ABSTRACT
A strain of Aspergillus niger PR-142 native to northern coast of Peru was subjected to successive processes of mutagenesis by ultraviolet light (UV) irradiation at 253.7 nm to increase the production of fructooligosaccharides (FOS). An initial selection was made by considering the mutants with increased invertase activity followed by the measurement of β-fructosyltransferase (FTase) activity both in mycelium and extracellular environment. Five selected mutants, which showed increased values of mycelium invertase activity (ranging from 101 to 128% as compared to the parent strain) at 40°C and sodium dodecylsulfate 0.15 (w/v), were grown in a fermentative medium in 50 mL conical tubes on a rotary shaker, and their FTase activity was determined. The 6-M69 mutant showed the most active mycelium activity of 1.5 fold as compared to the parent strain. When the same reaction was performed between 1 to 4 h, at the 3rd h, the mycelium FTase activity significantly increased up to 7 and 3 times in the mutant and parental strain, respectively. Finally, 4 mutants and the parental PR-142 were genetically characterized using inter simple sequence repeat polymerase chain reaction (ISSR-PCR) molecular markers. This analysis showed a significant 33% polymorphic bands between the parent and mutant markers, and 20 bands were unique to the mutants.
Key words: Aspergillus, mutagenesis, β-fructosyltransferase, fructooligosaccharides, inter simple sequence repeat polymerase chain reaction (ISSR-PCR).
Fructooligosaccharides (FOS) are prebiotics with known beneficial properties, including growth promotion of Bifidobacterium species, prevention of colon cancer, and reduction of cholesterol levels and triglycerides in the blood. It can also be considered as a calorie-free and non-cariogenic sweetener (Maiorano et al., 2008). FOS can be obtained from different vegetables such as garlic onions, artichokes, asparagus, bananas, rye wheat and tomatoes (Sangeetha et al., 2005). However, commercial FOS are enzymatically produced either by hydrolysis of inulin (to produce oligofructoses) catalyzed by inulinases (EC 3.2.1.7) or by transfructosylation of sucrose employing sucrose fructosyltransferases (FTase, EC 2.4.1.99) and β-D-fructofuranosidases (also termed invertases) (FFase, EC 3.2.1.26) (Maiorano et al., 2008). These classes of enzymes are widely distributed in plants, fungi and bacteria kingdoms and most of them belong to the Glycoside Hydrolase families 32 (GH32) and 68 (GH68) (Olarte et al., 2016). Although, the physical and chemical characteristics of the molecular structure vary in many microorganisms, they all have both hydrolytic and transfer activities (Dominguez et al., 2013).
Although, FOS has been produced by commercial microorganisms for more than twenty years, it is still necessary to continue to identify new FFase(s) of high yield as well as the genes involved in its synthesis. This is the reason why the search for microorganisms with these characteristics such as Penicillum, Aerobasidium, Fusarium and mainly Aspergillus species is continuously reported (Muñiz et al., 2016). It should be noted that Aspergillus niger is a filamentous fungus of the group of black Aspergillus that are widely disseminated in the environment and is widely used in biotechnology industries due to its great secretory capacity of a large amount of enzymes and organic acids, among other things. In addition, many of these products have the generally recognized as safe (GRAS) status by the US Food and Drug Administration (FDA). Different techniques have been used in order to increase the production of FOS and among them are recombinant DNA technologies that have allowed the expression of the FTase gene in plants (Heyer and Wendenburg, 2001), bacteria and yeasts (Zhang et al., 2015). In the specific case of increasing FOS productivity, although there are very few reports on the use of ultraviolet (UV) irradiation mutagenesis, Guilarte et al. (2009) obtained three mutants of Aspergillus oryzae, known as IPT-745, IPT-746 and IPT-748, which showed values ranging from 1.5 to 1.8-fold of activity, that was bound to the mycelium of the parent strain. The ability of UV light to produce genetic variation is known and has been used on numerous occasions to produce metabolic changes that increase the efficiency of some particular enzymes of interest. The inter simple sequence repeat polymerase chain reaction (ISSR-PCR) molecular markers have been successfully used to study the genetic variability of Aspergillus species, but there are no reports on the use of this marker in UV mutants in order to better compare them with the parent strain.
