ABSTRACT
Nanotechnology has rapidly developed as an important field of modern research, generating most promising applications in electronics and medicine. It involves different approaches for the synthesis and application of nanoparticles having dimension smaller than 100 nm. Nanoparticles are the fundamental building blocks for preparing many nanostructured materials and devices. Therefore, there is an enormous interest in developing safe, cost effective and ecofriendly techniques for synthesis of nanoparticles. Biological synthesis has emerged as an attractive alternative to overcome the side effects accompanied with physical and chemical methods of synthesis. This led to the development of new branch of nanotechnology called “green nanotechnology” or “nanobiotechnology” which combines biological principles with physical and chemical procedures to generate eco-friendly nano-sized particles with specific functions. Various biological entities could be employed for the biosynthesis of nanoparticles including plant, algae, fungi, yeast, bacteria and viruses. Recently, much attention has been given on exploring fungi as potent biofactories for synthesis of silver nanoparticles since they possess numerous bioactive properties which find variety of applications in the field of biomedicine. Its use in the form of effective antimicrobial agent and disinfectant is known since time immemorial. The present chapter emphasize on the richness of endophytic fungal diversity and their role in biosynthesis of silver nanoparticles. It gives detailed overview about the mechanism of synthesis, characterization techniques involved in analysis of silver nanoparticles and biomedical application of silver nanoparticles with special reference to their antimicrobial potential.
Key words: Nanobiotechnology, silver nanoparticles, endophytic fungi, antimicrobial potential, eco-friendly.
Nanotechnology has rapidly evolved as an important field of science as a result of its increasing applications in electronics and medicine (Boisselier and Astruc, 2009). Specially, nanotechnology has limitless potential in biomedical applications, since our own biological system is basically a complex of nano-machines. Nanotechnology is the branch of research involving novel strategies for the design, synthesis, manipulation and application of particles with dimension smaller than 100 nm. These particles are known as “nanoparticles”. Nanoparticles are considered as essential building blocks for nano-technology. They are the starting points for preparing many nano-structured materials and devices. Because of their extremely small size and high surface area to volume ratio, nanoparticles possess certain extraordinary physicochemical properties. These properties makes them applicable in different sectors viz., electronics and photonics, catalysis, information storage, chemical sensing and imaging, environmental remediation, drug delivery and biological labelling, thus enhancing their commercial value (Prabhu and Poulose, 2012).
In the last decade, application of nanoparticles has extensively increased leading to high demands for their synthesis. Nanoparticles of a wide range of materials can be prepared by a number of methods which are categorized mainly in three groups: chemical, physical and biological methods. Out of these biological methods are the most promising ones for synthesis of nanoparticles, since physical and chemical methods usually involves the use of toxic radiations and chemicals which has hazardous effects. This has led to the emergence of a new field called “Nanobiotechnology” as integration between biotechnology and nanotechnology for developing biosynthetic and environment friendly technology for synthesis of nanoparticles (Kalishwaralal et al., 2008). It is commercially viable, clean and nontoxic approach which involves the use of biological agent’s viz. bacteria, fungi and plants for the synthesis of metal nanoparticles. The use of fungi, particularly endophytic fungi in the synthesis of nanoparticles is potentially exciting since they secrete large amounts of enzymes and are simpler to deal with in the laboratory (Singh et al., 2013). Endophytic fungi are endosymbiont that live within a plant for least part of their life without causing apparent harm and are relatively unexplored potential source of biosynthesized silver nanoparticles (Petrini, 1991). These nanoparticles have recently emerged up as novel antimicrobial agents to overcome the outbreak of the infectious diseases caused by different antibiotic resistant pathogenic microorganisms (Kim et al., 2007). Moreover silver is known to be a health additive in traditional ayurvedic medicine. Biosynthesized silver nanoparticles thus present an important source of various therapeutic agents particularly antimicrobial agents. The present book chapter gives a detailed description about the mechanism of biosynthesis of silver nanoparticles by using endophytic fungi.
NANOPARTICLES: “AS BUILDING BLOCKS OF NANOTECHNOLOGYâ€
Nanoparticles are materials at nanoscale levels that is they have dimensions between 1 and 100 nm. Recently, they have attracted great scientific interest of researchers as they bridge the gap between bulk materials and atomic or molecular structures. Moreover they are the basic components of many nanoengineered products having varied applications (Saifuddin et al., 2009). At present, different types of metal nanoparticles are being produced using copper, zinc, titanium, magnesium, gold, alginate and silver. These particles exhibit unique properties at nanoscale of 1 to 100 nm. The changes in properties are due to their very small sizes and large surface area to volume ratio which leads to the dominance of quantum effects. Several examples which depict the difference in properties of metals at macroscale and nanoscale are as described in Table 1.
Some of their extraordinary properties which make them useful in various fields are as follows:
1. High tensile strength
2. Better thermal and electrical conductivity
3. Larger surface area to volume ratio
4. High reactivity
Metallic nanoparticles, including gold, silver, iron, zinc and metal oxide nanoparticles, have shown great potential in terms of biomedical applications (Bhattacharya and Mukherjee, 2008; Hirst et al., 2009). This is so because these particles not only possess large surface area to volume ratio, but also exhibit different biomedical activities. Several experiments have demonstrated bioactive properties of metal nanoparticles viz., application of gold and cerium oxide nanoparticles for the treatment of tumours and anti-inflammation respectively (Muangman et al., 2009). In the present scenario silver nanoparticles has gained more importance as compared to any other metallic nanoparticle since it finds significant use in the field of medicine as therapeutic agents. It possesses extraordinary antimicrobial and anti-inflammatory properties which are useful for the treatment of various diseases.
As the nano-revolution unfolds synthesis of safe, reliable and ecofriendly nanoparticles has become an important component of the rapidly growing research efforts in nanoscience and nanoengineering (Verma et al., 2010). The nanoparticles of a wide range of materials can be prepared by a number of methods which are categorized mainly in three groups: Chemical, physical and biological methods. These methods follow either of the two approaches for synthesis, that is, top-down or bottom-up approach. In top down approach the bulk materials are mechanically grinded and the resulting nanosized particles are stabilized by addition of colloidal stabilizing agents. On the other hand bottom-up approach includes the reduction of bulk metals by electrochemical methods (Amulyavichus et al., 1998).
The physicochemical methods involved in production of metal-based nanoparticles mainly include chemical reduction, thermal treatment, irradiation and laser ablation (Peterson et al., 2007; Tsuji et al., 2002; Sun and Luo, 2005; Shao and Yao, 2006). But these methods are usually associated with certain disadvantages such as requirement of expensive equipments and the use of toxic reducing agents like sodium borohydride and N,N dimethylformamide which produce hazardous effects on environment and health. Due to the drawbacks of physicochemical methods and growing usability of nanoparticles in biological systems especially as drug delivery vehicles into the cellular world, the need to prepare metal nanoparticles using nature friendly biological methods is on the rise.
Biosynthetic approach involves the use of diverse biological species including plant and plant products, algae, fungi, yeast, bacteria and viruses for synthesis of nanoparticles (Sunkar and Nachiyar, 2012). Various reports have earlier advocated the production of intra-cellular as well as extracellular biosynthesis of nanoparticles by unicellular and multicellular organisms. This led to the development of new branch of nanotechnology called “green nanotechnology” or “nanobiotechnology” which combines biological principles with physical and chemical procedures to generate eco-friendly nano-sized particles with specific functions.
BIOSYNTHESIS OF SILVER NANOPARTICLES
Recently biosynthesis of silver nanoparticles has received increasing attention of the researchers to overcome the drawbacks of physical and chemical methods of synthesis. Besides this the growing need of silver nanoparticles in the biomedical field has led to the search of biosynthetic methods for the generation of ecofriendly silver naanoparticles. It has emerged as an intersection between nanotechnology and biotechnology. First evidence of biosynthesis was reported using Pseudomonas stulzeri (Klaus et al., 1999) where the nanoparticles were deposited on the cell membrane. This study was followed by various other reports that demonstrated the use of different microorganisms including Bacillus licheniformis (Kalimuthu et al., 2008), Lactobacillus strains (Nair and Pradeep, 2002), Bacillus subtilis (Saifuddin et al., 2009), Fusarium oxysporium (Ahmad, 2003) and Aspergillus fumigatus (Bhainsa and D’Souza, 2006). The biological systems most commonly used for this purpose are herbal extracts, microalgae, fungi and bacteria. Biosynthetic methods come under the bottom-up approach wherein the silver nanoparticles are produced by reduction via, enzymes and other metabolites secreted by the biological agents. The experimental procedure for the synthesis of silver nanoparticles is usually the same with just slight differences depending upon the type of fungi being used (Figure 1).
