ABSTRACT
The objective of this study was to compare maggots’ production in different substrates following an experimental design of six treatments (S1, 1000 g of brewery wet grains - BWG; S2, 900 g of BWG and 100 g of blood; S3, 1000 g of dung; S4, 900 g of dung and 100 g of blood; S5, 1000 g of rumen content; and S6, 900 g of rumen content and 100 g of blood) repeated trice. 121 flies were collected and identified and 5 days after putting in place the experimental set up, larvae were collected. 6 families of flies, namely Calliphoridae (50.41%), Muscidae (18.18%), Sacrophagidae (12.40%), Strationomiydae (8.26%), Piophilidae (5.79%) and Syrphidae (4.96%) were identified. The highest fresh biomass (41.67±3.51 g) was obtained with S6 and S2 (21.33±15.63 g). The mean highest length (2.62±1.01 cm) was obtained with S3. The highest dry matter (DM) (23.89±2.90%) was obtained with S2 and the lowest DM with S6 (13.56±2.90%). The lowest values of crude proteins (CP) were obtained in S4 (32.82±10.05%) and S6 (33.82±0.18%). Highest fats contents were obtained in S2 (43.93±1.46%) and S4 (26.99±1.12%). In short, S2 can be used for maggots’ production.
Key words: Identification, flies, production, larvae, insect breeding, bovine blood.
In Africa, high costs of dietary protein sources constitute a real challenge on livestock development (Bouafou, 2000). This situation is largely due to the unavailability of inputs, competition between humans, animals and industries over basic feed ingredients for livestock (Dronne, 2018). Gabon in particular is not on the sidelines of this observation and FAO (2016) pointed out that this country imports 96% of its needs in animal products and due to the increasing demand, the local livestock sector is facing many challenges to be addressed, among which the limited access to expensive animal feed. So, efforts have been made in order to valorize new and cheap locally and easily accessible protein sources for livestock feed, namely, flours from maggots, cockroaches, termites, grasshoppers, earth worms, etc., in order to boost the animal production sector (Hardouin et al., 2000). Among all these insects, maggots’ production is easier and maggots’ flour has an interesting nutritive value. Maggots are rich in reserve nutrients such as fats and proteins (47.50 and 52.23%) and potentially in essential amino acids (Bouafou et al., 2007). They can contribute in improving feeding in non-ruminants such as fishes, poultry and rodents (Mensah et al., 2002). Nutrient content in maggots depends on the type of substrate used (Sogbessan et al., 2006; Bouafou et al., 2008). All fast decaying organic matters can serve as substrates for fly laying and maggot development (Hardouin et al., 2000). Considering the high cost and the scarcity of protein sources commonly used in livestock feeding and in local markets, it seems that getting the right substrates of maggots’ production can contribute in the reduction of animal production costs. The objective of this work was to determine best substrates for maggots’ production.
The substrates
The fresh cattle dung was collected at the stable of the National Higher Institute of Agronomy and Biotechnology (INSAB) of the University of Science and Techniques Masuku (USTM), at Franceville in the Haut-Ogooué province. This zone is situated at a latitude of 1°37’59’’ south, a longitude of 13°35’00’’and an altitude of 405 m above the sea level (FDNS, 2004).
The blood and the rumen content from cattle were collected fresh at a slaughter house of the town, and the BWG was also collected from a brewery industry of the same town. The blood is used as attractant meanwhile the BWG, the cattle dung and rumen content were used as basic substrates.
Experimental design
The completely randomized experimental design is made up of six treatments of 1000 g each, repeated 3 times in plastic basins of 86 cm of diameter and 14 cm of height. The substrates’ composition according to the treatments is as follows: S1, 1000 g of BWG; S2, 900 g of BWG + 100 g of blood; S3, 1000 g of cattle dung; S4, 900 g of cattle dung + 100 g of blood; S5, 1000 g of rumen content; S6, 900 g du rumen content + 100 g of blood.