The present study aimed to 1) increase FOS production, using a native A. niger strain, via UV irradiation, and 2) characterize the mutants in reference to the parent strain using an ISSR-PCR as molecular marker.
Microorganism and fermentative medium
The native strain of A. niger PR-142 isolated from sugarcane soil during 2015 at the north coast of Peru were used in this research. The isolate was identified in the Biotechnology Laboratory of the Continental University S.A.C, Huancayo, Peru (Gutarra et al., 2017). All strains including the mutants were grown on potato dextrose agar (PDA) medium at 30°C for 5 to 7 days. The produced spores were filtered and separated from the mycelium with the help of cotton swabs in sterile 0.1% (w/v) Tween 80 medium, and they were quantified using a Neubauer chamber. The fermentative tests were carried out in 250 ml flasks containing 50 ml of fermentative medium (g/L): sucrose 3.0, NaNO3 0.3, KH2PO4 0.2, MgSO4.7H2O 0.05, MnCl2.4H2O 0.02 and FeSO4.7H2O 0.001 with a pH of 5.5.
Growth studies
Adequate doses of sodium dodecylsulfate (SDS) were evaluated in order to restrict the growth of mycelia and to have isolated strains. The evaluation comprised a range of 0.001 to 0.3% (w/v), resulting in the growth of hyphae being measured for a period of between 3, 4 and 5 days. Different doses of ultraviolet (UV) radiation were evaluated by using two 253.7 nm germicidal UV lamp (15W G15T8, Philips Ltd.) within a time range of 1 to 10 min, fixed as an optimal time that allowed the survival of only 5% of the spores used.
Production of mutants
A concentration of 105 spores was spread on glass plates coated with PDA medium that was supplemented with SDS and placed at a distance of 15 cm from two germicidal UV lamps (15W G15T8, Philips Ltd.) for 4 min. The spores were maintained for 30 min in absolute darkness and incubated at 40°C overnight and, then, at 30°C for 5 to 6 days. The chosen colonies were those that at first glance, presented typical morphology while those of strange appearance were discarded.
Selection of mutants based on FFase activity
As an initial step, the initial selection of promising mutants was based on the activity of FFase (invertase) secreted by them according to the methodology reported by Guilarte et al. (2009) that consists of taking circular fragments of mycelium of 7 mm in diameter as a source of enzyme. In the second stage, 107 spores were grown in 250 ml flasks with 50 ml of the fermentation medium for 72 h at 30°C and 200 rpm in an orbital shaker. The obtained mycelia were vacuum filtered using a polysulfone (PSF) filtration kit (Nalgene Ltd.) and Whatman® grade 1 qualitative filter paper. The enzymatic reaction was carried out in a shaking water bath GFL 1083 (Biovendis Ltd.) at 100 rpm for 60 min at 50°C with 50 ml conical tubes containing 1.2 ml of 0.2 M tris-acetate buffer at pH 5.5 and using approximately 0.02 g of mycelium as the enzyme source. As the substrate, 3.7 ml of a sucrose concentration of 64% (w/v) was used. The amount of reducing sugars obtained was quantified by the 3,5-dinitrosalicylic acid (DNS) assay method as described by Miller (1959). In the first stage, given the considerable number of strains to be evaluated, only the absolute values of absorbance (optical density, OD 540 nm) were considered as the selection factor. In the second stage, FFase activity linked to mycelium was defined as that catalyzing the formation of 1 µmol reducing sugar per minute under the above conditions.
Selection of mutants with high FTase activity
The mycelial and extracellular FTase activities of the mutants that had the highest values of invertase activity were determined. For this purpose, the same methodology as described above was used, but with 0.3 ml of the fermentation broth as an enzyme source for the extracellular activity. The reaction products were analyzed by liquid chromatography with refractive index detector (LC-RID) on an Agilent Technologies 1220 Infinity LC system-1260 RID equipment (Boeblingen, Germany). Separation was performed on a HPLC column Kromasil® (100-NH2) (Akzo Nobel, Brewster, NY, USA) (250 x 4.6 mm, I.d. 5μm particle size) using 70:30 (v/v) acetonitrile/water as the mobile phase and isocratic elution with a flow rate of 1.0 ml min-1 for 120 min. One unit of fructosyltransferase activity was defined as the amount of enzyme producing 1 μmol of FOS (1-kestose and nystose) per min, under the assay conditions.
