed that essential oils significantly reduced the viability of MCF-7, HFS and HCT116 cells in a concentration-dependent manner. The evaluated essential oils could prove to be promising for future applications in the treatment of cancer-related diseases.
Key words: Anticancer activity, chemical composition, essential oil, cytotoxic effects, cell lines, Mcf-7, Hfs, Hct116, gas chromatography-mass spectrometry (GC–MS), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.
Medicinal plants have been used in healthcare since time immemorial for well-being and bio-prospecting of new plant-derived drugs. Cancer is a group of disease that endangers human life and has currently become the first leading cause of death in the world. The normal cells control the production and release of growth factors, which regulate cell growth/proliferation, thus ensuring cellular homeostasis and maintenance of normal tissue architecture; however, in the case of cancer, cells increase proliferatively because these signals are deregulated and thus, homeostasis within the cell is disrupted (Hanahan and Weinberg, 2011).
Cancer cells can signal normal neighboring cells, resulting in mutations/alterations in signaling pathways. These alterations stimulate the release of growth factors which are supplied back to the cancer cells, enhancing their proliferation (Bhowmick et al., 2004; Cheng et al., 2008). Breast cancer is the most common form of cancer affecting women in the Kingdom of Saudi Arabia (KSA), and there is a steady increase in its patients (Amin et al., 2009).
Many pharmaceutical agents have been discovered by screening natural products from plants and are currently successfully employed in cancer treatment (Da Rocha et al., 2001). The exploration into natural products offers great opportunity to evaluate new chemical classes of anticancer agents. The plant-derived secondary metabolites products are expected to induce lesser side effects compared to synthetic drugs. Among plant derived compounds, essential oils (EOs) from aromatic plants have been reported to possess anticancer properties (Yu et al., 2011). EOs has also been reported to improve the quality of life of the cancer patients by lowering the level of their agony (Bhalla et al., 2013).
Citruses such as oranges and lemons provide multitude of health benefits resulting from their constituent compounds like bitter limonods, carotenoids, flavonoids, and folic acid which have been shown to prevent a variety of cancers and cardiovascular diseases (Mohamed et al., 2010).
Lemon is used traditionally to reduce high blood pressure, mental health, respiratory problems, arthritis, and treatment of diabetes, rheumatism and headaches. The essential oil of lemon showed fungi-toxicity against some fungi, anticancer and antibacterial potency (Al-Jabri and Hossain, 2018). The majority of chemical compounds in lemon fruits are alkaloids (Oikeh et al., 2016). Similarly, Omani sour lemon essential oil was composed of limonene (53.57%), a-terpineol (14.69%), b-pinene (8.23%), a-pinene (1.84%), b-myrcene (1.51%), a-terpinolene (4.33%), terpinen-4-ol (3.38%), cymene (1.80%), b-bisabolene (1.43%), b-linalool (0.85%) and E-citral (1.08%) (Al-Jabri and Hossain, 2014; Tadtong et al., 2015).
The plant Rosmarinus officinalis L., a member of the mint family Lamiaceae has many culinary and medicinal uses. The main polyphenols found in rosemary extract (RE) include the diterpenescarnosic acid (CA) and rosmarinic acid (RA) (Cuvelier et al., 1994), and also contains numerous phytochemicals such as flavonoids, glycosides, phytosterols and polyphenols; phytochemicals that are claimed to have antioxidant activity. The aim of this study is to analyze the chemical composition of three different essential oils (lemon, orange and rosemary), in addition to evaluating their cytotoxic activity against human carcinoma; breast cancer (MCF-7); human foreskin fibroblasts (HFS) and colorectal carcinoma (HCT116).
Essential oils (EOs)
The following commercial EOs (lemon, orange and rosemary) were purchased from a GNC market (http://www.armalgnc.com/index.php?id_lang=1) in Makkah City, Saudia Arabia in October 2018, and the EOs were kept at 4°C in dark containers until they were used for the experiment. The oil samples were diluted in diethyl ether (1: 200) for GC-MS analysis.
Cell culture
The cell lines including, breast cancer (MCF-7), human foreskin fibroblasts (HFS) and colorectal carcinoma (HCT116) was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). Institutional ethical approval was obtained for the use of cancer cells in this study.
