ABSTRACT
Peels of sweet potato (Ipomoea batatas) were buried in the soil for 14 days and the isolates associated with the degradation of the peels were obtained using standard microbiological procedures. The bacterial isolates obtained were screened for amylolytic and cellulolytic activities under different pH and temperatures as parameters and optimized for enzyme production. Sixteen (16) bacterial isolates were obtained and characterized and screened for amylase and cellulase production. Bacillus pumilus has the highest frequency of occurrence (18.75%) followed by B. subtilis (12.50%). After 24 to 48 h of incubation, B. pumilus produced highest concentration of amylase at 55°C, pH 6 (5.4 U/mL) while B. subtilis had the best cellulase production of 0.75 U/mL at 55°C, pH 7. B. pumilus and Bacillus subtilis produced the highest amylase and cellulase concentrations and seem to be the potential sources of these enzymes for industrial application.
Key words: Sweet potato peel, amylase, cellulase, bacteria.
Amylases are class of enzymes, which are of important applications in the food, brewing, textile, detergent and pharmaceutical industries. Their most relevant effect is employed during starch liquefaction to reduce their viscosity, production of maltose, oligosaccharide mixtures, high fructose syrup and maltotetraose syrup (Jose and Arnold, 2014). During detergents production, they are applied to improve cleaning effect and are also used for starch de-sizing in textile industry (Aiyer, 2005). α-Amylase is characterized by its random hydrolysis of α-1,4-glucosidic bonds in amylose and amylopectin molecules, while amylopectin α-1,6-bonds are resistant to its cleavage (Parmar and Pandya, 2012). Many micro-organism such as Bacillus subtilis, Bacillus cereus, Bacillus polmyxa, Bacillus amyloliquefaciens, Bacillus coagulans, Lactobacillus, Escherichia, Proteus, Bacillus lincheniformis, Bacillus steriothermophilu, Bacillus megaterium, Strepotmyces sp., Pseudomonas sp. etc. were used in α- and β-amylases production. Although, among bacteria, Bacillus sp. was widely used for thermostable α-amylase production so as to meet industrial needs (Parmar and Pandya, 2012).
Cellulose is the most abundant biomass on Earth (Tomme et al., 1995). It is the primary product of photosynthesis in terrestrial environments and the most abundant renewable bioresource produced in the biosphere (Jarvis, 2003; Zhang and Lynd, 2004). Cellulose is commonly degraded by an enzyme called cellulase. This enzyme is produced by several microorganisms, mainly bacteria and fungi (Bahkali, 1996; Magnelli and Forchiassin, 1999; Shin et al., 2000; Immanuel et al., 2006). Cellulases from bacteria are more effective catalysts and less inhibited by the presence of material that has already been hydrolyzed. The greatest potential importance of using bacteria for cellulase production is the ease with which bacteria can be genetically engineered, high growth rate as compared to fungi, often more complex and in multi-enzyme complexes providing increased function and synergy, inhabit a wide variety of environmental and industrial niches (Ariffin et al., 2006; Sadhu and Maiti, 2013). However, the application of bacteria in producing cellulase is not widely used (Sonia et al., 2013). Some bacterial species used in cellulase production are Cellulomonas species, Pseudomonas species, Bacillus species and Micrococcus species (Nakamura and Kappamura, 1982). Cellulases are used: In the textile industry for cotton softening and denim finishing; in laundry detergents for colour care, cleaning; in the food industry for mashing; in the pulp and paper industries for drainage improvement and fibre modifi-cation (Cherry and Fidants, 2003).
Amylase and cellulase yields appear to depend upon a complex relationship involving a variety of factors like inoculums size, pH value, temperature, presence of inducers, medium additives, aeration, growth time, and so forth (Immanuel et al., 2006).
This study was therefore designed to isolate high amylase and cellulase producing bacteria from decaying sweet potato peels and to optimise for enzyme production.
Samples collection
Sweet potatoes (yellow skin) were purchased from Agbowo Market in Ibadan Metropolis, Oyo State, Nigeria.
Sample preparation
The peels of sweet potatoes were carefully scraped off so that the amount of corker removed was kept to a minimum. The scraped peels were buried inside the soil (14 cm deep) in Botanical Garden, University of Ibadan, Oyo State, Nigeria.