The breeding was conducted in a semi-hard building, isolated, ventilated, and sheltered from bad weather where flies could enter and leave freely. The entire design was placed in two boxes of 3.0 m length × 2.5 m width (7.5 m2). The boxes were left open during 24 h for colonization (Bouafou et al., 2006). 24 h after putting in place the experimental set up, basins remained open to allow the flies to lay eggs on the substrates, then they were covered using lids (Figure 1). These lids had small aeration holes made with the help of a nail. The daily follow up of the experimentation was done trice (at 8 am, 12 am and 5 pm). The basins were open during the periods for a good aeration of the culture media.
Data collection
Identification of flies
For the identification of different colonizing flies in the substrates, 121 flies were collected with the help of a cage covered with a mosquito net treated with a RAMBO brand insecticide (Figure 2) mixed with Trafluthrin (0.25%) and Permethrin (0.25%) active ingredients. The cage contained plates in which were placed small quantities of substrates (cattle dung, rumen content, BWG) as well as the attractant (bovine blood).
Collection of maggots
Once the flies were trapped in the cage, they were killed by the insecticide, collected and conserved in alcohol at 70°C. Collected flies were identified by observation of morphological characters using an OPTIKA brand binocular loupe at X2 magnification. Harvesting of fly larvae was done 5 days after setting up the treatments. Two harvesting methods were used:
(1) The first method consisted of spreading the substrate on a harvesting device made up of a sieve and a receptacle, both exposed to sunlight. Maggots fleeing the sunlight fall to the bottom of the receptacle and are collected. This method is only for the substrate S2 (BWG + blood). This substrate is very compact and makes manual sorting difficult.
(2) The second method is the manual sorting. It consists of soaking the substrates in hot water (60°C) in order to kill the maggots. Maggots’ mobility makes the collection difficult. Once the substrates are soaked, parts of the killed larvae float in hot water and are then picked up with the help of a strainer, meanwhile the others (dead and alive) are trapped in the substrate. For the maggots’ collection, 2 basins, one strainer, a plastic pot for each substrate and a filter were used. Trapped larvae were picked up by removing small quantities of substrates over time. After collection, all the maggots were transferred in 18 labelled pots according to different substrates. To kill the still alive larvae, hot water was introduced into the pots containing them.
Chemical analyses
For chemical analyses, dried maggots were ground manually with the help of a bowel and conserved in labelled plastic sachets (Figure 3). The analyses of flour samples were carried out in the laboratory to determine their chemical characteristics (water content (%); dry matter - DM (%), crude proteins - CP (%); fats content (%)) with two repetitions. The DM content was determined according to the method described by AOAC (1990). The water content was obtained via the determination of the humidity rate = [(fresh weight - dry weight) / fresh weight] × 100. The CP content was obtained using the Bradford method (1976) and the fats content using the extraction method with the help of the Soxhlet design.
Productivity of substrates: Fresh weight and dry weight
In order to determine the biomass of maggots produced, their fresh and dry weights were recorded according to substrates. After having removed water from pots, larvae are exposed on LOTUS® paper (Figure 4) during about 20 min before recording the fresh weight. This paper absorbs the water present on maggots’ body. Weighing was done using a digital SARTORIUS® brand balance (capacity 500 g and precision 0.1 g).
After recording fresh weight, larvae were dried in an oven at 55°C during 24 h. After cooling, larvae were once weighed with the same balance.
Measurements in larvae
The length of a maggot was determined using 10 fresh maggots randomly selected per substrate. The measurement is done using a ruler labelled in centimeters. A total of 60 maggots were measured for all the substrates.
Statistical analyses
Data on biomass, measurements and chemical composition of maggots were submitted to the analysis of variance at one factor (substrate) following the procedure of the general linear model (GLM proc.) with SPSS 20.0® software. In case of significant differences between the treatments, separation of means was done using the Waller Duncan test at 5% significant level (Steel and Torrie, 1980).
Results are related to the identification of colonizing flies in substrates, biomass and mean measurements of maggots produced and their chemical composition.