Temporal profile of the reaction
In a manner that is similar to that described above, the FTase reaction of the mycelium of the best mutant was evaluated for a period of 1 to 4 h using the portions of mycelia of about 0.02 g as the enzyme. The reaction was undertaken on a ThermoMixer® Comfort Shaker (Eppendorf, Cambridge, UK) at 800 rpm and 50°C using 2 mL tubes containing 0.42 ml of 0.2M tris-acetate buffer with pH at 5.5, and 1.3 ml of 64% (w/v) sucrose was used as substrate.
Statistical analysis
Data analysis was carried out using one-way analysis of variance (one-way ANOVA) and Turkey’s test by the Statistical Package for
the Minitan16. Statistical significance was set at P < 0.05 and the results were expressed as means ± standard error of mean.
Scrutiny of genetic variability
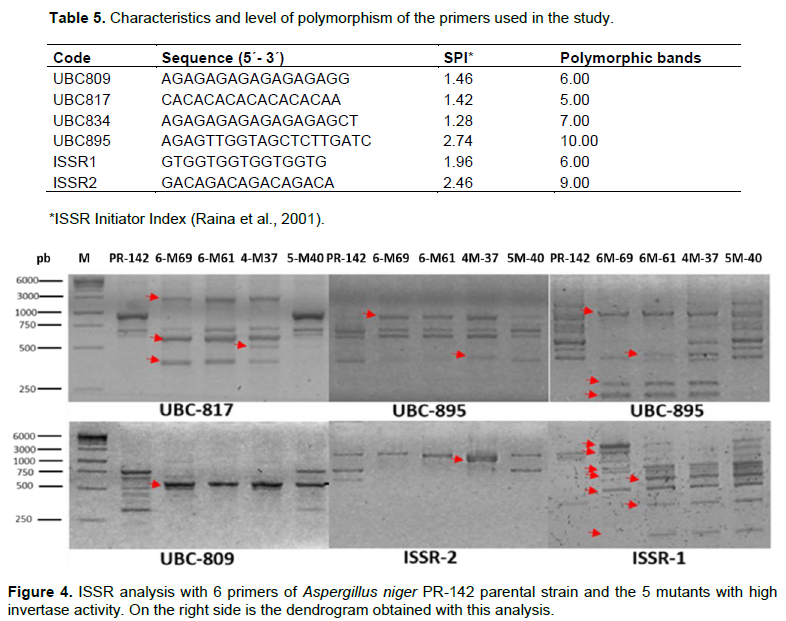
The inter simple sequence repetitive (ISSR) molecular markers were used to test the ability of UV irradiation to produce genetic differences between mutants and parental PR-142. Genomic DNA extraction was performed using the methodology developed by Liu et al. (2000). The amplification via polymerase chain reaction (PCR) was performed with a volume of 25 μL having the same number and concentration of components as described by Neal et al. (2011) in a Mastercycler® Nexus Gradient Thermal Cycler (Eppendorf, Germany) using 6 ISSR primers (Integrated DNA Technologies) (Table 5). The thermal cycle of the PCR was also similar to that described by Neal et al. (2011). The PCR products were resolved on 1.5% agarose gels after staining with 1% ethidium bromide. The ISSR band patterns were assigned values of 1 (band presence) and 0 (band absence). The analyses were performed using the NTSYSpc program version 2.11.