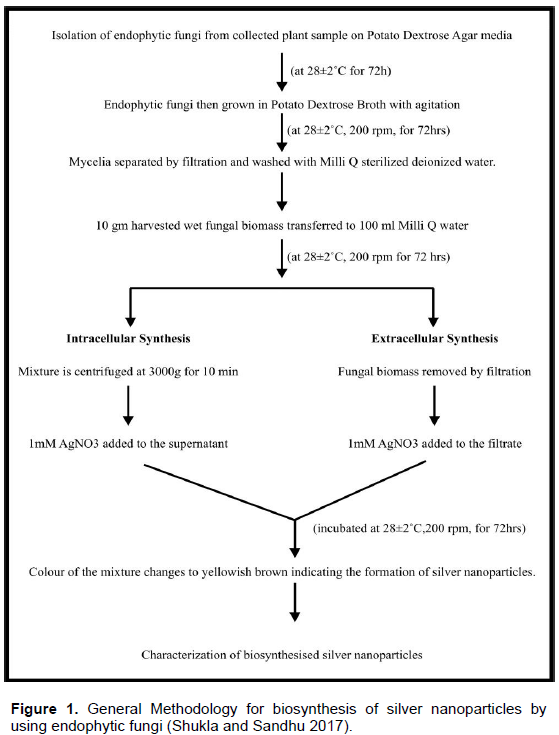
ENDOHYTIC FUNGI AS SOURCE OF SILVER NANOPARTICLES
The increasing problem of antibiotic resistant microbes has resulted in an urgent need for development of novel antimicrobial agents. The use of silver nanoparticles has emerged as most promising approach for overcoming antibiotic resistance of microorganisms. It has been known as effective antimicrobial agent since ancient times (Klasen, 2000). Silver is advantageous over other metals since it exhibits higher toxicity to broad spectrum of microorganisms and lower toxicity to mammalian cells. Silver ions are also known to be effective against a broad range of antibiotic-resistant microorganisms (Sharma et al., 2013). Their antimicrobial activity is due to higher surface to volume ratio compared to common metallic silver.
Silver nanoparticles show inhibitory effect over microorganisms by various mechanisms. They enter inside the microbial cells and disturb functions of cell membranes such as permeability and respiration. Besides this they inhibit enzyme functioning of the microbial cells by interacting with sulphur containing proteins and phosphorus-containing compounds such as DNA. Thus, silver nanoparticles interrupt the respiratory chain and cell division leading to cell death (Sondi and Sondi, 2004; Song et al., 2006). Such complex action mechanisms of silver decrease the probability of development of microbial resistance against them.
A number of reports are available on the biological synthesis of silver nanoparticles, but the potential of endophytic fungi in this respect has not yet been explored. These fungi lives inside the internal tissues of plants and do not cause any side effects (Wilson, 2000). Several researchers have reported endophytic fungal flora as a source of various bioactive compounds with potential activities but very few of them have used endophytic fungi for biosynthesis of nanoparticles. In one such study an endophytic fungus Pestaloptiopsis pauciseta isolated from the leaves of Psidium guajava Linn. was used for the extracellular synthesis of silver nanoparticles (Vardhana and Kathiravan, 2015).
Similarly, biosynthesis of silver nanoparticles was carried out by using endophytic fungi Aspergillus conicus, Penicillium janthinellum and Phomosis sp. isolated from Avicennia marina, Suaeda monica and Rhizophora mucronata plant leaf. Thereafter, the antibacterial efficacy of these silver nanoparticles was also examined (Bharthidasan et al., 2012). Fungi possess some advantages over bacteria in nanoparticles synthesis, as most of the fungi are easy to handle, require simple nutrients, possess high wall-binding capacity, as well as intracellular metal uptake capabilities (Dias et al., 2002; Sanghi and Verma, 2009). Compared to bacterial broth, fungal broth can be easily filtered by filter press thus saving considerable investment costs for specialized equipment which may be needed for other methods. As a result, for large-scale production of nanoparticles fungi is preferred over other biological systems. Moreover the use of fungi is potentially exciting since they secrete large amounts of enzymes and are simpler to deal with in the laboratory. The initial confirmation for the synthesis of silver nanoparticles is done by observing a colour change in the reaction mixture from pale yellow to brown. Rahi and Parmar (2014) confirmed the presence of silver nanoparticles when 10 g of Penicillium species biomass was exposed to 1 mM silver nitrate solution. The colour of the reaction mixture changed from transparent to brown after incubation at 28±2°C for 3 days under shaking condition. The fungus used for the experiment was isolated from Aloe roots. Similarly, three endophytic fungi namely, Aspergillus tamarii PFL2, Aspergillus niger PFR6 and Penicllium ochrochloron PFR8 isolated from an ethno-medicinal plant Potentilla fulgens L. were used for the biosynthesis of silver nanoparticles (Devi and Joshi, 2015).
Figure 2 clearly depicts that colour of the reaction mixture containing mycelia free water extract and 1 mM silver nitrate turns to yellowish brown due to the reduction of silver nitrate by certain reducing agents released in the water by the respective fungus. On the other hand there is no change in the colour of the control flask containing mixture of sterile distil water and 1 mM silver nitrate indicating no reduction of silver nitrate.

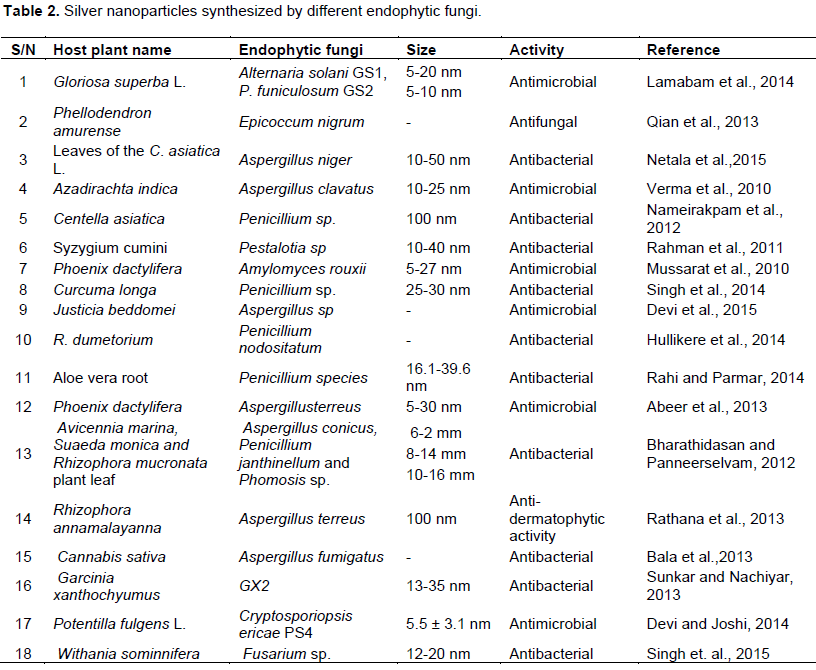
Thus, several experimental results (Table 2) suggest that endophytic fungi are potential bio factories for the synthesis of antimicrobial silver nanoparticles.
Diversity of endophytic fungi and their functional roles
Endophytic fungi are those microorganisms that resides inside plants especially leaves, stems, roots without causing apparent harm to host (Azevedo et al., 2000). They are found to inhabit almost all classes of vascular plants and grasses examined to date (Zhang et al., 2006). Besides fungi, different groups of organisms such as bacteria, actinomycetes and mycoplasma are reported as endophytes of plants (Bandara et al., 2006). Their existence has been known for over one hundred years. The word endophyte is derived from Greek word ‘endon’ meaning within, and ‘phyton’ meaning plant. The research on endophytic fungi and their huge diversity among plants has a long history. Recently it has been reported that each plant harbour one or more endophytic fungi (Verma et al., 2007; Kharwar et al., 2008). These endophytic fungi are an outstanding source of secondary metabolites which possess variety of bioactive potential. They have received considerable attention in last 20 years for their capacity to protect against deadly pathogens. Besides this much interest has been taken by Botanists to carry out research into the plant endophyte relation.
Host endophyte relationship
It is clear from some recent studies that endophytes are not host specific. One endophyte can invade number of host plants. Endophytic fungi isolated from different plants grow under different conditions and belongs to different families belongs to different families (Petrini, 1986). It has also been suggested through some research works that different strains of the same fungus isolated from different parts of the same host differ in their ability to utilize different substances. Therefore the host endophyte relationship is variable for different hosts and endophyte.
Fungal endophyte diversity
Most of the plants in natural ecosystems are symbiotic with fungal endophytes (Petrini, 1986). These fungal endophytes have intense effects on plant ecology, fitness, and evolution. They also have strong effects on the community structure and diversity of associated organisms for example bacteria, nematodes and insects. Fossil records indicates that endophytic fungi plants have been associated with plants (Krings et al., 2007) since hundreds of years, thus playing a long and important role in driving the evolution of life on land. Endophytic fungi grow within roots, stems and/or leaves of the host plant and sporulate at host-tissue senescence (Stone et al., 2004).
Two major groups of endophytic fungi have been recognized viz., the clavicipitaceous endophytes (C-endophytes) and the nonclavicipitaceous endophytes (NC-endophytes). C-endophytes infects some grasses and can be recovered from the same, while the NC-endophytes can be recovered from asymptomatic tissues of nonvascular plants, ferns, allies, conifers, and angiosperms.