GC/MS analysis of essential oils
The oil samples were diluted in diethyl ether (1: 200) for GC-MS analysis using a capillary gas Chromatography (model Trace GC ULTRA, Thermo Scientific) directly coupled to ISQ Single Quadruple MS.
The GC/MS analysis was performed on a TG-5MS non-polar 5% phenyl methylpolysiloxane capillary column (30 m × 0.25 mm ID × 0.25 µm). The oven temperature was maintained at 40°C for 3 min and heated slowly to 280°C at 5°C/min. The flow rate of carrier gas (Helium) was 1 ml/min, the injected sample volume was 10 µl, split less injection technique, and ionization energy of 70 eV in the electronic ionization (EI) mode.
Identification of essential oils components
The compounds were identified by the retention index and the fragmentation pattern of the authentic compound mass spectra data (Chien et al., 2009). Few compounds were also identified by Wiley 9 and NIST08 commercial catalogue library (Database of National Institute Standard and Technology) having more than 62,000 patterns.
Cell growth
Cells were cultivated in Dulbecco’s Minimal Essential Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (both GIBCO, Grand Island, NY, USA), 1% l-glutamine, 1% penicillin, and streptomycin, 10% serum, 100x, 1% streptomycin/penicillin/amphotericin B solution, and then periodically sub-cultured and transferred in a humid-CO2 incubator (ShelLab, USA) at 5% CO2, 37°C. Cell numbers were calculated using a Neubauer Chamber. The cells were thawed, allowed to grow, and subcultured until it reaches the number of cells needed which was 2,000,000 for all the plates for each type of cells.
This is based on the conversion of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to MTT-formazan. The cells (MCF-7, HFS and HCT116) were seeded with 200 µL DMEM/F12 medium containing bovine serum in 96-wells plate and incubated in 37°C for 24 h. Stock solutions of the three essential oils (Lemon oil, rosemary oil and orange oil) were prepared in DMSO (1%) and phosphate buffered saline (PBS) at different concentrations (50, 100, 150 and 200 µl/mL) for MTT assay.
After 72 h of cell incubation with different doses of the three essential oils (50, 100, 150 and 200 µl/mL), 20 µL MTT solution (5 mg/mL) was added to each well. After 4 h incubation at 37°C, the formazan was dissolved in DMSO. Finally, the optical density (OD) of wells was measured on a micro plate ELISA reader at 540 nm.
Normal human fibroblasts (wst-1) were used as control. In the first day, each well was seeded with 10,000 cells; and the next day, the media was replaced with drugs concentration. After 24 h, we apply with WST-1 kit for cell viability assay, 10 µl was added on each wells, incubated for 2 h, then read with the ELISA READER MACHIN at 540 nm. Similar approach was followed in the 48 h and 72 h incubation (Thani et al., 2014).
Each oil concentration was repeated eight times, standard curves for each sample (absorbance value against cell number) were plotted, and cell viability was calculated based on the standard curves. Cell survival in the negative control was assumed to be 100% and IC50 was calculated using analysis of regression. Analysis of Variance (ANOVA) followed by the Student-Newman-Keuls test was used to identify the differences among the groups. Significance was assumed at 5% level.
Essential oils by GC-MS
The analytical results of lemon, orange and rosemary essential oils are shown in Tables 1 to 3 respectively. The main components of the lemon oil were diethyl phthalate (60.61%) and D-Limonene (4.86%) followed by Ø¡-d-Mannofuranoside, Ogeranyl-Squalene (4.86%) as shown in (Table 1). Monoterpenes derived from citrus have been shown to inhibit human cancer cell proliferation and tumor growth through various mechanisms such as promoting cell apoptosis, inhibition of the expression of growth factors and cell cycle arrest (Chidambara Murthy et al., 2012). For the orange oil, the Cyclohexanol, 5-methyl-2-(1-methylethyl) was detected at a level of (28.84%) as a major compound; followed by Butanoic acid, 2-methyl-, ethyl ester (16.54%), 1-Heptatriacontanol (13.79%) and Camphor (10.65%). On the other hand, Squalene (27.34%) was the major percentage of the rosemary oil followed by trans-Geranylgeraniol (24.82%) and Lucenin-2 (7.10%) as shown in Table 3.