Identification colonizing flies in substrates
The proportions of identified fly families are presented in Figure 5. This shows that the most represented family is Calliphoridae (50.41%) followed by Muscidae (18.18%). Less represented families are Piophilidae (5.79%) and Syrphidae (4.96%).
Biomass and average measurements of maggots produced
The biomass distribution in fresh and dry maggots as well as the respective mean length according to substrates is shown in Table 1. It reveals that the maggots from the substrate S6 had the highest fresh matter content (41.67 ± 3.51 g) followed by maggots from the substrate S2 (21.33 ± 15.63 g). There is no significant difference (p>0.05) for the values presented by the substrates S1 and S4 (3.33 ± 2.52a versus 3.33 ± 1.53a, p>0.05) and between the substrates S3 and S5 (13.33 ± 3.21ab versus 11.67 ± 6.11ab p>0.05). The same observation was made for the dried larvae whose weights are higher in the substrates S2 and S6 which are comparable, the couple of substrates S3 and S5, same for S1 and S4. So, except in S3 (only cattle dung), the addition of blood caused an increase in fresh biomass from the substrates S2 and S6, respectively BWG + blood and the rumen content + blood. The growth in length of maggots has been more pronounced in the substrate S3 compared to the maggots from the other substrates. The difference could be due to the quality of substrates: addition of blood could lead to the reduction in maggots’ length against the substrate containing only dung.
Chemical composition of maggots’ flour
The Table 2 presents the chemical composition of maggots’ flour according to different substrates. Maggots from the substrate S6 had the highest water content (86.44 ± 12.77%) while the lowest water content (76.11 ± 6.66%) was observed in the substrate S2. The addition of blood had no effect on the water content of maggots in all the substrates. The opposite trend is observed in terms of DM content, and inversely revealed that the substrate S2 has the highest DM content (23.89 ± 2.90%), meanwhile the lowest DM content is from the substrate S6 (13.56 ± 2.90%). These results showed that the addition of blood in the substrate made up of rumen content caused a decrease in DM content of maggots’ flour. The CP content in substrates is ranked as follows: S5>S2>S1>S6>S4. It can be concluded that the addition of blood in substrates (dung and rumen content) caused a decrease in CP content of maggots’ flour. Concerning fats, the highest content was obtained with maggots’ flour from the substrate S2 (43.93 ± 1.46%) and the lowest content was obtained in the substrate S6 (14.02 ± 0.51%). This means that in substrates made up of BWG and dung, the addition of blood led to an increase in lipid content unlike the level of substrate which basically constituted rumen content.
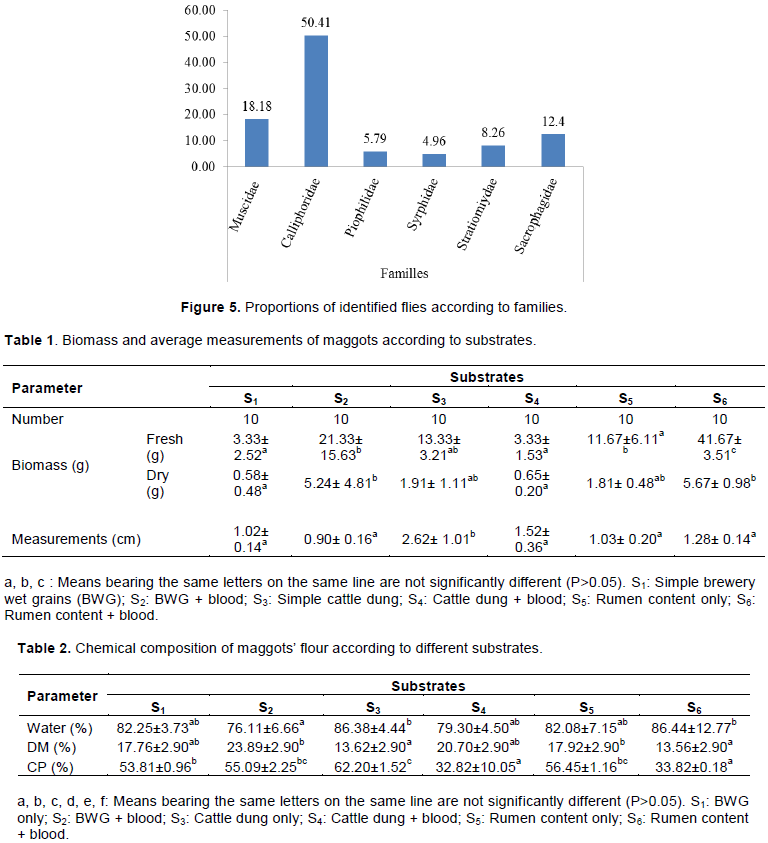