C-endophytes also known as Class 1 endophytes are fastidious in culture and limited to some cool- and warm-season grasses. They represent a small number of phylogenetically related clavicipitaceous species (Bischoff and White, 2005). Usually these endophytes form systemic intercellular infections within plant shoots. Three types of clavicipitaceous endophytes have been recognized viz., symptomatic and pathogenic species (Type I), mixed interaction (Type II) and asymptomatic endophytes (Type III).
Class I endophytes are transmitted vertically, when maternal plants pass them on to offspring via seed infections. The potential of these fungi varies depending upon the host species, host genotype and environmental conditions (Faeth et al., 2006).
Class 2 endophytes have quite limited diversity in individual host plants. Most of their species are members of the Dikarya (Ascomycota or Basidiomycota). They confer habitat specific stress tolerance to host plants (Rodriguez et al., 2008). According to a proposed hypothesis, Clavicipitaceous endophytes are defensive mutualists of host grasses and this hypothesis is widely accepted and followed by number of researchers (Koulman et al., 2007).
Class 3 endophytes on the other hand are transmitted horizontally and distinguished on the basis of their occurrence. They usually occur in vascular, nonvascular plants, woody and herbaceous angiosperms in tropical forest and antarctic communities (Davis and Shaw, 2008). They are present in great diversity within individual host plant. Individual leaves may harbor number of species at the rate of one isolate per 2 mm of leaf tissue. Therefore hundreds of different endophytic fungi are associated with single host plant.
Class 4 endophytes are mainly found in plant roots and have darkly melanized septa. They form structures like inter and intracellular hyphae and microsclerotia in the roots and are generally Ascomycetous fungi. They harbour usually nonmycorrhizal host plants particularly belonging to antarctic, arctic, alpine, sub-alpine, temperate zones and tropical ecosystems.
Isolation of endophytic fungi
Various methods are available in literature for isolation of endophytic fungi from their host plant. Most commonly used method involves dipping of tissues in 70% alcohol for few seconds or in 0.5 to 3.5% sodium hypochlorite for 1 to 2 min followed by rinsing in sterile double distilled water. These tissue pieces are then plated on a nutrient medium for isolation of endophytic fungi (Maheshwari, 2006) in another method surface sterilization of plant tissues is performed by dipping sequentially in 70% ethanol (1-3 min), 4% sodium hypochloride (3-5 min), 70% ethanol (2-10 s) and finally rinsing with double distilled water (Verma et al., 2007). The tissue pieces are air dried inside the laminar air flow and then place on potato dextrose agar media plates containing 50 mg/l chloramphenicol to suppress bacterial growth (Kharwar et al., 2008). The tissues are cut into pieces of specific diameter with the help of sterile knife blade (Strobel and Daisy, 2003). Plates containing tissue pieces are kept in incubator at temperature of about 25 to 30ºC for fungal growth. Suitable media for isolation of endophytic fungi are water agar, potato dextrose agar, yeast extract agar, Rose bengal chloramphenicol agar, Luria bertani agar, humic acid vitamin agar.
Bioactive compounds from endophytic fungi
Endophytic fungi have been the source of several important compounds possessing potential bioactivities. In 1993, a novel anticancer compound taxol was produced from fungus Taxomyces andreanae, isolated from the yew Taxus brevifolia (Huang et al., 2008). In the same way anti-inflammatory compounds pestacin and isopestacin were isolated from Pestalotiopsis microspora of the plant Terminalia morobensis (Strobel et al., 2002). An antimicrobial compound clavatol was obtained from endophytic fungi Aspergillus clavatonanicus isolated from Torreya mairei (Verma et al., 2009). Two novel compounds cytonic acid A and B have been isolated from the endophytic fungus Cytonaema sp. These compounds show antiviral property by inhibiting human cytomegalovirus (hCMV) and protease (Guo et al., 2000).
Many more such compounds from endophytic fungi have been isolated, purified and characterized by various researchers.
THE MECHANISM OF SILVER NANOPARTICLE PRODUCTION USING ENDOPHYTIC FUNGI
Synthesis of nanoparticles using biological entities has attracted much attention in the last decade because of different unusual properties possessed by nanoparticles viz., optical, chemical, photoelectrochemical and electronic properties (Krolikowska et al., 2003; Kumar et al., 2003; Chandrasekharan and Kamat, 2000). Although the exact mechanism of nanoparticle biosynthesis is not yet clearly defined but several hypothesis have been proposed by many research scientists. According to Mehra and Winge (1991) certain fungi have the ability of producing extracellular metabolites and enzymes when exposed to such environmental stresses like toxic materials (such as metallic ions), predators and temperature variations. These secreted materials serve as agents for the survival of the respective fungus and reduces silver ions present in its vicinity to nanosized particles. Therefore, for the biosynthesis of metal nanoparticles by a fungus, the fungus mycelium is exposed to the metal salt solution. This solution creates osmotic stress conditions for the fungus and prompts it to produce enzymes and metabolites for its own survival. These extracellular enzyme and metabolites of the fungus then catalyzes the reduction of toxic metal ions to the non-toxic metallic solid nanoparticles. Thus, biosynthesis of nanoparticles by fungi is a two-step mechanism which is as follows (Figure 3):

Step I: Involves trapping of metal ions in close vicinity of the fungal cells;
Step II: Enzymes secreted by the cell reduce silver ions.
Besides these extracellular enzymes, several naphthoquinones and anthraquinones with excellent redox properties have been reported in some fungi that could act as electron shuttle in metal reductions. The size of nanoparticles and their rate of synthesis can be controlled by manipulating parameters such as pH, temperature, substrate concentration and exposure time to substrate (Gericke and Pinches, 2006).
CHARACTERIZATION TECHNIQUES INVOLVED IN THE ANALYSIS OF BIOSYNTHESISED SILVER NANOPARTICLES
After preliminary confirmation of the formation of silver nanoparticles by observing colour change of reaction mixture from pale white to yellowish brown, several characterization techniques are applied to further confirm the presence of silver nanoparticles in the solution (Sunkar and Nachiyar, 2012). It is the most important part of biosynthesis of silver nanoparticles because it not only reveals information about the size and shape of silver nanoparticles formed, but also describes about the presence of any biomolecules associated with these particles. Some of the most commonly used techniques for characterization of nanoparticles are as follows:
UV-visible spectroscopic analysis
This technique confirms the presence of silver nanoparticles by measuring the absorbance of bioreduced solution at wavelengths between 200 and 800 nm. It is a well known fact that exposure of silver nanoparticles (brown solution containing reduced silver nitrate in water extracts of fungi) to light leads to electron dipolar oscillation and appearance of a strong absorption peak at the wavelength range from 390 and 420 nm whereas no absorption peak is observed at this wavelength range in the control (pale white solution containing silver nitrate in deionized Milli-Q water). This confirms the reduction of silver nitrate in water extracts of endophytic fungi (Kleemann, 1993). Many researchers have used this technique for the analysis of biosynthesised silver nanoparticles.
Biosynthesis of silver nanoparticles using A. conicus, Penicillium janthinellum and Phomosis was monitored in the UV-Vis spectrophotometer by Bhartathidasan et al. (2012). The UV-Vis spectra recorded after 24, 48 and 72 and 96 h incubation showed strong peak at 420 nm indicating the presence of silver nanoparticles.
The reaction mixture containing fungal cell filtrate of endophytic fungus Pestalotia sp. after treatment with aqueous silver ions when subjected to optical analysis using UV-Vis spectrophotometer showed a sharp peak at 415 nm which lies in the characteristic wavelength range (390 to 420 nm) for silver nanoparticle (Rehman et al., 2011).
Verma et al. (2010) also reported the formation of silver nanoparticles by A. clavatus isolated from the stem of Azaidrecta indica. This was confirmed with the UV spectroscopic study of the colloidal silver nanoparticle solution, which showed strong absorption peak at 415 nm in comparison with the aqueous silver nitrate (AgNO3) solution.
Similarly, the mycelia free filtrates of three endophytic fungal isolates namely, Aspergillus tamarii PFL2, Aspergillus niger PFR6 and Penicllium ochrochloron PFR8 were analysed via, UV-Visible spectrophometer. These samples gave strong absorption peaks at 419, 430 and 430 nm respectively, thus indicating the presence of reduced silver nanoparticles in them (Devi et al., 2015).
Electron microscopic analysis
The size and shape of nanoparticles are important factors which are responsible for their function. These factors vary with the type of microorganism used, temperature and pH of the medium etc. Therefore, considerable varieties of nanoparticles are produced by different fungal species. Electron microscopy plays an important role in measuring the size and determining the shape of nanoparticles. It is done by applying scanning electron microscopy or transmission electron microscopy. Several reports have been generated regarding the size of nanoparticles by microscopic techniques. Scanning electron microscope analysis was used to measure the size of silver nanoparticles synthesized by fungi A. conicus, P. Janthinellum and Phomosis. In this analysis the size of nanoparticles was found to be 80 and 120 μm (Bharathidasan and Panneerselvam, 2012). In the same way oval shape of the silver nanoparticles synthesized by endophytic fungus Penicillium sp. was confirmed by scanning electron microscopy (Devi et al., 2012).