Evaluation of the effect of SDS concentration on hyphal growth
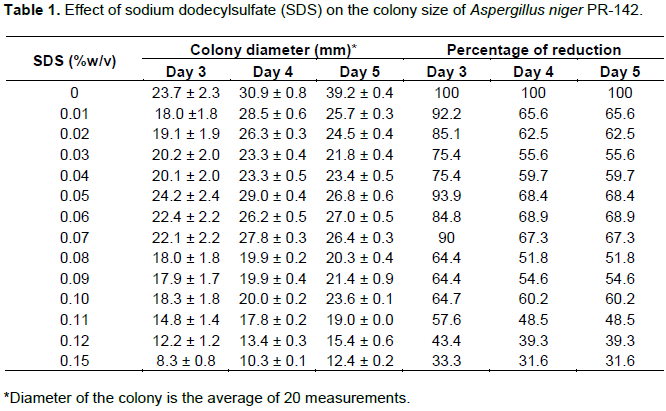
A reduction of 68.4% in the diameter of the colonies was achieved at a SDS concentration of 0.15% (w/v) on the fifth day of counting (Table 1). This size was sufficient and facilitated the separation of individual colonies averaging 12 to 15 colonies per plaque.
Resistance to ultraviolet light
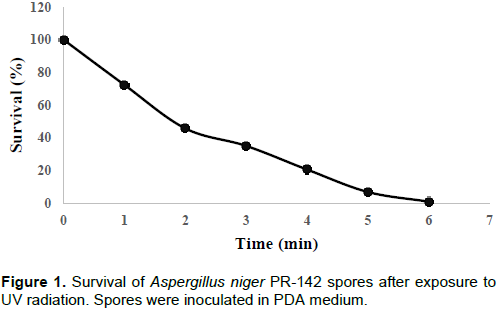
The parental strain was characterized by presenting different doses of resistance to UV irradiation which reached a lethality of 50% at 1.5 min (Figure 1). Optimal time occurs at 5 min (5% survival). Consequently, this time was used in all mutagenesis assays.
Selection based on FFase activity
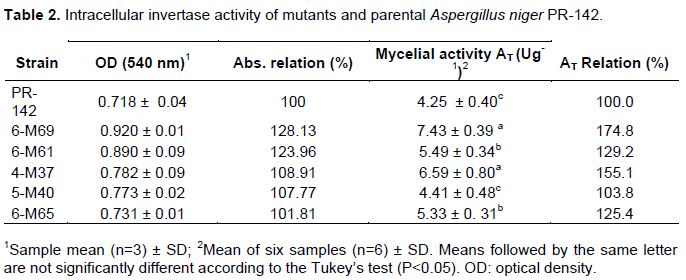
From 666 surviving lineages, 5 of them had higher absorbance values than the parent strain and the 6-M69, 6-M61 and 4-M37 mutants had higher mycelial activity (Table 2). The FFase activity showed that the mutant 6-M69 was the most active but it did not present statistically significant differences with the 4-M37 strain. The other 5 strains had lower activity values without observing significant differences neither between the 6-M65 and 6-M61 strains nor between the parental and the 5-M40 strain (Table 2). All the assays were carried out in sextduplicate.
Selection of mutants with high FTase activity
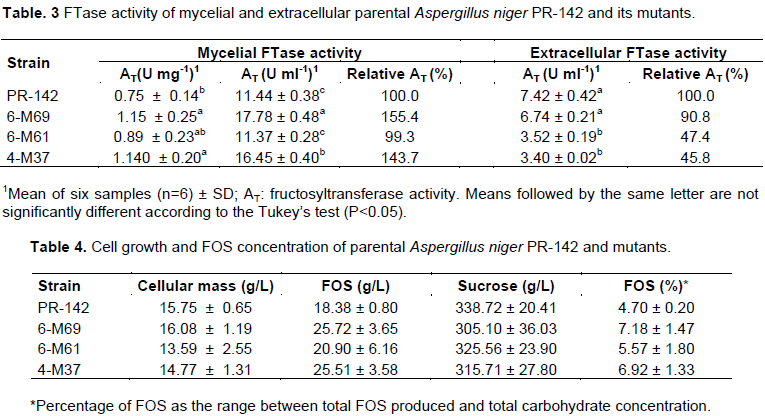
The 6-M69 and 4-M37 mutants showed the highest values of mycelial activity of between 1.5 and 1.4 times the parental activity, respectively (Table 3). No significant difference in extracellular activity was observed between the parent strain and the 6-M69 mutant except with respect to strains 6-M61 and 4-M37 (Table 3). The cell mass had high values above 15 g/L-1 with a slight advantage for the 6-M69 mutant over the parent. The highest FOS production was presented by the 6-M69 mutant strain, being 1.4 times higher than the parental production (Table 4) and the FOS composition was GF2 (1-kestose) and GF3 (1-nistose), which in the case of 6-M69 was 86.2% GF2 and 13.8% GF3.