Isolation of organism
The buried scrapped peels were exhumed carefully after 14 days and put in a sterile nylon bag and carried to the laboratory. The adhering sand was shaken off and 1 g of the peel was homogenized aseptically using a sterilized mortar and pestle. Serial dilution was carried out and 1 mL of dilution 104 and 106 were mixed with 20 mL of plate count agar, poured on plate and allowed to set. This was incubated for 24 h at 37°C and observed for bacterial growth. Colonies with different morphology (shape, texture and colour) were isolated and purified by sub-culturing several times till pure cultures were obtained. Isolation was carried out in triplicates.
Identification of isolates
Organisms were identified based on their macroscopic, micro-scopic, physiological and biochemical characteristics of the isolates with reference to Bergey’s Manual of Systematic Bacteriology (Sneath et al., 1986). The biochemical tests carried out are starch hydrolysis, catalase test, Voges Prokauer test, citrate utilization and endospore test.
Growth on carboxymethylcellulose (CMC)
CMC (2%) was prepared with nutrient agar, sterilized and allowed to cool to 45°C. It was poured into Petri dishes. The plates were inoculated with single streak of test organism and incubated at 37°C for 48 h. Presence of clear zones along line of growth indicates that the organism can utilize or break down cellulose and this was used to screen for cellulase production ability of the isolates.
Growth on starch
Starch agar was prepared by adding 1 g of soluble starch to 100 mL of nutrient agar. The mixture was homogenized and sterilized at 121°C for 15 min. This was then dispensed into sterile plates and allowed to set. A single streak of culture was made on the plate and incubated at 37°C for 48 h. After incubation, the plates were flooded with Gram’s iodine. A positive result was indicated by retention of the iodine colour as a clear zone around the growth region indicating starch hydrolysis while unhydrolyzed starch formed a blue and black colouration with iodine. This was used to screen the bacterial isolates for amylase production.
Extraction of enzymes
The medium used was nutrient broth in which soluble starch and CMC (1%) was added respectively. It was sterilized at 121°C for 15 min, allowed to cool and the test organisms inoculated into it. It was then incubated at 30°C for 48 h after which the culture was centrifuged at 10,000 rpm for 15 min using a refrigerated centrifuge (IEC centra, MP4R model). The cell free culture supernatant was then assayed for amylase and cellulase production and activity. One unit (U) of enzyme activity is expressed as the quantity of enzyme, which is required to release 1 mol of glucose per minute under standard assay conditions (Muhammad et al., 2012).
Amylase assay
Amylase assay was determined using DNSA reagent method of Bernfeld (1955) as modified by Giraud et al. (1991). To 1 mL of culture supernatant was added 1 mL of the substrate containing 1.2% w/v soluble starch in 0.1 N phosphate buffer, pH 6.0. The enzyme substrate reaction was incubated at 45°C for 1 h. The reaction was brought to halt by adding a drop of 5 M NaOH. The amount of reducing sugar produced was determined with 3,5-dinitrosalicylic acid (DNS). 1 mL of DNS reagent was added to filtrate-substrate reaction mixture and was heated in a boiling water bath at 100°C for 10 min. It was cooled with distilled water. The absorbance was measured at 540 nm using spectrophotometer
(Unipec 23 D, Uniscope England). One millilitre of uninoculated blank similarly treated was used to set spectrophotometer at zero. Standard maltose concentrations were prepared within the range of 0.2 - 3.0mg/mL maltose into the requisite medium. The results were then used to construct a standard curve. The spectrophotometer values were then extrapolated as maltose equivalent from the standard curve plotted (Bernfield, 1955).
Cellulase assay
Cellulase assay was determined using the method of Mandel et al., (1976). 1 mL of culture supernatant was added to 9 mL of the substrate containing 0.55% w/v of CMC (carboxymethylcellulose) in 0.55 M acetate buffer, pH 5.5. It was incubated at 45°C for 1 h. The reaction was brought to halt by adding a drop of 5 M NaOH. 1 mL of DNS was added to 1 mL of the filtrate in order to estimate the reducing sugar that was released. The mixture was boiled at 100°C for 10 min in water bath. After cooling, the absorbance was determined at 540 nm using Unispec 23D spectrophotometer.
Effect of different temperatures on amylase and cellulase productions
Nutrient broth was prepared and 10 ml each dispensed into screw capped bottles and sterilized at 121°C for 15 min and allowed to cool. Bacillus isolates were inoculated into each bottle and incubated at different temperatures (25, 37, 45, 55 and 65°C) for 24 h. Amylase and cellulase activities were then determined as described earlier.