According to Musarrat et al. (2010) the Transmission Electron Microscopic image of silver nanoparticles synthesised by endophytic fungus Amylomyces rouxii strain KSU-09 showed variable, but predominantly spherical nanoparticles. The average size determined was 20 and 14 nm. Similarly, TEM analysis finally confirmed the synthesis of spherical and polydispersive silver nanoparticles in the reaction mixture containing the endophytic fungus Pestalotia sp. The particles were in the range of 10 to 40 nm with average diameter of 12.40 nm (Raheman et al., 2011). Likewise the average particle size of silver nanoparticles synthesised by endophytic fungi A. tamarii PFL2, A. niger PFR6 and P. ochrochloron PFR8 was determined to be 3.5 ± 3.3, 8.7 ± 6 and 7.7 ± 4.3 nm, respectively by using Transmission Electron Microscope (Devi et al., 2015).
Recently, silver nanoparticles synthesized by endophytic fungus A. tenuissima PGL#71 were analyzed via, transmission electron microscope. These particles were found to be spherical and polydispersed ranging from 2 to 20 nm in size (Shukla and Sandhu, 2017).
X-Ray diffraction analysis
Silver nanoparticles coalesce to form large aggregates after long time of incubation period (over 70 h). These aggregates possess well defined morphologies and are responsible for the crystalline nature of silver nanoparticle which is further confirmed by X-Ray Diffraction analysis (XRD). The crystalline nature of the silver nanoparticles formed by endophytic fungi A. clavatus was observed by XRD analysis (Verma et al., 2010). Peaks were obtained at 38.06Ëš (111) and 44.1392Ëš (200) corresponding to the diffraction facets of silver and indicating that the precipitate is composed of pure crystalline silver.
Similarly, Devi et al. (2012) performed the XRD analysis of silver nanoparticles synthesised by the endophytic fungus Penicillium sp. The XRD patterns revealed that silver nanoparticles were not well crystallized and the crystalline peak corresponds to cubic shapes of particles with an average grain size of 32 nm.
FTIR analysis
This technique gives detailed information about the molecules associated with the silver nanoparticles which are responsible for their formation and stability. It reveals the molecular structures and chemical bonds of these molecules which are probably secreted by the fungus involved in the biosynthesis of nanoparticles.
FTIR spectrum revealed that the silver nanoparticles synthesized from endophytic fungus Pestalotia sp. showed the presence of peak at 1651.4, 1542, 1387 and 1057 cm-1. The bands at 1651.4 and 1542.4 corresponds to the bonding vibrations of the amide I and amide II bands of proteins while the bands obtained at 1387 and 1057 cm-1 are due to presence of C-N stretching vibrations of aromatic and aliphatic amines (Raheman et al., 2011). These results resembles with the findings of Gole et al. (2001) who reported that proteins can bind to nanoparticles either through free amine groups or cystein residues or through the electrostatic attraction of negatively charged carboxylate groups in enzymes present in cell-wall of mycelia. FTIR spectroscopy has confirmed that amino acid residues and peptides of proteins has the stronger ability to bind with metal, so that the proteins could most possibly form a coat covering the metal nanoparticles, that is, capping of silver nanoparticles to prevent agglomeration of the particles and stabilizing in the medium (Basavaraja et al., 2008).
SILVER NANOPARTICLES: “IN CLINICAL MEDICINEâ€
Over the past decade, silver has been used for the treatment of a variety of diseases viz., epilepsy, venereal infections, acnes and leg ulcers. Besides this the use of silver foil for improved healing of surgical wounds and reduced post-operative infections has been practised since ancient times. While pencils containing silver nitrate mitigated with potassium nitrate were used for wart removal and ulcer debridement (Klasen, 2000). Though these approaches were useful but they have been shown to be very impractical to use on large wounds or for extended time periods due to instability. These drawbacks were overcome with the advancement of nanotechnology. It has enabled the application of pure silver in the form of silver nanoparticles in medical practice. The efficacy of silver nanoparticles depends upon their size, shape and composition. Therefore extensive research is going on synthesizing and characterizing silver nanoparticles. The application of nanosilver can be broadly divided into diagnostic and therapeutic uses.
Nanosilver in diagnosis and imaging
Early diagnosis of any disease plays an important role in the early treatment of the same. Surface-enhanced Raman spectroscopy (SERS) has emerged as a powerful analytical tool for the diagnosis of respiratory infections. It utilizes metallic nanostructures particularly silver nanorods to enhance the vibrational spectra of molecules adsorbed to the surface that extends the possibilities of vibrational spectroscopy. SERS therefore differs from standard Raman scattering. Recently four strains of respiratory syncytial virus (RSV) were readily detected at very low detection limits by using SERS composed of silver nanorods (Shanmukh et al., 2008).
In another study, Au-Ag nanorods were used in detection of cancer. It behaves as a nanoplatform for multivalent binding by multiple aptamers, and helps in cancer cell recognition. The molecular assembly of aptamers on the nanorods was shown to lead to a 26-fold higher affinity than the original aptamer probes (Huang et al., 2008). Thus, these nanorods exhibit most promising results in terms of for cell studies, disease diagnosis, and therapy.
Nanoparticles in therapeutics
Anti-inflammatory properties of silver nanoparticles
Along with excellent anti-bacterial properties, the silver nanoparticles also have anti-inflammatory properties. Several reports on burn cases reveals that significantly lower levels of the pro-inflammatory cytokine IL-6 are found in animals treated with silver nanparticles using quantitative real-time RT-PCR. On the other hand mRNA levels of IL-10, an anti-inflammatory cytokine, remains higher in the silver nanoparticles treated cells (Wong et al., 2009). Likewise findings of Nadworny et al. (2008) and Bhol and Schechter (2007) clearly confirms that silver nanoparticles had direct anti-inflammatory effects and improved the healing process significantly when compared with controls.
Wound dressing
The use of silver in wound dressings (Fong and Wood, 2006) has been documented in several researches. Acticoat®, made up of two layers of polyamide ester membranes covered with nanocrystalline silver ions, is the first commercial dressing. Various in vitro studies reveals that Acticoat® has lowest MIC and MBC values, and possesses the fastest Kill kinetics against the five bacteria. Further, the sustained release of silver particles minimizes the likelihood of bacteria developing resistance to silver. In a clinical trial including 30 burnt it was found that the frequency of burn wound sepsis, as well as secondary bacteraemia was lower in patients with treated with silver nanoparticles (Tredget et al., 1998). According to Sibbald et al. (2007) the use of silver nanoparticles dressing on a variety of chronic non-healing wounds has a beneficial effect of protecting the wound site from bacterial contamination. Similarly, Liu et al. (2010) proved that silver nanoparticles could promote wound healing through facilitating the proliferation and migration of keratinocyte.
Silver-impregnated catheters
Central venous catheters: Central venous catheters (CVC) are widely used in infective complications such as bloodstream infections at around 80000 cases annually (Mermel, 2000). Recently, silver-impregnated catheters have been developed for clinical use, on which silver ions are bonded with an inert ceramic zeolite. On comparing these silver-impregnated catheters with standard catheters in terms of incidence of catheter-related blood stream infections, it was shown that overall colonization rate was significantly lower in the silver-impregnated CVC tips.
Vascular prosthesis: Recently, the use of the InterGard Silver® bifurcated polyester graft coated with collagen and silver has been shown to achieve excellent patency rates over a long-term period with a low rate of graft infection (Ricco, 2006).
Ventricular drainage catheters: It is a commonly used procedure in intensive care patients for the management of acute occlusive hydrocephalus. The use of silver-impregnated ventricular catheters have resulted in an increased clinical efficacy in the treatment of neurological and neurosurgical patients requiring external CSF drainage. Furthermore, aseptic meningitis due to inflammation was not seen in patients with the silver-impregnated biomedical material (Galiano et al., 2008).
Silver in orthopaedics
Recently, bone cement loaded with nanosilver (Alt et al., 2004a) has shown high antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA). On the other hand these nanoparticles were not found to be cytotoxic against osteoblasts grown in vitro. It is clear from the above study that silver nanoparticles could play a critical role in orthopaedics.
Surgical mesh
Surgical meshes are commonly used for bridging large wounds, as well as acting as reinforcements to tissue repair. The use of silver nanoparticles polypropylene mesh has been studied recently. In a study it was found that silver nanoparticles polypropylene mesh had significant bactericidal efficacy against S. aureus. Furthermore, it was shown that silver nanoparticles could continue to diffuse off the mesh and had sustained activity (Cohen et al., 2007). These results clearly warrant that silver nanoparticles-coated polypropylene mesh can decrease the prosthetic infection rate and the host inflammatory response in the clinical setting.
Future therapeutic directions
Antiviral drug
The antiviral properties of metal nanoparticles are of significant medicinal interest. According to a study (Sun et al., 2005), it was found that silver nanoparticles not only possess anti-retrovirus activities but also inhibit HIV-1 replication. Further, these nanoparticles did not show acute cytotoxicity to either the Hut/CCR5 cells or to normal peripheral blood mononuclear cells.