Cell viability of essential oils
The cell viability was examined at a different concentration (50, 100, 150 and 200 µl/ml) of essential oils against 3 cancer cells (MCF-7, HFS and HCT116), and the results showed that the more sensitive cancer cell for the used essential oils was MCF-7 (breast cancer) cell line, followed by HFS (human foreskin fibroblasts) and HCT116 (colorectal carcinoma) with comparison to normal human fibroblasts using MTT assay after 24 h (Figure 1). Essential oils significantly reduced the viability of MCF-7, HFS and HCT116 cells in a concentration- dependent manner.
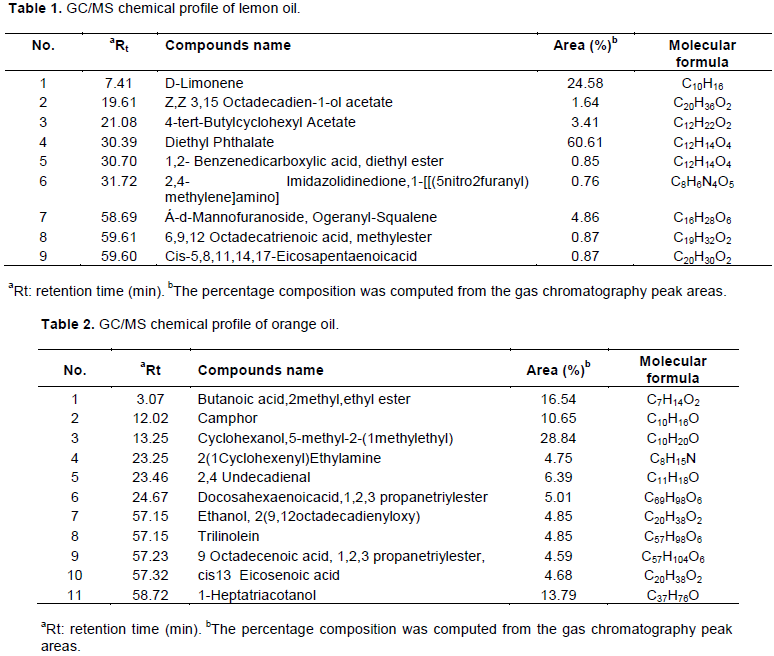
Cell viability of essential oils after incubation with MCF-7 cell line
Initial results showed that essential oils had a clear effect on the MCF-7 cell lines from other cells, hence the focus was on this type in particular to complete the experiments compared to the other cells.
In this study, the percentage viability of MCF-7 cell line incubated with different concentrations of lemon, rosemary, and orange oil for 24 h were tested. The cell viability was decreased in a dose-dependent manner where the IC50 were 84 ± 1.3, 57 ± 2.8, and 59 ± 2.8 µl/ml for lemon, rosemary and orange oil respectively (Figure 2 and Table 1). ANOVA analysis of IC50 shows that the f-ratio value is 27.03666, the p-value is 0.000996, and the result is significant at p ≤ 0.05. This indicates that both rosemary and orange oils are more effective than lemon oil (with p-value of 0.001943 and 0.002468). In addition, there is no significant difference between the effect of rosemary and orange at 24 h incubation period (p-value is 0.756019) (Table 1).
Cell viability after 48 h incubation with MCF-7 cell line
In MCF-7 cell line incubated with different concentrations of lemon, rosemary, and orange oil for 48 h, percentage viability was observed to decrease in a dose-dependent manner where the IC50 were 77 ± 1.9, 78 ± 10.6, and 69 ± 2.8 µl/ml for lemon, rosemary and orange oil respectively (Figure 3 and Table 1). ANOVA analysis of IC50 shows that the f-ratio value is 0.39042; the p-value is 0.692792; and the result is not significant at p ≤ 0.05. This indicates that lemon, rosemary, and orange oils are nearly equally effective at 48 h incubation period (Table 1).