The most represented fly family was Calliphoridae with 50.41% of identified individuals. The same observation was made by Bouafou et al. (2006) during a study on fly inventory carried out on different by-products. The marked presence of this family could be justified by its large size, its social and cosmopolitan character. In fact, according to Byrd and Castner (2010), this family contains more than 1000 species and the members can be found worldwide; in addition, Calliphoridae like flying in groups and when a fly detects an excrement to lay on, it sends out pheromones to prevent the others (Claude and Daniel, 2013). Then, they arrive massively and firstly colonize the medium. Muscidae is the second most represented family (18.18%). The site chosen for this study and the great height of these species could justify that position. In fact, according to Byrd and Castner (2010), Muscidae are the flies belonging to a large synanthropic family, having a cosmopolitan distribution with more than 4000 species in the world. Both Piophilidae (5.79%) and Syrphidae (4.96%) are less represented. The height of Piophilidae as well as their uneven distribution in the world could justify this low rate. In fact, according to Byrd and Castner (2010), Piophilidae represents a small family with 69 species found in the world. As the family Syrphidae is concerned, the scarcity of flowers on the study site could justify their low abundance; in fact, the adults from this family live on flowers (Dussaix, 2009).
The addition of blood has caused an increase in fresh biomass from the substrates S2 and S6. These results are in the same line with those of Tendonkeng et al. (2017) who showed that the addition of 10% of fresh bovine blood in rumen content, in swine dung and in the mixture of both improves on the biomass of maggots produced. These authors obtained a fresh biomass of 66.10 g in the rumen content + blood versus 31.30 g in simple rumen content, 189.00 g in swine dung + blood versus 89.40 g in simple swine dung and 128.00 g in the mixture of both (rumen content + swine dung) + blood versus 58.50 g in the mixture of both (rumen content + swine dung) without blood. The fresh biomass obtained with the maggots from substrate S6 (41.67 ± 3.51 g) is higher than that of Ndadi (2010), obtained with bovine rumen content (10.05 g) and of Keyi (2014) with simple fowl droppings; but close to that obtained by this last author (47.00 ± 5.4 g) with fowl droppings + blood. This difference could be explained by the type of substrates used (Ekoue and Hadzi, 2000; Bouafou, 2007). On the other hand, the substrate S4 revealed less productive (3.33 ± 1.53 g); this low biomass obtained could be explained by the fact that, cattle dung being already attractive, addition of blood could have led to an increase in the number of flies on the substrate, hence, increasing the number of eggs laid and the number of maggots, hence limiting the nutrient content; so, many larvae die and the resistant ones do not develop well due to insufficient nutriments in the substrates. The addition of blood attracts not only flies but also improves on the nutritional value of the rumen content and the brewery wet grains, favoring the proper development of larvae.
The attractiveness of substrates and the nutrient availability could equally justify the results on measurements. It appears that the greatest length (2.62 ± 1.01 cm) was obtained with maggots from the substrate S3.