Anti-platelet agent
According to Shrivastava et al. (2009) silver nanoparticles are effective in treatment of thrombotic disorders by causing inhibition of platelet functional responses like aggregation, secretion, adhesion etc. A similar study exhibited anti-platelet properties of silver nanoparticles wherein significant inhibition of platelet functions was observed via, in vivo mouse models.
Effects on stem cells
The epidermal stem cells, which reside in the dermal layer in the skin, play the most important roles for repairing the epidermis, regenerating hair and maintaining tissue homeostasis after injury. In recent years, many scientists have found that proliferation of epidermal stem cells in skin is promoted by silver nanoparticles at low concentrations (Ito et al., 2007). However the exact mechanism behind this theory is yet to be explored.
SILVER NANOPARTICLES AS THERAPEUTIC AGENTS WITH SPECIAL REFERENCE TO THEIR ANTIMICROBIAL POTENTIAL
Silver has been used in the field of medical sciences since ancient time. This precious metal was known mainly as an effective antimicrobial agent and disinfectant with no side effects (San Chan et al., 2012). But since 1990's, it has been used as an alternative medicine for treatment of various diseases (Fung and Bowen, 1996). It is usually consumed in the form of metallic silver and silver sulfadiazine for the treatment. However, with the development of modern antibiotics the use of silver agents in the field of medicine was restricted mainly to topical silver sulfadiazine cream (Shrivastava et al., 2007; Su et al., 2010). Silver returned to prominence recently due to the emergence of antibiotic-resistant microorganisms as a result of the overuse of antibiotics (Madhumathi et al., 2010).
In the form of nanoparticles silver has attracted much attention of researchers towards itself. The diameter of silver nanoparticles is generally smaller than 100 nm and contains 20 to 15000 silver atoms (Lok et al., 2007). At such small sizes they exhibit remarkably unusual physicochemical and biological properties (Yen et al., 2009). These properties make them exclusively applicable in the field of medical science. Silver nanoparticles possess large surface area to contact with the target cells and hence induce increased effects on them. Besides this their nanosize provides them high penetration potential into the target cell (Prabhu and Poulose, 2012). Inspite of so many therapeutic properties the antimicrobial nature of silver nanoparticles is the most exploited one followed by anti-inflammatory nature.
Antimicrobial properties
The silver nanoparticles are found to be effective against both aerobic and anaerobic microorganisms. They usually do so by precipitating cellular proteins and by blocking the microbial respiratory chain system (Leaper, 2006; Barreiro et al., 2007; Gravante et al., 2009). Before the advent of silver nanoparticles, silver nitrate was an effective antibacterial agent used clinically (Monteiro et al., 2009; Chen et al., 2008). With the advancement of nanotechnology, the interest in the use of the anti-microbial efficiency of silver nanoparticles has increased as compared to silver compounds (Lok et al., 2007). This has raised the demand for the development of sustainable and ecofriendly methods for the synthesis of silver nanoparticles on large scale. Biosynthetic approaches utilizing endophytic fungi have come up as a promising method leading to the green synthesis of silver nanparticles. Rathna et al. (2013) isolated endophytic fungus Aspergillus terreus and examined the anti-dermatophytic activity of the silver nanoparticles synthesised by it. The activity was tested against three dermatophytes namely, T. rubrum, E. floccosum and T. mentagrophytes by well diffusion method at different concentration levels. The synthesised silver nanoparticles exhibited maximum activity against E. floccosum followed by T. rubrum and T. mentagrophytes.
Similarly, Rahi and Parmar (2014) evaluated the antibacterial efficacy of silver nanoparticles synthesised by Penicillium species isolated from Aloe vera roots. 15 μl of silver nanoparticles was found to be active against bacterial pathogens viz, Escherichia coli, Methicillin resistant S. aureus (MRSA), Pseudomonas aeruginosa and S. aureus. In a recent study antibacterial activity of silver nanoparticles synthesised by the endophytic fungus Fusarium sp. isolated from healthy leaves of Withania sominnifera (Ashwagandha) was examined. Maximum zone of inhibition of 26, 26 and 28 mm respectively at 60 μl concentrations of silver nanoparticles against E. coli, S. typhi and S. aureus was observed (Singh et al., 2015). In the same way Shukla and Sandhu (2017) examined the antimicrobial activity of silver nanoparticles synthesized by endophytic fungal isolate A. tenuissima PGL#71. Maximum zone of inhibition was observed against E. coli MTCC#82 (32±0.82 mm), followed by Bacillus subtilis MTCC#441 (28±0.41 mm) and Salmonella typhimurium MTCC#3904 (28±0.28 mm). On the other hand highest antifungal activity was obtained against Candida albicans MML#25 (18±0.25 mm) followed by Candida kruezi MML#10 (10±0.23 mm) and Candida glabrata MML#32 (6.63±0.22 mm).
Possible mechanisms of action of silver nanoparticles
1. Because of their extremely large surface area silver nanoparticles get attached to the microbial cell wall and subsequently penetrate it after causing structural changes which leads to cell lysis (Rai et al., 2009).
2. Silver nanoparticles bind to the sulphur and phosphorous containing bases of the DNA of microorganisms perhaps inhibiting their function (Matsumura et al., 2003).
3. They release silver ions which interact with the thiol groups of many vital respiratory enzymes and inactivates them resulting in microbial cell death (Sondi and Sondi, 2004).
4. They get accumulated inside the microbial cells and have a sustained release of Ag+ inside the cells which may create free radicals and induce oxidative stress, thus
further enhancing their killing activity (Song et al., 2006)
(Figure 4).
Other therapeutic properties
1. Silver nanoparticles causes reduction in the activity of local matrix metalloproteinase (MMP) thus reducing the inflammation (Kirsner et al., 2001).
2. They are used in article joint replacements as bone cements along with polymethyl methacrylate. (Alt et al., 2004b).
3. Due to their unique size and shape nano silver is used as biosensors for the detection of various diseases which could not be detected by normal biosensors (Zhou et al., 2011).
4. Silver nanoparticles act as excellent bioimaging materials because of their plasmonic properties (Lee et al., 2007).
5. They are used in wound dressings and are known to speed up the healing process as a result of their better effect on bacteria and easy penetration into the body fluids in wound dressing (Burrell et al., 1995).
6. Silver nanoparticles have ample of applications in the field of medical science viz., in drug delivery, silver-impregnated central venous catheters, silver-impregnated ventricular drainage catheters, orthopaedics, surgical mesh, antiviral drug and anti-platelet agents
Silver nanoparticles are undoubtedly the most widely used nanomaterials among all. They show great promise in terms of biomedical applications viz, wound healing properties, antimicrobial properties, anti-inflammatory properties etc. Moreover the application of silver nanoparticles in drug delivery systems and diagnostic purpose has attracted the attention of researchers worldwide. This has urged upon the need to develop safe, reliable and ecofriendly approaches for large scale production of silver nanoparticles. The use of biological agents for synthesis of silver nanoparticles has emerged as an attractive approach. This gave rise to a new branch of nanotechnology called nanobiotechnology which combines biological principles with physical and chemical procedures to generate nano-sized particles with specific functions. Biosynthesis of nanoparticles provides an attractive alternative for the hazardous chemical and physical methods of nanoparticles synthesis. Recently the use of endophytic fungal cells has emerged as a novel approach for the biosynthesis of metal nanoparticles. Advantages of this method include highly reproducible synthesis, easy scale up process, economic viability, tolerance towards high metal nanoparticle concentration in the medium, easy management in large-scale production of nanoparticles, good dispersion of nanoparticle and much higher amounts. As a result, for large-scale production of nanoparticles fungi is preferred over other methods. While a number of reports are available on the biological synthesis of nanoparticles, the potential of endophytic fungi has still not been explored completely. Endophytic fungus Colletotrichum sp. isolated from geranium leave, A. clavatus isolated from sterilized stem tissues of A. indica, Pestalotia sp. isolated from leaves of Syzygium cumini are some examples of recently used fungi for the synthesis of nanoparticles with antimicrobial properties.
It is clear from the present chapter that endophytic fungi isolated from various plants synthesize silver nanoparticles of varying size and morphology. These nanoparticles show potential antimicrobial properties and therefore can be employed as nanomedicines for the treatment of various diseases. Therefore, endophytic fungi should be explored more as prospective biofactories for the green synthesis of silver nanoparticles.
The authors have not declared any conflict of interests.
REFERENCES
|
Abeer RM, Aziz AE, Monira R, Alothmana AL, Saleh AE, Mohamed A, Mahmoud M, Majrashic M (2013). Green synthesis of silver nanoparticles using Aspergillus terreus (KC462061). Dig. J. Nanomat. Biostruct. 8:1215-1225.
|
|
|
|
Ahmad A, Mukherjee P, Senapati S, Mandal D, Khan MI, Kumar R, Sastry M (2003). Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. B Biointerfaces 28:313-318.