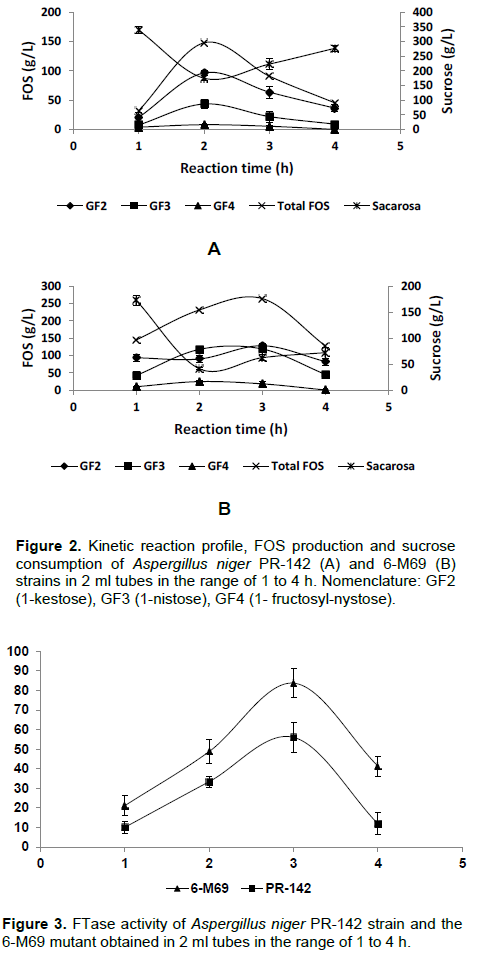
Temporal profile for 6-M69
Figure 2 shows the course of consumption of sucrose and the synthesis of FOS of the 6-M69 and PR-142 strains. The process reached the maximum FOS production at 2 h (147.7 g/L) in the case of PR-142 and at 3 h (263.78 g/L) in the case of 6-M69 and after that, a gradual FOS decrease concomitant with an increase in sucrose was observed. In both cases, the sucrose underwent a maximum depletion between 2 and 3 h and at the end of this period its concentration increased. During the maximum production of FOS in the PR-142 strain, the final product contained: 1-kestose (GF2-65.2%), 1-nystose (GF3-29.3%) and 1-fructosyl-nystose (GF4-5.4%), while the 6-M69 strain contained: 1-kestose (GF2-48.4%), 1-nystose (GF3-44.8%) and 1-β-fructofuranosyl nystose (GF4- 6.7%) and after that, a gradual decrease of all these products took place. The dramatic increase of FOS under these conditions is far superior to the tests in conical tubes of 50 ml on a rotating shaker, which became evident from the first h of reaction. The FTase activity increased and reached the maximum value at 3 h, of 83.8 and 56.1 U/mL for 6-M69 and PR-142, respectively (Figure 3). This increase kept the activity ratio of 1.5-fold between the mutant and the parental strains which was, in turn, rather similar to that obtained in the test using conical tubes.