Cell viability after 72 h incubation with MCF-7 cell line
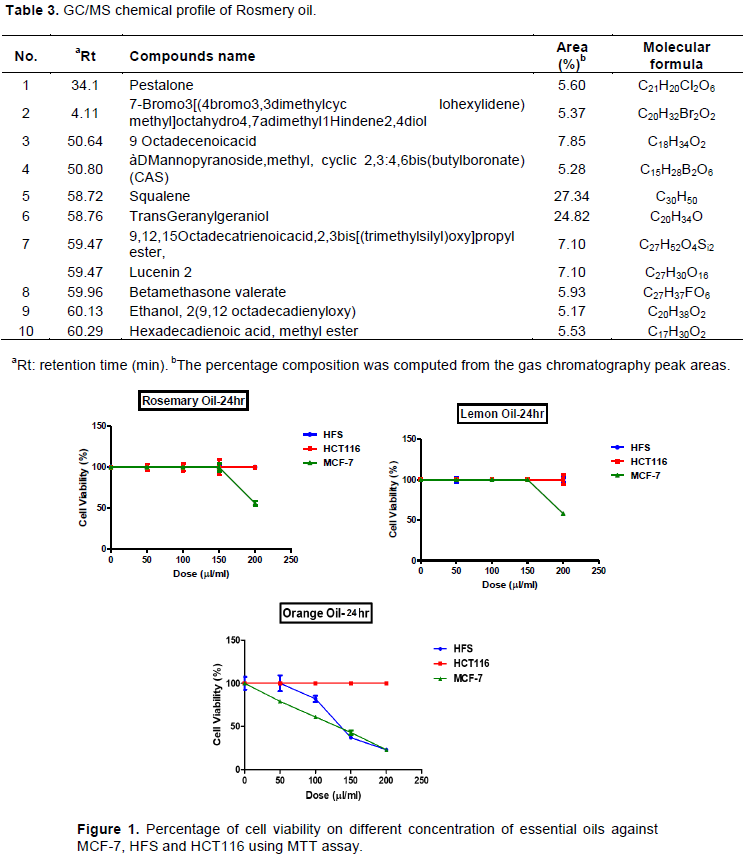
Finally, the percentage viability of MCF-7 cell line incubated with different concentrations of lemon, rosemary, and orange oil for 72 h was found to decrease in a dose-dependent manner where the IC50 was 94 ± 6.5, 63 ± 3.9 and 63 ± 2.5 µl/ml for lemon, rosemary and orange oil, respectively (Figure 4 and Table 4). ANOVA analysis of IC50 shows that the f-ratio value is 9.8061; the p-value is 0.012856; and the result is significant at p ≤ 0.05. This indicates that both rosemary and orange oil are more effective than lemon oil (p-value is 0.028979 and 0.023842). In addition, there is no significant difference between the effect of rosemary and orange at 72 h incubation period (with p-value of 0.902487) (Table 4).
In the present study, the constituents of the essential oils of rosemary, lemon and orange were identified to include monoterpenes, sesquiterpenes, alcohols and esters. Diethyl phthalate was demonstrated to account for the highest percentage of the components in lemon oil (60.61%).
A significant inhibitory effect was noted in the cell lines following treatment with the essential oils. This observation indicated that apoptosis may be a major contributor to the biological efficacy of the MCF-7 cells. The apoptosis rate was higher in the lemon essential oil group compared with that of other essential oil groups at three concentrations (P<0.01).
In lemon oil, the result is in consensus with previous studies of Rabi and Bishayee (2009) that identified D-Limonene, as one the components of lemon essential oil by using GC-MS. In this study, it was reported that D-Limonene can prevent human prostate cancer cells and enhance antioxidant activities. It is well known that the differences between the results of the present study and the chemical profile of previously investigated lemon oils are in the concentrations and types of the essential components which appeared to somewhat agree with some reports in Hamdan et al. (2013). Essential oils showed high variation in terms of composition even among closely related species (that is, species of the same genera), which is in agreement with previous reports on several plants (Sharma and Cannoo, 2013). A number of recent studies investigated the anticancer activities of several compounds. Lee et al. (2006) found that camphor suppresses the proliferation of cancer cells by inducing apoptosis. The diterpene alcohol, geranylgeraniol which was present in a relatively considerable amount (24.82%) in rosemary oil is reputed for broad spectrum of biological activities, as a potentially useful chemopreventive agent in hepatocarcinogenesis (Katuru et al., 2011). Among identified compounds, carboxylic acids, dominated by n-Hexadecanoic acid and cis-9-hexadecanoic acid, constituted the largest group of compounds. The higher content of carboxylic acids in the essential oil, compared to other groups of compounds, is an unusual finding, as most other essential oils reportedly contain terpenes and other hydrocarbons. The anticancer properties of monoterpenes are discovered every now and then. For instance, limonene, a monoterpene, has been shown to exhibit anticancer properties against different types of cancers (Gautam et al., 2014).