Crossref
|
|
|
|
|
Alt V, Bechert T, Steinrücke P, Wagener M, Seidel P, Dingeldein E, Scheddin D, Domann E, Schnettler R (2004a). Nanoparticulate silver: A new antimicrobial substance for bone cement. Orthopade 33:885-892.
|
|
|
|
|
Alt V, Bechert T, Steinrücke P, Wagener M, Seidel P, Dingeldein E, Domann E, Schnettler R (2004b). An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials 25:4383-4391.
Crossref
|
|
|
|
|
Amulyavichus A, Daugvila A, Davidonis R, Sipavichus C (1998). Study of chemical composition of nanostructural materials prepared by laser cutting of metals. Fizika Met. Met. 85:111-117.
|
|
|
|
|
Azevedo JL, Maccheroni W, Pereira JO, Araujo WL (2000). Endophytic microorganism a review on insect control and recent advances on tropical plants. Electron. J. Biotechnol. 3:1-36.
Crossref
|
|
|
|
|
Bala M, Arya V (2013). Biological synthesis of silver nanoparticles from aqueous extract of endophytic fungus Aspergillus fumigates and its antibacterial action. Int. J. Nanomat. Biostruct. 3:37-41.
|
|
|
|
|
Bandara WMMS, Seneviratne G, Kulasooriya SA (2006). Interactions among endophytic bacteria and fungi: Effects and potentials. J. Biosci. 31(5):645-650.
Crossref
|
|
|
|
|
Barreiro E, Casas JS, Couce MD (2007). Synthesis and antimicrobial activities of silver (I) sulfanylcarboxylates: Structural isomers with identically or unequally coordinated Ag centers in an Ag4S4 ring. Dalton Trans. 28:3074-3085.
Crossref
|
|
|
|
|
Basavaraja S, Balaji SD, Lagashetty A, Rajasab AH, Venkataraman A (2008). Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium semitectum. Mater. Res. 43:1164-1170.
Crossref
|
|
|
|
|
Bhainsa KC, D'Souza SF (2006). Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids Surf. 47:160-164.
Crossref
|
|
|
|
|
Bharathidasan R, Panneerselvam A (2012). Biosynthesis and characterization of silver nanoparticles using endophytic fungi Aspergillus concius, Penicillium janthinellum and Phomosis sp. Int. J. Pharm. Sci. Res. 3:3163-3169.
|
|
|
|
|
Bhattacharya R, Mukherjee P (2008). Biological properties of "naked" metal nanoparticles. Adv. Drug Deliv. Rev. 60:1289-1306.
Crossref
|
|
|
|
|
Bhol KC, Schechter PJ (2007). Effects of nanocrystalline silver (NPI 32101) in a rat model of ulcerative colitis. Dig. Dis. Sci. 52:2732-2742.
Crossref
|
|
|
|
|
Bischoff JF, White JF (2005). Evolutionary development of the Clavicipitaceae. In. The Fungal Community, Its Organisation and Role in the Ecosystem (eds. J. Dighton, J.F. White, and P. Oudemans, Jr.), CRC Taylor & Francis, Inc. USA. pp. 505-518.
|
|
|
|
|
Boisselier E, Astruc D (2009). Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 38:1759-82.
Crossref
|
|
|
|
|
Burrell RE, McIntosh CL, Morris LR (1995). Process of activating anti-microbial materials. US Patent 5454886. 3.
|
|
|
|
|
Chandrasekharan N, Kamat PV (2000). Improving the photoelectrochemical performance of nanostructured TiO2 films by adsorption of gold nanoparticles. J. Phys. Chem. B. 104:10851-10857.
Crossref
|
|
|
|
|
Chen X, Schluesener HJ (2008). Nanosilver: A nanoproduct in medical application. Toxicol. Lett. 176:1-12.
Crossref
|
|
|
|
|
Cohen MS, Stern JM, Vanni AJ, Kelley RS, Baumgart E, Field D, Libertino JA, Summerhayes IC (2007). In vitro analysis of a nanocrystalline silver-coated surgical mesh. Surg. Infect. 8:397-403.
Crossref
|
|
|
|
|
Davis EC, Shaw A (2008). Biogeographic and phylogenetic patterns in diversity of liverwort-associated endophytes. Am. J. Bot. 95:914-924.
Crossref
|
|
|
|
|
Devi LS, Bareh DA, Joshi SR (2014). Studies on Biosynthesis of Antimicrobial Silver Nanoparticles Using endophytic Fungi isolated from the Ethno-medicinal Plant Gloriosa superba L. Proc. Nat. Acad. Sci. India. 84.
Crossref
|
|
|
|
|
Devi LS, Joshi SR (2014). Evaluation of antimicrobial potency of silver nanoparticles biosynthesized by using endophytic fungus Cryptosporiopsis ericae PS4. J. Microbiol. 52: 667-674.
Crossref
|
|
|
|
|
Devi LS, Joshi SR (2015). Ultrastructures of silver nanoparticles biosynthesized using endophytic fungi. J. Micro. Ultrastruc. 3: 29-37.
Crossref
|
|
|
|
|
Devi NN, Dheeban Shankar P, Sutha S (2012). Biomimetic synthesis of silver nanoparticles from an endophytic fungus and their antimicrobial efficacy. Int. J. Biomed. Adv. Res. 3:409-415.
|
|
|
|
|
Devi P, Niveditha R, Vaishnavie R (2015). Antimicrobial activity of silver nanoparticles synthesized by endophytic Aspergillus sp. isolated from Justicia beddomei. J. Chem. Pharma Res. 7:784-788.
|
|
|
|
|
Dias MA, Lacerda ICA, Pimentel PF, Castro D, Rosa HF (2002). Removal of heavy metals by an Aspergillus terreus strain immobilized in a polyurethane matrix. Lett. Appl. Microbiol. 34:46-50.
Crossref
|
|
|
|
|
Faeth SH, Gardner DR, Hayes CJ, Jani A, Writtlinger SK, Jones TA (2006). Temporal and spatial variation in alkaloid levels in Achnatherum robustum, a native grass infected with the endophyte Neotyphodium. J. Chem. Ecol. 32:307-324.
Crossref
|
|
|
|
|
Fong J, Wood F (2006). Nanocrystalline silver dressings in wound management: a review. Int. J. Nanomed. 1:441-449.
Crossref
|
|
|
|
|
Fung MC, Bowen DL (1996). Silver products for medical indications: risk-benefit assessment. J. Toxicol. Clin. Toxicol. 34:119-126.
Crossref
|
|
|
|
|
Galiano K, Pleifer C, Engelhardt K, Brossner G, Lackner P, Huck C, Lass-Flörl C, Obwegeser A (2008). Silver segregation and bacterial growth of intraventricular catheters impregnated with silver nanoparticles in cerebrospinal fluid drainages. Neurol. Res. 30:285-287.
Crossref
|
|
|
|
|
Gao S, Zhao Y, Gou P, Chen N, Xie Y (2003). Preparation of CuAlO2 nanocrystalline transparent thin films with high conductivity. Nanotechnology 14:538-541.
Crossref
|
|
|
|
|
Gericke M, Pinches A (2006a). Biological synthesis of metal nanoparticles. Hydrometallurgy 83:132-140.
Crossref
|
|
|
|
|
Gravante G, Caruso R, Sorge R (2009). Nanocrystalline silver: a systematic review of randomized trials conducted on burned patients and an evidence based assessment of potential advantages over old silver formulations. Ann. Plast. Surg. 63:201-205.
Crossref
|
|
|
|
|
Guo B, Jin-Rui D, Siewbe Ng, Huang Y, Leong C, Ong W, Carte BK (2000). Cytonic acids A and B: Novel tripepside inhibitors of hCMV protease from the endophytic fungus Cytonema sp. J. Nat. Prod. 63(5):602-604.
Crossref
|
|
|
|
|
Hirst SM, Karakoti AS, Tyler RD, Sriranganathan N, Seal S, Reilly CM (2009). Anti-inflammatory properties of cerium oxide nanoparticles. Small 5:2848-2856.
Crossref
|
Huang YF, Chang HT, Tan WH (2008). Cancer Cell Targeting Using Multiple Aptamers Conjugated on Nanorods. Anal. Chem. 80:567-572.
Crossref
|
|
|
|
Hullikere MM, Joshi CG, Raju NG (2014). Biogenic synthesis of silver nano particles using endophytic fungi Penicillium nodositatum and its antibacterial activity. J. Chem. Pharm. Res. 6:112-117.
|
|
|
|
|
Ito M, Yang Z, Andl T (2007). Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 447:316-320.
Crossref
|
|
|
|
|
Kalimuthu K, Babu SR, Venkataraman D, Bilal M, Gurunathan S (2008). Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf. B Biointerfaces 65:150-153.
Crossref
|
|
|
|
|
Kalishwaralal K, Deepak V, Ramkumarpandian S, Nellaiah H, Sangiliyandi G (2008). Extracellular biosynthesis of silver nanoparticles by the culture supernatant of Bacillus licheniformis. Mater. Lett. 62:4411-4413.