Scrutiny of genetic variability
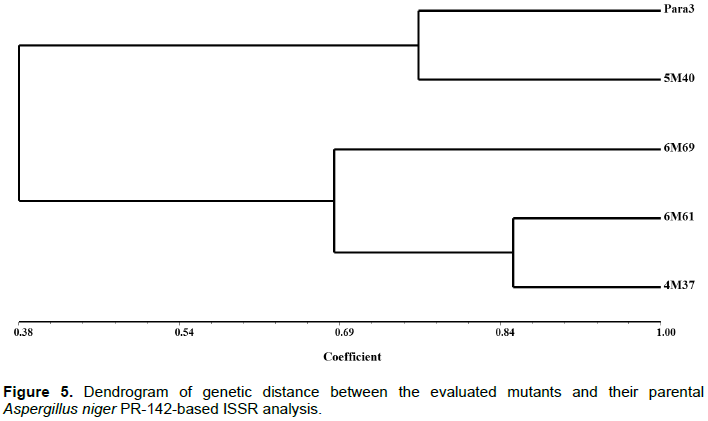
The 6 primers (Table 5) gave a total of 42 loci with an average of 26.5 bands per individual of which 33% were polymorphic. A total of 20 bands were present only in the mutants (Figure 4). Initiators UBC-895 and UBC-834 had the highest number of polymorphic bands and the highest ISSR primer index (SPI) (Raina et al., 2001). The dendrogram obtained (Figure 5) at a similarity coefficient of 0.65 shows two groups, one of which consists of PR-142 parental and the 5-M40 mutant and the other group consists of two subgroups where the first one consists of the 4-M37 and 6-M61 mutants and the second one consists of only the 6-M69 mutant. The highest genetic similarity (0.86) occurred between PR-142 and the 4-M37 mutant and the lowest similarity (0.23) occurred between the PR-142 strain and 5-M40.
The 6-M69 and 4-M37 mutants have been shown to have higher FTase activity than the parental strains and this demonstrates the potential of these strains for use in cell immobilization techniques for the production of FOS (Chien et al., 2001). This increase of about 1.5 fold in FTase activity can be compared to the results obtained by Guilarte et al. (2009) for A. oryzae strain 1303 and by Skowronek and Fiedurek (2003) for A. niger strain 13/36.
The FTase activity and, consequently, the production of FOS increased dramatically by performing the reaction in 2 mL tubes in a ThermoMixer®. This demonstrates that the reaction conditions in 50 ml conical tubes in a rotary shaker were not fully optimal. It must be taken into account that hydrolytic processes are influenced by many factors such as particle size, concentration of the reaction medium, and the geometric and operational form of the medium (Abd Rahim et al., 2015). It is probable that in this case, several factors such as the environment, the type of equipment used, and the agitation elevated to 800 rpm could increase the collision-producing cellular autolysis and morphological changes in the mycelium (Purwanto et al., 2009). These phenomena could allow a proportion of the enzymes to be free to react with the substrate by drastically changing the results since the cells per se would be unable to prolong the reaction for an extended period of time. This would explain the higher FTase activity in the PR-142 strain and M-69M being approximately 3 h in duration, greater than that reported for the Aspergillus strain sp.N74 by Sanchez et al. (2008), and which presented a greater performance for up to 1.25 h. A similar finding was reported by Virgen-Ortiz. (2016) for a pure fructosyltransferase from Aspergillus aculeatus which reached a maximum yield at 2 h. The decrease of the concentration of FOS after 2 to 3 h of reaction and the gradual increase of sucrose concentration after 2 h was probably due to a loss of hydrolysis capacity of the enzyme produced by the accumulation of glucose which acts as a competitive inhibitor (Sangeetha et al., 2004). However, the typical pattern of FOS formation of double-deletion (Ruiz et al., 2013) comprising the reduction of GF2 and a simultaneous increase of GF3 and GF4 does not seem to occur here after 2 and 3 h in both mutants. This fact could be due to the negative effect of the enzymes shear caused by very high agitation that would be partially inhibiting this behavior. The concentration of 0.15% (w/v) SDS is 10 times higher than that used for the same purposes in A. oryzae (Guilarte et al., 2009) which demonstrates the higher resistance of the PR-142 strain. The lethal dose of UV reached 50% at 1.5 min, which is less than the 3 min obtained by other A. niger strains as reported by De Nicolás-Santiago et al. (2006) for the production of mutants with high production capacity of xylans and mannans. This reduction could be expected by considering the higher dose of UV rays used in the current work.