Effect of different pH on amylase and cellulase production
Buffer was used to adjust the pH of nutrient broth to 3.0, 4.0, 5.0, 6.0 and 7.0 accordingly. 10 mL of the adjusted nutrient broth was dispensed into screw capped bottles and sterilized at 121°C for 15 min. After cooling, test isolates were inoculated into each bottle and incubated at 37°C for 24 h. Enzymes activities were determined as earlier described.
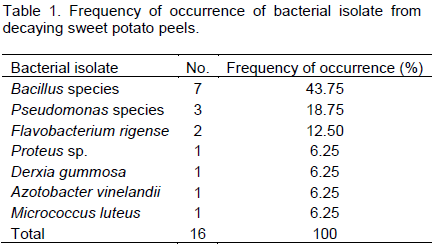
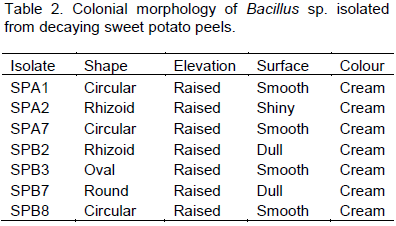
Bacillus species had highest occurrence of bacterial isolate from buried potatoes peels after 14 days (Table 1). Bacillus sp. recorded 43.75% of occurrence; followed by Pseudomonas with 18.75%. Other bacteria isolated were Flavobacterium rigense, Proteus sp., Derxia gummosa, Azotobacter vinelandii and Micrococcus luteus. The colonial morphologies of Bacillus species isolated were represented on Table 2, they all have raised elevations and cream colour while their texture are either smooth, dull or shiny. Also, they exhibit different colony shapes on the plate, B. pumilus is circular, B. licheniformis is rhizoid, B. megaterium is oval and B. subtilis is round.
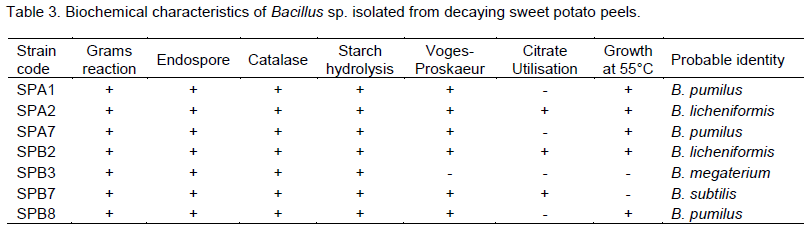
Table 3 shows the biochemical tests for bacillus isolates. The Bacillus spp. are Gram positive, rod shaped and endospore positive. All the bacillus isolates have the ability to hydrolyse starch and utilize citrate except B. pumilus which is negative to hydrolysis citrate utilization. B. subtilis was positive to Voges-Proskaeur test, citrate utilization but no growth was recorded at 55°C. B. licheniformis is positive to Voges-Proskaeur test, citrate utilization and also has the ability to grow at 55°C. B. megaterium is negative to both Voges-Proskauer and citrate utilization test.
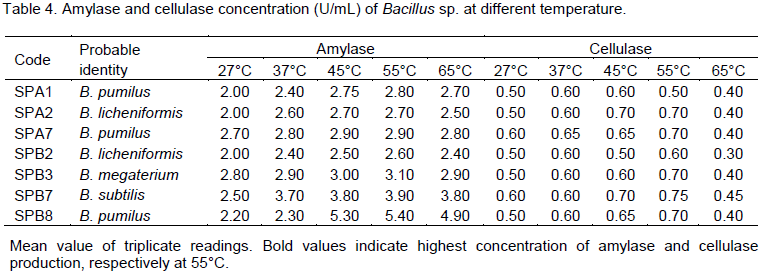
The effect of different temperatures on amylase and cellulase production of the Bacillus species are presented in Table 4, for all the isolates, there was a gradual increase in enzymes activities as the temperature increases with maximum concentration produced at 55°C before a general decline at 65°C. B. pumilus SPB8 produced highest concentration of amylase at 55°C (5.4 U/mL) while B. subtilus SPB7 produced cellulase best also at 55°C with 0.75 U/mL concentration. Least production of enzymes was noticed at 27°C for all isolates. Figure 1 shows the effect of pH, at pH 6, B. pumilus SPA7 produced the highest concentration of amylase (5.2U/mL) followed by B. megaterium SPB3 (4.2 U/mL) and the lowest producer at that pH is B. subtilis SPB7 (3.1 U/mL). The highest concentration of cellulase was produced by B. pumilus SPB8 (1.8 U/mL) at pH 5 followed by B. pumilus SPA1 (1.5 U/mL), while B. licheniformis SPB2 produced the least concentration at this pH (Figure 2). All the organisms recorded their highest cellulase production at either pH 5 or 6 with the exception of B. licheniformis SPB2 that recorded its highest cellulase production at pH 3.
