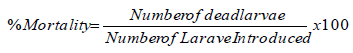ABSTRACT
Larvicidal active pure chemical compound was isolated from a Trametes species using conventional chemistry techniques like solvent-solvent extraction and liquid-solid adsorption techniques. Larvicidal assays carried out on the purified compound, LC50 and LC90 values were calculated and found to be 23.5 and 67.4 ppm, respectively. The chemical structures of the purified compound was elucidated using standard spectroscopic techniques: 1D (1H; 13C) and 2D (HSQC, COSY, NOESY, HMBC; DEPT) NMR experiments, assignments. The results indicate the potential of novel compounds with mosquito larvicidal activity from the Trametes species commonly found in the Kenyan forest ecosystems.
Key words: Mosquito larvicidal, Aedes aegypti, trihydroxylindene derivative, Trametes species.
Mosquito-borne diseases have an economic impact, including loss in commercial and labor outputs, particularly in countries with tropical and subtropical climates; however, no part of the world (both the tropics and non-tropics) is free from vector-borne diseases (Fradin and Day, 2002). One main method for the control of mosquito-borne diseases is the use of insecticides, most of which are synthetic that affect the non-target population and also the mosquitoes are constantly developing resistance. Hence, there is a constant need for developing biologically active natural materials as larvicides, which are expected to reduce the hazards to human and other organisms by minimizing the accumulation of harmful residues in the environment. Natural products are generally preferred because of their less harmful nature to non-target organisms and due to their innate biodegradability (Rahuman et al., 2008). Bioactive compounds from nature have been the most consistent successful source for new drugs (De Silva et al., 2013).
Trametes species are group polypore fungi in the basidiomycetes division that causes wood rot and produces laccase enzymes (Isikhuemhen and Mikiashvili, 2009; Zjawiony, 2004). These are in plenty in Kenyan forests particularly during the rainy seasons and areas of high altitude like Mount Kenya forest. Whereas there are many literature reports on laccase-producing fungi, Trametes species is one of the best studied white-rot
fungi, and is known to secrete several laccase isoforms (Kim et al., 2012; Montazer et al., 2009). Trametes species are known as some of the most efficient lignin- degrading species due to their ability to produce lignin-modifying enzymes (Baldrian, 2006). These enzymes enable degradation of lignin and a wide range of compounds with structural similarities to lignin, in addition to the reported resistance of Trametes species to toxic or mutagenic chemicals.
A wood rotting basidiomycete that colonized a piece of wood was collected from Mt. Kenya forest in July 2005 and immediately brought into pure culture. The fungus was serialized JO5066 and has been preserved in Integrated Biotechnology Research Laboratory (IBRL) at Egerton University as a herbarium material and pure culture. The morphological and discernable microscopic features confirmed that the fungus had clamp connections – the characteristic distinction of the basidiomycetes. The basidiomycete has been further studied using 18S RNA (ITS technique) and has been found to belong to Trametes species. This study clearly shows the extraction of the pure compound that was isolated from a Trametes species and its activity against Aedes aegeptii.
Re-growth of the Trametes species (JO5066)
From agar slants, agar pieces with mycelia were cut from the slants and inoculated onto PDA plates under sterile conditions inside the laminar flow hood. Then the Petri dishes were sealed using the Para film and left to grow under sterile ambient laboratory conditions of 12 h light/dark cycles for 21 days.
Preparation of liquid media
Malt extract 1%, yeast 0.4% and glucose 0.4% were dissolved in 250 ml of tap water to form starter cultures. Then replicates of 1 L scale in 2 L flasks with the pH of each adjusted to 5.5. They were corked with cotton wool plugs, wrapped with aluminium foil and autoclaved at 121°C and pressure 1.5 bars for 15 min. The media was sterilized twice after which let to cool.
Inoculation of the Trametes species (JO5066) strain in the liquid media
A well grown pure culture of the Trametes species (JO5066) strain on PDA plate was cut into several agar plugs using sterile inoculating blade and in each conical flask (250 ml) four agar plugs with mycelium of the Trametes species (JO5066) was introduced then allowed to grow as still cultures with regular agitations at ambient conditions. The growth of the culture was then closely monitored and evaluated daily to check the biomass build up and the presence of any contamination and stopped after 7 days. A well grown 250 ml starter culture was then used to inoculate into a 1 L scale. This was allowed to grow until the glucose level in the culture was exhausted. It was done in replicates (at least fifty flasks) to ensure a high yield of the crude extracts.
Preparation of crude extracts
After growth was stopped, the culture filtrate was then separated from mycelium by filtration using a Buchner filtration system and both extracted as follows.
Culture filtrate crude extract (Kex)
The combined culture filtrate was passed through a Mitsubishi HP21-DIAION resin packed in a glass column thrice. The column was then eluted with 100% acetone, followed by 100% methanol and the eluents collected. Each organic extracts was concentrated under reduced pressure using rotary evaporator to remove acetone and methanol, respectively. The aqueous rest concentrate was extracted with equal volume of ethyl acetate thrice and the combine ethyl acetate extract dried with anhydrous sodium sulphate. The dried organic extract was concentrated using the rotary evaporator at temperatures not exceeding 50°C. The crude extract left was re-dissolved in 1 ml of methanol and kept in screw capped vials at 4°C awaiting tests and analysis.