It is known that UV rays are inductors of mutations since the pyrimidine bases (thymine and cytosine) of DNA are very sensitive to their effects. These mutations distort the double helix of DNA affecting future replication (Sambrook et al., 2000) and creating genetic variance. In the present study, each strain was differentially affected in its ability to produce FOS after exposure to UV light. The high genetic variability in similar mutants has been previously reported in amylolytic strains of A. niger (Shafique et al., 2010) and Alternaria tennuissima for hyper-active alpha amylase (Shafique et al., 2009). The presence of unique bands can be considered positive for the mutants, since they could be used in selection programs assisted by molecular markers as previously suggested by Afifi et al. (2013) in mutants of Penicillium chrysogenum with a high production of protease.
In this study, the potential of UV radiation mutagenesis to increase FOS production in an A. niger strain was reported. The increase of the enzymatic activity and the production of FOS have increased up to 1.5 times and this improvement is dramatically noticeable under conditions of high agitation in 2 ml microtubes. The process of mutagenesis by UV irradiation has been shown to have high capacity to generate great variability with respect to the native strain and the formation of unique molecular patterns.
The authors have not declared any conflict of interests.
REFERENCES
|
Abd Rahim SN, Sulaiman A, Hamid KHK, Edama NA, Baharuddin AS (2015). Effect of Agitation Speed for Enzymatic Hydrolysis of Tapioca Slurry Using Encapsulated Enzymes in an Enzyme Bioreactor. Int. J. Chem. Eng. Appl. 6(1):38-41.
Crossref
|
|
|
|
Afifi AF, Abo-Elmagd HI, Housseiny MM (2013). Improvement of alkaline protease production by Penicillium chrysogenum NRRL 792 through physical and chemical mutation, optimization, characterization and genetic variation between mutant and wild type strains. Ann. Microbiol. 64(2):521-530.
Crossref
|
|
|
|
|
Chien CS, Lee WC, Lin TJ (2001). Immobilization of Aspergillus japonicus by entrapping cells in gluten for production of fructooligosaccharides. Enzyme Microb. Technol. 29(4):252-257.
Crossref
|
|
|
|
|
De Nicolás-Santiago S, Regalado-González C, García-Almendárez B, Fernández FJ, Téllez-Jurado A, Huerta-Ochoa S (2006). Physiological, morphological, and mannanase production studies on Aspergillus niger uam-gs1 mutants. Electronic J. Biotechnol. 9(1):10.
Crossref
|
|
|
|
|
Dominguez AL, Rodrigues LR, Lima NM, Teixeira JÁ (2013). An Overview of the Recent Developments on Fructooligosaccharide Production and Applications. Food Bioprocess Technol. 7(2):324-337.
Crossref
|
|
|
|
|
Guilarte B, Gutarra B, Cuervo R, Sabino da silva E, Maiorano AE, De Andrade MF (2009). Mutagenesis of Aspergillus oryzae IPT-301 to improve the production of β-fructofuranosidase. Braz. J. Microbiol. 41(1):1194-1203.
|
|
|
|
|
Gutarra B, Diez-Municio M, Cisneros de la Cruz J, Moreno FJ (2017). Diversity of Aspergillus isolates and selection of an isolate with high β-fructofuranosidase activity that is native to the Peruvian coast. Afr. J. Biotechnol. 16(21):1221-1229.
Crossref
|
|
|
|
|
Heyer AG, Wendenburg R (2001). Gene cloning and functional characterization by heterologous expression of the fructosyltransferase of Aspergillus sydowi IAM 2544. Appl. Environ. Microbiol. 67(1):363-370.
Crossref
|
|
|
|
|
Liu D, Coloe S, Baird R, Pedersen J (2000). Rapid Mini-Preparation of Fungal DNA for PCR. J. Clin. Microbiol. 38(1):471.
|
|
|
|
|
Maiorano AE, Piccoli RM, Sabino da Silva E, De Andrade MF (2008). Microbial production of fructosyltransferases for synthesis of pre-biotics. Biotechnol. Lett. 30:1867-1877.
Crossref
|
|
|
|
|
Miller GL (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31(3):426-428.