Using cancer cell apoptosis induction trials, previous studies have identified that specific components of essential oils are capable of inducing cancer cell apoptosis. For example, sesquiterpenes have anticancer activities that are likely to arrest the proliferation of prostate cancer cells (Shoemaker et al., 2005; Sharifi-Rad et al., 2017). In addition, geranyl acetate and geranylgeraniol are two important monoterpenes found in the essential oils of several plant species (Qi et al., 2018; Dosoky and Setzer, 2018).
Geranylgeraniol have been shown to be of tremendous pharmacological potential activity. Moreover, previous studies have identified antitumor activity in two compounds with slightly greater contents of volatile oil, D-limonene, and n-Octanol (Zhang et al., 2017; Sun et al., 2010). However, the activities and mechanisms of specific compositions must be investigated in future studies.
From the EOs tested in this study, it was noticed that some had potential activities against the cancer cell tested. Factors such as processing, handling, and storage conditions may be responsible for the faintness in the biological and pharmacological properties of the commercial EOs, which originally possess significant biological activities, suggesting that more investigation on commercial Eos is required before recommending their use to the public.
The authors have not declared any conflict of interests.
REFERENCES
|
AL-Jabri NN, Hossain MA (2014). Comparative chemical composition and antimicrobial activity study of essential oils from two imported lemon fruits samples against pathogenic bacteria. Beni-Suef University Journal of Basic and Applied Sciences 3:247-253.
Crossref
|
|
|
|
Al-Jabri NN, Hossain MA (2018). Chemical composition and antimicrobial potency of locally grown lemon essential oil against selected bacterial strains. Journal of King Saud University-Science 30:14-20.
Crossref
|
|
|
|
|
Amin TT, Al Mulhim A, Al Meqihwi A (2009). Breast cancer knowledge, risk factors and screening among adult Saudi women in a primary health care setting. Asian Pacific Journal of Cancer Prevention 10:133-138.
|
|
|
|
|
Bhalla Y, Gupta VK, Jaitak V (2013). Anticancer activity of essential oils: A review. Journal of the Science of Food and Agriculture 93:3643-3653.
Crossref
|
|
|
|
|
Bhowmick NA, Neilson EG, Moses HL (2004). Stromal fibroblasts in cancer initiation and progression. Nature 432(332).
Crossref
|
|
|
|
|
Cheng N, Chytil A, Shyr Y, Joly A, Moses HL (2008). Transforming growth factor-β signaling-deficient fibroblasts enhance hepatocyte growth factor signaling in mammary carcinoma cells to promote scattering and invasion. Molecular Cancer Research 6:1521-1533.
Crossref
|
|
|
|
|
Chidambara Murthy KN, Jayaprakasha G, Mantur SM, Patil BS (2012). Citrus monoterpenes: potential source of phytochemicals for cancer prevention. Emerging Trends in Dietary Components for Preventing and Combating Disease. ACS Publications pp. 545-558.
Crossref
|
|
|
|
|
Chien SC, Young PH, Hsu Y J, Chen C-H, Tien Y-J, Shiu S-Y, Li T-H, Yang C-W, Marimuthu P, Tsai LF-L, Yang W-C (2009). Anti-diabetic properties of three common Bidens pilosa variants in Taiwan. Phytochemistry 70:1246-1254.
Crossref
|
|
|
|
|
Cuvelier ME, Berset C, Richard H (1994). Antioxidant constituents in sage (Salvia officinalis). Journal of Agricultural and Food Chemistry 42:665-669.
Crossref
|
|
|
|
|
Da Rocha AB, Lopes RM, Schwartsmann G (2001). Natural products in anticancer therapy. Current Opinion in Pharmacology 1:364-369.
Crossref
|
|
|
|
|
Dosoky NS, Setzer WN (2018). Chemical Composition and Biological Activities of Essential Oils of Curcuma Species. Nutrients 10(9):1196.
Crossref
|
|
|
|
|
Gautam N, Mantha AK, Mittal S (2014). Essential oils and their constituents as anticancer agents: A mechanistic view. BioMed Research International 154106.
Crossref
|
|
|
|
|
Hamdan D, Ashour ML, Mulyaningsih S, El-Shazly A, Wink M (2013). Chemical composition of the essential oils of variegated pink-fleshed lemon (Citrus x limon L. Burm. f.) and their anti-inflammatory and antimicrobial activities. Zeitschrift für Naturforschung C. 68:275-284.