Mycelium crude extract (Mex)
The mycelium was soaked in 3 L acetone immediately after filtration for 4 h under constant agitation using a magnetic stirrer to ensure all the compounds are extracted. It was then filtered using Buchner filtration system and the wet mycelium was dried. The dry extract was re-dissolved in 4 L water and extracted with 10 L of ethyl acetate. The combined ethyl acetate solution obtained was then dried with anhydrous sodium sulphate and concentrated using rotary evaporator. The crude extract obtained was kept in screw capped vials at 4°C awaiting tests and further analysis.
Larvicidal assay
In order to establish LC50 and LC90 values (that is, the concentrations of extracts in parts per million required to kill 50 and 90% of Aedes aegypti larvae, within 24 h), multiple 10-fold dilutions of the extract stock solution was prepared to provide a working concentration range. Two replicate assays were carried out for every sample concentration, each with 10 larvae. Larvae were observed at the start of the assay, 2, 4, and 8 h interval and after 24 h and considered dead when they did not respond to stimulus or when they did not rise to the surface of the solution. Negative controls accompanied each assay and involved treating larvae with water and methanol. The LC50 and LC90 were calculated only for the most active extracts. The dead larvae counted after every 2 h, was used to calculate the percentage mortality reported from the average for the three replicates taken.
Cultivation and purification of the basidiomycete, Trametes (JO5066)
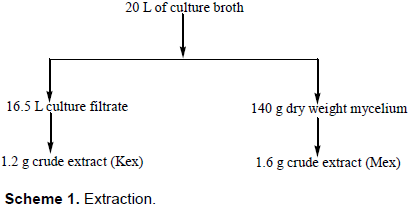
The basidiomycete JO5066 was cultivated as still cultures under laboratory ambient conditions and the growth was stopped after 21 days when the glucose levels were depleted. Immediately growth was stopped, crude extracts were prepared for both intra- and extra-cellular secondary metabolites as summarized in Scheme 1. The crude extract from culture filtrate (Kex) was 1.2 g while the mycelium crude extract (Mex) was 1.6 g.
The crude extracts were tested for larvicidal activity and Kex was found to have activity for as low as 50 ppm concentration within the range 30 to 50% mortality for the whole period of mortality. It was also observed that 100% mortality occurred for concentrations from 500 ppm except for 200 ppm after 8 h. While for Mex, activity was observed from 500 ppm in the range 30 to 70% for the whole period the experiment was evaluated. It is only at 1000 ppm that 100% mortality was observed.
Bioactivity activity guided purification of compounds from Kex
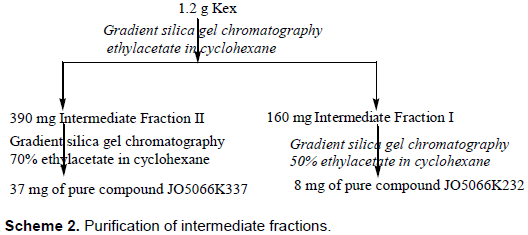
The biologically active compounds in culture filtrate (Kex) were targeted for purification using bioactivity guided purification. This was done using silica gel chromate-graphy. The crude extract was purified according to the scheme summarized in Scheme 2.
The 1.2 g yield of the crude extract (Kex) was subjected to chromatography with gradient elution with increasing polarity of ethyl acetate in cyclohexane. The active compounds eluted with gradient mobile phase (cyclohexane and ethylacetate) to afford 160 mg of an intermediate FI and 390 mg fraction labelled II respectively. These intermediate fractions were further subjected to chromatographic separation, which led to elution of 8 and 37 mg of pure compounds JO5066K232 with 50% ethyl acetate in cyclohexane and JO5066K337 with 70% ethyl acetate in cyclohexane respectively.
Mosquito larvicidal activity of the pure compounds
The purified compound, JO5066K337 (2,3-dihydro-3,3-dimethyl-1H-indene-1,2,6-triol), was tested for larvicidal activity against A. aegypti. There was no observable activity up to 8 h since the start of the experiment. From the results, the mortality data at 24 h was correlated to obtain LC50 = 235 ppm and LC90 = 674 ppm.
The pure compound JO5066K337 was obtained as a yellow liquid weighing 37 mg. The chemical structure of the compound was determined based on 1D and 2D NMR experiments. From this the structure of compound JO5066K337 was discerned and proposed to be 2, 3-dihydro-3, 3-dimethyl-1H-indene-1, 2, 6-triol. This is a new compound being reported for the first time from cultures of a Trametes species. From 13C-NMR spectrum there were 11 discernable carbon signals, which when analyzed together with 1H-NMR and DEPT spectra indicated that there were 4 quaternary carbons, 5 methine carbons and 2 methyl carbons. It was evident that the compound had no methylene carbons. The information was found to be consistent with the molecular formula, C11H14O3, with unsaturation index (UI) of 5.