Crossref
|
|
|
|
|
Mu-iz-Márquez DB, Contreras JC, Rodríguez R, Mussatto SI, Teixeira JA, Aguilar CN (2016). Enhancement of fructosyltransferase and fructooligosaccharides production by A. oryzae DIA-MF in Solid-State Fermentation using aguamiel as culture medium. Bioresour. Technol. 213:276-282.
Crossref
|
|
|
|
|
Neal CO, Richardson AO, Hurst SF, Tortorano AM, Viviani MA, Stevens DA, Balajee SA (2011). Global population structure of Aspergillus terreus inferred by ISSR typing reveals geographical subclustering. BMC Microbiol. 16(11):203.
Crossref
|
|
|
|
|
Olarte S, Rodríguez A, Pati-o JD, Almeciga CJ, Sanchez OF (2016). In Silico Analysis of the Structure of Fungal Fructooligosaccharides-Synthesizing Enzymes. Interdiscip. Sci. Comput. pp. 1-15.
Crossref
|
|
|
|
|
Purwanto LA, Ibrahim D, Sudrajat H (2009). Effect of Agitation Speed on Morphological Changes in Aspergillus niger Hyphae During Production of Tannase. World J. Chem. 4(1):34-38.
|
|
|
|
|
Raina SN, Rani V, Kojima T, Ogihara Y, Singh KP, Devarumath RM (2001). RAPD and ISSR fingerprints as useful genetic markers for analysis of genetic diversity, varietal identification, and phylogenetic relationships in peanut (Arachishypogaea) cultivars and wild species. Genome 44:763-772.
Crossref
|
|
|
|
|
Ruiz Y, Klotz B, Serrato J, Guio F, Bohórquez J, Sánchez OF (2013). Use of spent osmotic solutions for the production of fructooligosaccharides by Aspergillus oryzae N74. Food Sci. Technol. Int. 20(5):365-372.
Crossref
|
|
|
|
|
Sanchez O, Guio F, García D, Silva E, Caicedo L (2008). Fructooligosaccharides production by Aspergillus sp. N74 in a mechanically agitated airlift reactor. Food Bioprod. Process 86:109-115.
Crossref
|
|
|
|
|
Sangeetha PT, Ramesha MN, Prapulla SG (2005). Recent trends in the microbial reduction, analysis and application of Fructooligosaccharides. Trends Food Sci. Technol. 16:442-457.
Crossref
|
|
|
|
|
Sangeetha PT, Rameshb MN, Prapulla SG (2004). Production of fructo-oligosaccharides by fructosyl transferase from Aspergillus oryzae CFR 202 and Aureobasidium pullulans CFR 77. Process Biochem. 39:753-758.
Crossref
|
|
|
|
|
Shafique S, Bajwa R, Shafique S (2009). Mutation of Alternaria tenuissima FCBP-252 for hyperactive alpha-amylase. Indian J. Exp. Biol. 47(7):591-596.
|
|
|
|
|
Shafique S, Bajwa R, Shafique S (2010). Mutagenesis and genetic characterization of amylolytic Aspergillus niger. Nat. Prod. Res. 24(12):1104-1114.
Crossref
|
|
|
|
|
Skowronek M, Fiedurek J (2003). Selection of biochemical mutants of Aspergillus niger resistant to some abiotic stresses with increased inulinase production. J. Appl. Microbiol. 95:686-692.
Crossref
|
|
|
|
|
Virgen-Ortíz JJ, Ibarra-Junquera V, Escalante-Minakata P, Centeno-Leija S, Serrano-Posada H, Ornelas-Paz JJ, Pérez Martínez JD, Osuna-Castro JA (2015). Identification and Functional Characterization of a Fructooligosaccharides-Forming Enzyme from Aspergillus aculeatus. Appl. Biochem. Biotechnol.179:497-513.
Crossref
|
|
|
|
|
Zhang G, Yang J, Shi J, Quian S, Chao Y (2015). Molecular cloning and over-expression of a fructosyltransferase from Aspergillus niger QU10. Chin J. Biotechnol. 31(4):512-522.
|
|