Crossref
|
|
|
|
|
Kharwar RN, Verma VC, Strobel GA, Ezra D (2008). The endophytic fungal complex of Catharanthus roseus (L.) G. Don. Curr. Sci. 95:228-232.
|
|
|
|
|
Kim JS, Kuk E, Yu KN (2007). Antimicrobial effects of silver nanoparticles. Nanomedicine 3:95-101.
Crossref
|
|
|
|
|
Kirsner R, Orsted H, Wright B (2001). Matrix metalloproteinases in normal and impaired wound healing: a potential role of nanocrystalline silver. Wounds 13:5-10.
|
|
|
|
|
Klasen HJ (2000). A historical review of the use of silver in the treatment of burns II. Renewed interest for silver. Burns 26:131-138.
Crossref
|
|
|
|
|
Klaus T, Joerger R, Olsson E, Granqvist CG (1999). Silver based crystalline nanoparticles, microbially fabricated. Proc. Nat. Acad. Sci. 968:13611-13614.
Crossref
|
|
|
|
|
Kleemann W (1993). Random-field induced antiferromagnetic, ferroelectric and structural domain states. Int. J. Mod. Phys. 7:2469-2507.
Crossref
|
|
|
|
|
Koulman A, Lane GA, Christensen MJ, Fraser K, Tapper BA (2007). Peramine and other fungal alkaloids are exuded in the guttation fluid of endophyte-infected grasses. Phytochemistry 68:355-360.
Crossref
|
|
|
|
|
Krings M, Taylor TN, Hass H, Kerp H, Dotzler N, Herman EJ (2007). Fungal endophytes in a 400-million-yr-old land plant: infection pathways, spatial distribution, and host response. New Phytol. 174:648-657.
Crossref
|
|
|
|
|
Krolikowska A, Kudelski A, Michota A, Bukowska J (2003). SERS studies on the structure of thioglycolic acid monolayers on silver and gold. Surf. Sci. 532:227-232.
Crossref
|
|
|
|
|
Kumar A, Mandal S, Selvakannan PR, Parischa R, Mandale AB, Sastry M (2003). Investigation into the interaction between surface-bound alkylamines and gold nanoparticles. Langmuir 19:6277-6282.
Crossref
|
|
|
|
|
Leaper DJ (2006). Silver dressings: their role in wound management. Int. Wound J. 3:282-294.
Crossref
|
|
|
|
|
Lee KJ, Nallathamby PD, Browning LM, Osgood CJ, Xu XHN (2007). In vivo imaging of transport and biocompatibility of single silver nanoparticles in early development of zebra fish embryos. ACS Nano 1:133-143.
Crossref
|
|
|
|
|
Liu X, Lee PY, Ho CM, Lui VC, Chen Y, Che CM, Tam PK, Wong KK (2010). Silver nanoparticles mediate differential responses in keratinocytes and fibroblasts during skin wound healing. Chem. Med. Chem. 5:468-475.
Crossref
|
|
|
|
|
Lok CN, Ho CM, Chen R (2007). Silver nanoparticles: partial oxidation and antibacterial activities. J. Biol. Inorg. Chem.12:527-534.
Crossref
|
|
|
|
|
Luo J, Maye MM, Kariuki NN, Wang L, Njoki P, Lin Y, Schadt M, Naslund HR, Zhong CJ (2005). Electrocatalytic oxidation of methanol: carbon supported gold-platinum nanoparticles catalysts prepared by two-phase protocol. Catal. Today 99:291-297.
Crossref
|
|
|
|
|
Madhumathi K, Sudheesh Kumar PT, Abhilash S, Sreeja V, Tamura H (2010). Development of novel chitin nanosilver composite scaffolds for wound dressing applications. J. Mater. Sci. Mater. Med. 21:7-13.
Crossref
|
|
|
|
|
Maheshwari R (2006). What is an endophytic fungus. Curr. Sci. 90(10):1309.
|
|
|
|
|
Matsumura Y, Yoshikata K, Kunisaki S I, Tsuchido T (2003). Mode of Bactericidal Action of Silver Zeolite and its Comparison with That of Silver Nitrate. Appl. Environ. Microbiol. 69:4278-4281.
Crossref
|
|
|
|
|
Mehra RK, Winge DR (1991). Metal Ion Resistance in Fungi: Molecular Mechanisms and their Regulated Expression. J. Cell. Biochem. 45:30-40.
Crossref
|
|
|
|
|
Mermel LA (2000). Prevention of intravascular catheter-related infections. Ann. Intern. Med. 132:391-402.
Crossref
|
|
|
|
|
Monteiro DR, Gorup LF, Takamiya AS (2009). The growing importance of materials that prevent microbial adhesion: antimicrobial effect of medical devices containing silver. Int. J. Antimicrob. Agents 34:103-110.
Crossref
|
|
|
|
|
Muangman P, Muangman S, Opasanon S, Keorochana K, Chuntrasakul C (2009). Benefit of hydrocolloid SSD dressing in the outpatient management of partial thickness burns. J. Med. Assoc. Thailand 92:1300-1305.
|
|
|
|
|
Musarrat J, Dwivedi S, Singh BR, Al-Khedhairy AA, Azam A, Naqvi A (2010).Production of antimicrobial silver nanoparticles in water extracts of the fungus Amylomyces rouxii strain KSU-09. Bioresour. Technol. 101:8772-8776.
Crossref
|
|
|
|
|
Nadworny PL, Wang JF, Tredget EE (2008). Anti-inflammatory activity of nanocrystalline silver in a porcine contact dermatitis model. Nanomed. Nanotechnol. Biol. Med. 4:241-251.
Crossref
|
|
|
|
|
Nair B, Pradeep T (2002). Coalescence of Nanoclusters and Formation of Submicron Crystallites Assisted by Lacto-bacillus Strains. Cryst. Growth Des. 2:293-298.
Crossref
|
|
|
|
|
Netala VR, Bobbu P, Ghosh SB, Tartte V (2015). Endophytic fungal assisted synthesis of Silver Nanoparticles, Characterization and Antimicrobial Activity. Asian J. Pharm. Clin. Res. 8:113-116.
|
|
|
|
|
Patel PP (2008). Turning waste heat into power: Research shows that silicon is as efficient as pricier materials. Technol. Rev. pp.1-2.
|
|
|
|
|
Peterson MSM, Bouwman J, Chen A, Deutsch M (2007). Inorganic metallodielectric materials fabricated using two single-step methods based on the Tollen's process. J. Colloid Interface Sci. 306:41-49.
Crossref
|
|
|
|
|
Petrini O (1986). Taxonomy of endophytic fungi of aerial plant tissues. In. N.J. Fokkema and J. van den Heuvel (eds.) Microbiology of the Phyllosphere. Cambridge University, Cambridge. pp. 175-187.
|
|
|
|
|
Petrini O (1991). Fungal endophytes of tree leaves. In. Andrews J., Hirano S., editors. Microbial ecology of leaves. New York. pp. 179-197.
Crossref
|
|
|
|
|
Prabhu S, Poulose EK (2012). Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2(1):32.
Crossref
|
|
|
|
|
Qian Y, Yu H, He D, Yang H, Wang W, Wan X, Wang L (2013). Biosynthesis of silver nanoparticles by the endophytic fungus Epicoccum nigrum and their activity against pathogenic fungi. Bioproc. Biosys. Eng. 36:1613-9.
Crossref
|
|
|
|
|
Raheman F, Deshmukh S, Ingle A, Gade A, Rai M (2011). Silver Nanoparticles: Novel Antimicrobial Agent Synthesized from an Endophytic Fungus Pestalotia sp. Isolated from Leaves of Syzygium cumini (L). Nano Biomed. Eng. 3:174-178.
Crossref
|
|
|
|
|
Rahi DK, Parmar AS (2014). Mycosynthesis of silver nanoparticles by an endophytic Penicillium species of Aloe vera root, evaluation of their antibacterial and antibiotic enhancing activity. Int. J. Nanomat. Biostruct. 4:46-51.
|
|
|
|
|
Rai M, Yadav A, Gade A (2009). Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 27(1):76-83.
Crossref
|
|
|
|
|
Rathna GS, Elavarasi A, Peninal S, Subramanian J, Mano G, Kalaiselvam M (2013). Extracellular Biosynthesis of Silver Nanoparticles by Endophytic Fungus Aspergillus terreus and its Anti-dermatophytic Activity. Int. J. Pharm. Biol. Arch. 4:481-487.
|
|
|
|
|
Ricco JB (2006). InterGard silver bifurcated graft: features and results of a multicenter clinical study. J. Vasc. Surg. 44:39-46.
Crossref
|
|
|
|
|
Rodriguez RJ, Henson J, Van E, Volkenburgh M, Hoy L, Wright F, Beckwith Y, Kim RS Redman (2008). Stress tolerance in plants via habitat-adapted symbiosis. Int. Soc. Microbiol. Ecol. 2:404-416.