The cattle dung being attractive, the quantity of eggs laid and the number of hatched eggs, proportional to the nutrient availability in the substrate could justify the good development of larvae. The mean lowest length (0.90 ± 0.16 cm) was recorded with the substrate S2; this value is comparable to the norm indicated by Hardouin et al. (2000), Hardouin and Mahoux (2003), Bouafou et al. (2006), and Keyi (2014). According to these authors, larvae can measure averagely 1 cm (a length comprised between 0.4 and 1.5 cm according to their age in days). The greatest length (2.62 ± 1.01 cm) was recorded with the substrate S3; this value is higher than that obtained by Tendonkeng et al. (2017) (1.22 cm); this difference could be explained by the good nutritional value in cattle dung and that consequently permits the increase in height of maggots over time (Ekoue and Hadzi, 2000; Bouafou et al., 2006).
The highest humidity rate (86.44 ± 12.77%) from maggots in the substrate S6 was comparable (p > 0.05) to those from maggots in substrates S5 (82.08 ± 7.15%), S4 (79.30 ± 4.50%), S3 (86.38 ± 4.44%) and S1 (82.25 ± 3.73%) that were also comparable (p > 0.05) to the lowest rate obtained with maggots from the substrate S2 (76.11 ± 6.66%). Generally, these values were lower than that recorded by Bouafou et al. (2007) (92.51%); this difference could be explained by the quantity of water present in these substrates. In another hand, this value of 86.44 ± 12.77%, obtained with maggots from S6, was close to that obtained by Keyi (2014) who recorded a water content of 87.47 ± 4.49% with maggots from the substrate constituting swine dung + blood. The highest DM (23.89 ± 2.90%) was obtained with maggots’ flour from the substrate S2 and the lowest DM (13.56 ± 2.90%) with flour of maggots from the substrate S6. It appears from all these values that the DM content is inversely proportional to the humidity rate.
The highest CP content (62.20 ± 1.52%) was obtained with maggots’ flour from the substrate S3; it was comparable (p > 0.05) to those in the maggots’ flours from substrates S2 (55.09 ± 2.25%) and S5 (56.45 ± 1.16%); these values are superior to those found by Sogbessan et al. (2006), Bouafou et al. (2007) and Bouafou et al. (2008), respectively comprised between 47.50 and 54.00% and 52.23 and 50.17% but comparable to those recorded by Tendonkeng et al. (2017) and Keyi (2014), respectively 53.10 and 57.14%. Fats contents of maggots’ flour were significantly different (p < 0.05) in all the substrates. The lowest value (14.02 ± 0.51%) was recorded with the maggots’ flour from S6. This value is inferior to those obtained by Sogbessan et al. (2006), Bouafou et al. (2007) and Bouafou et al. (2008), respectively 19.30, 24.43, and 35.41%. As far as CP and fats contents are concerned, the difference at the level of the results could be justified not only by the nutrient content in different substrates but also by the developmental stage of the larvae. In fact, Ekoue and Hadzi (2000) and Bouafou et al. (2007) outlined that the chemical composition of maggots’ flour may depend on the developmental stage of the larvae. So, the most interesting stage is the maggot but the closest stage prior to its transformation into pupa so as to have all the nutritive elements (protides, lipids and minerals) (Hardouin et al., 2000).
At the end of this study, six families of flies namely, Calliphoridae, Muscidae, Sacrophagidae, Stratiomiydae, Piophillidae and Syrphidae were identified. The most represented family was Calliphoridae. The addition of blood has influenced the biomass, the average length and the chemical composition of maggots’ flour.
Based on these results obtained, the substrate S2 consist of brewery wet grains associated with blood which can be used to produce maggots for the non-ruminants’ diets.
The authors have not declared any conflict of interests.