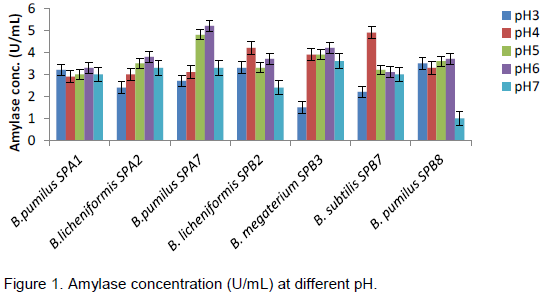
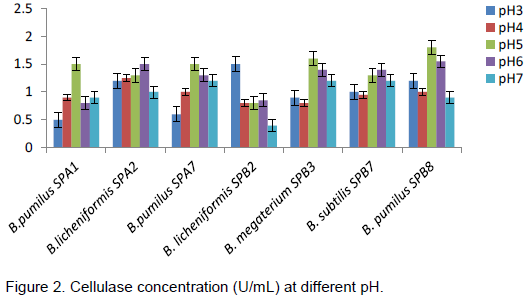
The most predominant bacterial isolates obtained from decaying sweet potatoes peels were identified as B. pumulis, B. licheniformis, B. subtilis and B. megaterium. The prevalence of B. pumulis and B. subtilis isolated in this work conforms to the findings of Lorena et al. (2001) and Madigan et al. (2005) which states that these two organisms are natural inhabitant of soil.
In this study, B. pumilus produced the highest concentration of amylase (5.4 U/mL) at 55°C and pH 6 which was also reported by Andrea et al. (2007) which states that B. pumilus produced amylase between the pH of 5.8 and 7.5 and at a temperature of 55°C. Effect of temperature on amylase production was observed by varying growth temperature of isolates and optimum temperature was found to be 55°C. This findings agrees with the behaviour of amylases from Bacillus spp. isolated from soils as reported by Cordeiro et al. (2003) and Vipal et al. (2011) who reported 50°C as optimum temperature. The effect of temperature on cellulase production was also observed when temperature of the production medium was varied. Cellulase production was highest in the temperature range of 45 - 55°C, with an optimum temperature of 55°C. Similarly, Shaikh et al. (2013) observed that Bacillus sp. produced cellulase optimally at 50°C and affirm that the thermostable property of cellulase has been shown to be of interest for industrial applications. Optimum pH for the production of cellulase by all the organisms used in this study ranged from 5 - 7 with pH 5 been the most predominant. This result was in agreement with the findings of others like Goya and Soni (2011), Azzeddine et al. (2013) and Trinh et al. (2013) who reported pH 5, 6 and 7 respectively as the optimum pH for production of cellulase from Bacillus spp.
This study inferred that decaying sweet potato peels harbour amylolytic and cellulolytic Bacillus species and the enzymes produced by these bacteria can be harnessed for industrial application. Optimum temperature for amylase and cellulase production was 55°C, whereas optimum medium pH for amylase and cellulase was 6 and 5, respectively. B. subtilis and B. pumilus produced the highest concentration of amylase (5.4 U/mL) and cellulase (0.75 U/mL), respectively.
No conflict of interest among the authors.
REFERENCES
Andrea CB, Mana AEW, Aneh B, Launval AV, Ana CB, Robert D, Emanuel MS, Mana HPF (2007). Structural characterization of the bglH gene encoding a beta-glucosidase-like enzyme in an endophytic Bacillus pumilus strain. Genet. Mol. Biol. 30(1):100-104
Crossref |
|
|
|
Aiyer PV (2005). Amylases and their application. Afr. J. of Biotech. 4(8):1525-1529.
|
|
|
|
|
Ariffin H, Abdullah N, Umi K, Shirai Y, Hassan MA (2006). Production and characterisation of cellulase from Bacillus pumilus EB3. Int. J. Eng. Technol. 3:47-53.
|
|
|
|
Azzeddine B, Abdelaziz M, Estelle C, Mouloud K, Nawel B, Nabila B, Francis D, Said B (2013). Optimisation and partial characterisation of endoglucanase produced by Streptomyces sp. B - PNG 23. J. Arch. Biol. Sci. Belgrade 65(2):549-558.