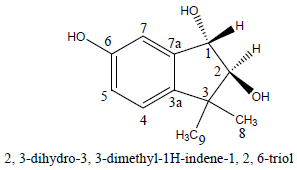
From the 11 signals there were 6 typical aromatic sp2 hybridized carbons; 111.8, 124.3, 129.3, 132.8, 135.7 and 168.3ppm, with the last one (C-6) being oxygenated typified by the chemical shift in the deshielded range and consistent with a phenol moiety. From these carbons C-3a (124.3 ppm) and C-7a (132.8ppm) are quaternary and C-4 (135.7 ppm), C-5 (129.3 ppm) and C-7 (111.8 ppm) are bearing a proton each.
The remaining 5 carbons were accounted for by 2 methyl carbons, 11.4 ppm (C-8) and 16.8 ppm (C-9), with both attached to a quaternary carbon 22.1 ppm (C-3). This leaves 2 carbons that are accounted for as oxygenated sp3 hybridized that are part of a rigid ring system, C-1 (83.3 ppm) and C-2 (78.9 ppm). Carbons C-1, C-2 and C-3 are part of five-membered ring that is fused to the aromatic system as deciphered from the 2-D NMR experiments COSY, NOESY and HMBC. The two protons (4.72 and 3.72 ppm) attached to C-1 and C-2, respectively are in a trans- orientation supported by a 3J-value of 2.1 Hz characteristic of such a stereochemistry common in sugars (Friebolin, 2005). It is on this basis that the relative configuration on C-1 and C-2 were proposed.
The research findings in this work revealed that the basidiomycete, Trametes sp. (JO5066) produced biologically active compounds when grown in liquid submerged cultures for 21 days. The crude extract from culture filtrate showed both larvicidal activity against A. aegyptii and antibacterial and antifungal activities. The crude extract showed 100% mortality of the mosquito larvae for a concentration of 50 ppm within 24 h. These observations clearly demonstrated that the Trametes sp (JO5066) was producing biologically active compounds.
The crude extracts when fractionated and purified for the active compounds responsible for the observed biological activities afforded the compound 2, 3-dihydro-3, 3-dimethyl-1H-indene-1, 2, 6-triol whose structure was elucidated based on NMR experiments. 2, 3-Dihydro-3, 3-dimethyl-1H-indene-1, 2, 6-triol showed clear larvicidal activity while it had weak and insignificant antimicrobial activities. The compound had a considerable activity with LC50 of 23.5 ppm and LC90 of 67.4 ppm against the third instar larvae of A. aegyptii. This is a major finding for this study given that the compound is a new compound reported for the first time from cultures of a fungal source.
The authors confirm that there is no conflict of interests. The funding agencies had no role in the review framework, concepts, interpretation of literature and the final conclusions. The conceptualization, design of the research, the execution of the research methods, collection, analysis and interpretation of data leading to the derived conclusions is wholly our responsibility as a research team.
REFERENCES
|
Baldrian P (2006). Fungal laccases-occurrence and properties. Microbiology 30:215-242.
Crossref
|
|
|
|
De Silva DD, Rapior S, Sudarman E, Stadler M, Xu J, Alias SA (2013). Bioactive metabolites from macrofungi: Ethnopharmacology, biological activities and chemistry. Fung. Divers. 62:1-40.
Crossref
|
|
|
|
|
Fradin MS, Day JF (2002). Comparative efficacy of insect repellents against mosquitoes bites. New Engl. J. Med. 347:13-18.
Crossref
|
|
|
|
|
Friebolin H (2005). Basic one-and two-dimensional NMR spectroscopy. Wiley-vch Verlag GmbH & Co.
|
|
|
|
|
Isikhuemhen OS, Mikiashvili NA (2009). Lignocellulolytic enzyme activity, substrate utilization, and mushroom yield by Pleurotus ostreatus cultivated on substrate containing anaerobic digester solids. J. Ind. Microbiol. Biotechnol. 36:1353-1362.
Crossref
|
|
|
|
|
Kim H, Lee S, Ryu S, Choi HT (2012). Decolorization of remazol brilliant blue R by a purified laccase of polyporus brumalis. Appl. Biochem. Biotechnol. 166:159-164.
Crossref
|
|
|
|
|
Montazer M, Dadashian F, Hemmatinejad N, Farhoudi K (2009). Treatment of wool with laccase and dyeing with madder. Appl. Biochem. Biotechnol. 58:685-693.
Crossref
|
|
|
|
|
Rahuman AA, Venkatesan P, Geetha K, Gopalakrishnan G, Bagavan A, Kamaraj C (2008). Mosquito larvicidal activity of gluanol acetate, a tetracyclic triterpenes derived from Ficus racemosa Linn. Parasitol. Res. 103:333-339.
Crossref
|
|
|
|
|
Zjawiony JK (2004). Biologically Active Compounds from Aphyllophorales (Polypore) Fungi. J. Nat. Prod. 67:300-310.
Crossref
|
|