Crossref
|
|
|
|
|
Hanahan D, Weinberg RA (2011). Hallmarks of cancer: The next generation. Cell 144:646-674.
Crossref
|
|
|
|
|
Katuru R, Fernandes NV, Elfakhani M, Dutta D, Mills N, Hynds DL (2011). Mevalonate depletion mediates the suppressive impact of geranylgeraniol on murine B16 melanoma cells. Experimental Biology and Medicine 236:604-613.
Crossref
|
|
|
|
|
Lee HJ, Hyun EA, Yoon WJ, Kim BH, Rhee MH, Kang HK (2006). In vitro anti-inflammatory and anti-oxidative effects of Cinnamomum camphora extracts. Journal of Ethnopharmacology 103:208-216.
Crossref
|
|
|
|
|
Mohamed AA, El-Emary GA, Ali HF (2010). Influence of some citrus essential oils on cell viability, glutathione-S-transferase and lipid peroxidation in Ehrlich ascites carcinoma cells. Journal of American Science 6:820-826.
|
|
|
|
|
Oikeh EI, Omoregie ES, Oviasogie FE, Oriakhi K (2016). Phytochemical, antimicrobial, and antioxidant activities of different citrus juice concentrates. Food Science and Nutrition 4:103-109.
Crossref
|
|
|
|
|
Qi F, Yan Q, Zheng Z, Liu J, Chen Y, Zhang G (2018). Geraniol and geranyl acetate induce potent anticancer effects in colon cancer Colo-205 cells by inducing apoptosis, DNA damage and cell cycle arrest. Official Journal of the Balkan Union of Oncology 23:346-52.
|
|
|
|
|
Rabi T, Bishayee A (2009). d-Limonene sensitizes docetaxel-induced cytotoxicity in human prostate cancer cells: Generation of reactive oxygen species and induction of apoptosis. Journal of Carcinogenesis, P. 8.
Crossref
|
|
|
|
|
Sharifi-Rad J, Sureda A, Tenore GC, Daglia M, Sharifi-Rad M, Valussi M, Tundis R, Sharifi-Rad M, Loizzo MR, Ademiluyi AO, Sharifi-Rad R, Ayatollahi SA, Iriti M (2017). Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 1;22(1):70.
Crossref
|
|
|
|
|
Sharma A, Cannoo DS (2013). Phytochemical composition of essential oils isolated from different species of genus nepeta of labiatae family: A review. Pharmacophore, P. 4.
|
|
|
|
|
Shoemaker M, Hamilton B, Dairkee SH, Cohen I, Campbell MJ (2005). In vitro anticancer activity of twelve Chinese medicinal herbs. Phytotherapy Research. An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives 19:649-651.
Crossref
|
|
|
|
|
Sun S, Du GJ, Qi LW, Williams S, Wang CZ, Yuan CS (2010). Hydrophobic constituents and their potential anticancer activities from Devil's Club (Oplopanax horridus Miq.). Journal of Ethnopharmacology 132:280-285.
Crossref
|
|
|
|
|
Tadtong S, Kamkaen N, Watthanachaiyingcharoen R, Ruangrungsi N (2015). Chemical components of four essential oils in aromatherapy recipe. Natural Product Communications 10:1934578X1501000673.
Crossref
|
|
|
|
|
Thani NAA, Keshavarz S, Lwaleed BA, Cooper AJ, Rooprai HK (2014). Cytotoxicity of gemcitabine enhanced by polyphenolics from Aronia melanocarpa in pancreatic cancer cell line AsPC-1. Journal of Clinical Pathology jclinpath-2013-202075.
Crossref
|
|
|
|
|
Yu JQ, Lei JC, Zhang X-Q, Yu HD, Tian DZ, Liao ZX, Zou G-L (2011). Anticancer, antioxidant and antimicrobial activities of the essential oil of Lycopus lucidus Turcz. var. hirtus Regel. Food Chemistry 126:1593-1598.
Crossref
|
|
|
|
|
Zhang L, Yang Z, Chen D, Huang Z, Li Y, Lan X, Su P, Pan W, Zhou W, Zheng X, Du Z (2017 ). Variation on Composition and Bioactivity of Essential Oils of Four Common Curcuma Herbs. Chemistry and Biodiversity 14(11).
Crossref
|
|