REFERENCES
|
Association of Official Analytical Chemist (AOAC) (1990). Official method of analysis 15th edition. AOAC. Washington D. C. 10 p.
|
|
|
|
Bouafou KGM (2000). Putting in place Common External Tarification (C.E.T.) within U.E.M.O.A.: Influence on Ivorian aviculture. Maîtrise Dissertation 35 p.
|
|
|
|
|
Bouafou KGM, Kouame KG, Offoumou AM (2007). Nitrogen balance in growing rat fed dried maggots' flour. Tropicultura 25:70-74.
|
|
|
|
|
Bouafou KGM, Kouamé KG, Amoikon EK, Offoumou AM (2006). Potentials for maggots' production on by-products in Côte d'Ivoire. Tropicultura 24:157-161.
|
|
|
|
|
Bouafou KGM, Zannou-Tchoko V, Konan BA, Kouame KG (2008). A study of the nutritional value of dried maggots' flour on growing rats. Ivorian Review of Science and Technology 12:215-225.
|
|
|
|
|
Bradford M (1976). A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry (USA) 72:248-254.
Crossref
|
|
|
|
|
Byrd JH, Castner JL (2010). Insects of forensic importance. Forensic entomology: The utility of arthropods in legal investigations pp. 43-79.
Crossref
|
|
|
|
|
Claude W, Daniel C (2013). Treaty of forenstine entomology: Insects on the scene of crime.
|
|
|
|
|
Dronne Y (2018). Raw agricultural materials for human and animal feeding: the world. INRA 31(3):165-180.
Crossref
|
|
|
|
|
Dussaix C (2009). Syrphidae Diptera, ed. Diptera Insects in continental invertebrates in the la Loire - Greta country.
|
|
|
|
|
Ekoue SE, Hadzi YA (2000). Maggots' production as source of proteins for young poultry in Togo. Preliminary observations. Tropicultura 18:212-214.
|
|
|
|
|
Food and Agriculture Organization (FAO) (2016). Boost the livestock sector in Gabon by capacity reinforcement in cooperatives of breeders. www.fao.org, consulted on 12/09/2018.
|
|
|
|
|
FDNS (2004). Information for MSKU as of august 2004.
View
|
|
|
|
|
Hardouin J, Mahoux G (2003). Livestock and utilization to the benefit of human and certain animals. Bureau for the Exchange and the Distribution of Information on Micro-Livestock (BEDIM). Gembloux 30:83-93.
|
|
|
|
|
Hardouin J, Dongmo T, Ekoue SK, Loa C, Malekani M, Malukisa M (2000). Technical guide of livestock n°7 on maggots. Bureau for the Exchange and the Distribution of Information on Micro-Livestock (B.E.D.I.M.), ed. J. Hardouin BEDIM.
|
|
|
|
|
Keyi RP (2014). Comparative evaluation of production and nutritive value of maggots from fowl droppings and swine dung. Master of Science thesis. University of Dschang. 57p.
|
|
|
|
|
Mensah GA, Pomalegni SCB, Koudjou AL, Cakpovi JCG, Adjahoutonon KYKB, Agoundo A (2002). Maggots' flour from fly, a protein source well valorized in barbarie duck feeding. In: Report of the 1st UAC Colloquium of Science and Culture. Natural and Agronomic Sciences. Abomey-Calavi (Benin).
|
|
|
|
|
Ndadi NK (2010). Contribution to the study of adequate substrates for production of maggots as feed for poultry in Kinshasa, TFE in Zootechny, Faculty of Agronomic Sciences/Unikin 25p.
|
|
|
|
|
Sogbessan AO, Ajuonu N, Musa BO, Adewole AM (2006). Harvesting techniques and evaluation of maggot meal as animal dietary protein source for heteoclarias in outdoor concrete tanks. World Journal of Agriculture Science 4:394-402.
|
|
|
|
|
Steel JGD, Torrie JH (1980). Principles and procedures of statistics, 2nd. ed., McGraw-Hill, New York.
|
|
|
|
|
Tendonkeng F, Miégoué E, Lemoufouet J, Mouchili M, Matimuini NF, Mboko AV, Zogang BF, Mweugang NN, Zougou TG, Boukila B, Pamo TE (2017). Production and chemical composition of maggots according to the type of substrate. Livestock Research for Rural Development 29(67). Retrieved October 4, 2020, from
View
|
|