Crossref
|
|
|
|
|
Saifuddin N, Wong CW, Nur Yasumira AA (2009). Rapid Biosynthesis of Silver Nanoparticles Using Culture Supernatant of Bacteria with Microwave Irradiation. E-J. Chem. 6:61-70.
Crossref
|
|
|
|
|
San Chan Y, Don MM (2012). Charactrization of silver nanoparticles produced by white rot fungi and it in vitro antimicrobial activities. Int. Arabic J. Antimicrob. Agents 2:1-8.
|
|
|
|
|
Sanghi R, Verma P (2009). Biomimetic synthesis and characterisation of protein capped silver nanoparticles. Bioresour. Technol. 100:501-504.
Crossref
|
|
|
|
|
Shafirovich E, Diakov V, Varma A (2006). Combustion of novel chemical mixtures of hydrogen generation. Combust. Flame 144:415-418.
Crossref
|
|
|
|
|
Shanmukh S, Jones L, Zhao YP, Driskell JD, Tripp RA, Dluhy RA (2008). Identification and classification of respiratory syncytial virus (RSV) strains by surface-enhanced Raman spectroscopy and multivariate statistical techniques. Anal. Bioanal. Chem. 390:1551-1555.
Crossref
|
|
|
|
|
Shao K, Yao J (2006). Preparation of silver nanoparticles via a non-template method. Mater. Lett. 60:3826-3829.
Crossref
|
|
|
|
|
Sharma S, Kumar S, Bulchandani BD, Taneja S, Banyal S (2013). Green Synthesis of Silver Nanoparticles and their antimicrobial activity against Gram positive and Gram Negative Bacteria. Int. J. Biotechnol. Bioeng. Res. 7: 711-714.
|
|
|
|
|
Shrivastava S, Bera T, Roy A, Singh G, Ramachandrarao P, Dash D (2007). Characterization of enhanced antibacterial effects of novel silver nanoparticle by using some Endophytic Fungi with special reference to their Antimicrobial Potential. Int. J. Nanotechnol. Appl. 7:7-22.
|
|
|
|
|
Sibbald RJ, Contreras-Ruiz J, Coutts P, Fierheller M, Rothman A, Woo K (2007). Bacteriology, inflammation, and healing: a study of nanocrystalline silver dressings in chronic venous leg ulcers. Adv. Skin Wound Care 20:549-558.
Crossref
|
|
|
|
|
Singh AK, Rathod V, Singh D, Ninganagouda S, Kulkarni P, Mathew J, Haq M (2015). Bioactive Silver Nanoparticles from Endophytic fungus Fusarium sp. Isolated from an Ethno medicinal Plant Withania somnifera (Ashwagandha) and its Antibacterial Activity. Int. J. Nanomat. Biostruc. 5: 15-19.
|
|
|
|
|
Singh D, Rathod V, Ninganagouda S, Hiremath J, Singh AK, Mathew J (2014). Optimization and Characterization of Silver Nanoparticle by Endophytic Fungi Penicillium sp. isolated from Curcuma longa (Turmeric) and Application Studies against MDR E. coli and S. aureus. Bioinorg. Chem. Appl. 2014:1-8.
Crossref
|
|
|
|
|
Singh D, Rathod V, Ninganagouda S, Hiremath J, Singh AK, Mathew J (2013). Biosynthesis of silver nanoparticle by endophytic fungi Pencillium sp. isolated from Curcuma longa (turmeric) and its antibacterial activity against pathogenic gram negative bacteria. J. Pharm. Res. 7:448-453.
Crossref
|
|
|
|
|
Sondi I, Sondi B (2004). Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 275:177-182.
Crossref
|
|
|
|
|
Song HY, Ko KK, Oh LH, Lee BT (2006). Fabrication of Silver Nanoparticles and their Antimicrobial Mechanisms. Eur. Cell. Mat. 11(Suppl 1):58.
|
|
|
|
|
Stone JK, Polishook JD, White JR (2004). Endophytic fungi, In. Biodiversity of fungi, Inventory and Monitoring Methods, Eds. Mueller GM, Bills GF, Foster MS, Elsevier Academic Press: Burlington. MA, USA. Pp. 241-270.
Crossref
|
|
|
|
|
Strobel G, Daisy B (2003). Bioprospecting for microbial endophytes and their natural products. Microbiol. Mol. Biol. Rev. 67:491-502.
Crossref
|
|
|
|
|
Strobel GA, Ford E, Worapong J, Harper JK, Arif AM, Grant DM, Fung PCW, Chan K (2002). Isopestacin, an isobenzofuranone from Pestalotiopsis microspora, possessing antifungal and antioxidant activities. Phytochemistry 60:179-183.
Crossref
|
|
|
|
|
Su Y, Qiao S, Yang H, Yang C, Jin Y, Stahr F, Sheng J, Cheng L, Ling C, Lu GQ (2010). Titanate-Silica mesostructured nanocables: synthesis, structure analysis and biomedical applications. Nanotechnology 21(6):065604.
Crossref
|
|
|
|
|
Sun RW, Chen R, Chung NP, Ho CM, Lin CL, Che CL (2005). Silver nanoparticles fabricated in Hepes buffer exhibit cytoprotective activities toward HIV-1 infected cells. Chem. Commun. 40:5059-5061.
Crossref
|
|
|
|
|
Sun X, Luo Y (2005). Preparation and size control of silver nanoparticles by a thermal method. Mater. Lett. 59:3847-3850.
Crossref
|
|
|
|
|
Sunkar S, Nachiyar CV (2012). Microbial synthesis and characterization of silver nanoparticles by using endophytic bacterium Bacillus cereus: A novel source in benign synthesis. Glob. J. Med. Res. 2(12):953-959.
|
|
|
|
|
Sunkar S, Nachiyar CV (2013). Endophytic fungi mediated extracellular silver nanoparticles as effective antibacterial agents. Int. J. Pharm. Pharm. Sci. 5:95-100.
|
|
|
|
|
Tian N, Zhou ZY, Sun SG, Ding Y, Wang ZL (2007). Synthesis of tetra hexahedral platinum nanocrystals with high-index facets and high electro-oxidation activity. Science 316:732-735.
Crossref
|
|
|
|
|
Tredget EE, Shankowsky HA, Groeneveld A, Burnell R (1998). A matched-pair, randomized study evaluating the efficacy and safety of Acticoat silver-coated dressing for the treatment of burn wounds. J. Burn Care Rehabil. 19:531-537.
Crossref
|
|
|
|
|
Tsuji T, Iryo KN, Watanabe Tsuji M (2002). Preparation of silver nanoparticles by laser ablation in solution: influence of laser wavelength on particle size. Appl. Surf. Sci. 202:80-85.
Crossref
|
|
|
|
|
Vardhana J, Kathiravan G (2015). Biosynthesis of Silver Nanoparticles by Endophytic Fungi Pestaloptiopsis pauciseta Isolated From the Leaves of Psidium guajava Linn. Int. J. Pharm. Sci. Rev. Res. 31:29-31.
|
|
|
|
|
Verma VC, Gond SK, Kumara A, Kharwar RN, Strobel GA (2007). Microbial Ecology, The endophytic mycoflora of bark, leaf, and stem tissues of Azadirachta indica A. Juss (Neem) from Varanasi (India). Microb. Ecol. 54:119-125.
Crossref
|
|
|
|
|
Verma VC, Kharwar RN, Strobel GA (2009). Chemical and Functional Diversity of Natural Products from Plant Associated Endophytic Fungi. Nat. Prod. Comm. 4:1-22.
|
|
|
|
|
Verma VC, Kharwar RN, Gange AC (2010). Biosynthesis of antimicrobial silver nanoparticles by the endophytic fungus Aspergillus clavatus. Nanomedicine 5:33-40.
Crossref
|
|
|
|
|
Wilson D (2000). Ecology of woody plant endophytes In microbial Endophytes (eds. C.W. Bacon and J.F. White, Jr), Marcel Dekker, Inc: New York: 389-420.
|
|
|
|
|
Wong KK, Cheung SO, Huang LM, Niu J, Tao C, Ho CM, Che CM, Tam PK (2009). Further evidence of the anti-inflammatory effects of silver nanoparticles. Chem. Med. Chem. 4:1129-1135.
Crossref
|
|
|
|
|
Yen HJ, Hsu SH, Tsai CL (2009). Cytotoxicity and immunological response of gold and silver nanoparticles of different sizes. Small 5:1553-1561.
Crossref
|
Zhang HW, Song CY, Tan RX (2006). Biology and chemistry of endophytes. Nat. Prod. Rep. 23:753-771.
Crossref
|
|
|
|
Zhou W, Ma YY, Yang HA, Ding Y, Luo XG (2011). A label-free biosensor based on silver nanoparticles array for clinical detection of serum p53 in head and neck squamous cell carcinoma. Int. J. Nanomed. 6:381-386.
Crossref
|
|
|
|
|
Zong RL, Zhou J, Li B, Fu M, Shi SK, Li LT (2005). Optical properties of transparent copper nanorod and nanowire arrays embedded in anodic alumina oxide. J. Chem. Phys. 123(9):94710.
Crossref
|
|
|
|
|
|