Crossref |
|
|
|
Bahkali AH (1996). Influence of various carbohydrates on xylanase production in Verticillium tricorpus. Bioresour. Technol. 57(3):265-268.
Crossref |
|
|
|
|
Bernfield P (1955). Methods in Enzymology. Ed by Kaplan, M.O and Colowicty, S.P. Academic Press, N.Y.P.149.
|
|
|
|
Cherry JR, Fidants AL (2003). Directed evolution of industrial enzymes: an update. Curr. Opin. Biotechnol. 4:438-443.
Crossref |
|
|
|
|
Cordeiro CAM, Martinas MLL, Lucaino A (2003). Production and properties of alpha amylase from thermophylic Bacillus species. Braz. J. Microbiol. 33:1-3.
|
|
|
|
Giraud EA, Keleke S, Lelory B, Rainbault M (1991). Isolation and physiological study of an amylase strains of Lactobacillus plantarum. Appl. Microbiol. Biotechnol. 36:379-383
Crossref |
|
|
|
|
Goya M, Soni G (2011). Production and characterisation of cellulolytic enzymes by Pleurotus florida. Mycosphere 2. 3:249-254
|
|
|
|
Immanuel G, Dhanusha R, Prema P, Palavesam A (2006). Effect of different growth parameters on endoglucanase enzyme activity by bacteria isolated from coir retting effluents of estuarine environment. Int. J. Environ. Sci. Technol. 3:25-36.
Crossref |
|
|
|
Jarvis M (2003). Cellulose stacks up. Nature 426:611-612.
Crossref |
|
|
|
Jose LA, Arnold LD (2014). Microbial enzymes: Tools for biotechnological processes. J. Biomol. 4:117-139.
Crossref |
|
|
|
|
Lorena DS, Toshihiko S, Kanefumi K, Yoshihito F, Hayao T (2001). Degradation of cell wall materials from sweet potato, cassava and potato by a bacterial protopectinase and terminal sugar analysis of the resulting solubilised products. J. Biosci. Bioeng. 93:64-72.
|
|
|
|
|
Madigan M, Martinko J (2005). Brocks Biology of Microorganisms 11th Ed, Prentice Hall.
|
|
|
|
Magnelli P, Forchiassin F (1999). Regulation of the cellulase complex production by Saccobolus saccoboloides: induction and repression by carbohydrates. Mycologia 91:359-364.
Crossref |
|
|
|
|
Mandel M, Andreotti R, Roche C (1976). Measurement of saccharifying cellulose. Biotechnol. Bioeng. Symp. (6): 21 - 23.
|
|
|
|
Muhammad Irfan, Asma Safdar, Quratulain Syed, Muhammad Nadeem (2012). Isolation and screening of cellulolytic bacteria from soil and optimization of cellulase production and activity. Turk. J. Biochem. 37(3):287 293.
Crossref |
|
|
|
|
Nakamura K, Kappamura K (1982). Isolation and identification of crystalline cellulose hydrolyzing bacterium and its enzymatic properties. J. Ferment. Technol. 60(4):343-348.
|
|
|
|
|
Parmar Dipali, Pandya Ajit (2012). Characterization of amylase producing bacterial isolates. Bull. Environ. Pharmacol. Life Sci. 6:42-47.
|
|
|
|
Sadhu S, Maiti TK (2013). Cellulase production by bacteria: A review. Brit. Microbiol. Res. J. 3:235-258.
Crossref |
|
|
|
|
Shaikh NM, Patel AA, Mehta SA, Patel ND (2013). Isolation and Screening of cellulolytic bacteria inhabiting different environment and optimization of cellulase production. Univ. J. Environ. Res. Technol. 3:39-49
|
|
|
|
Shin CS, Lee JP, Lee JS, Park SC (2000). Enzyme production of Trichoderma reesei rut C-30 on various lignocellulosic substrates. Appl. Biochem. Biotechnol. 84-86:237-245.
Crossref |
|
|
|
|
Sneath PHA, Nair NA, Sharpe ME (1986). Bergeys manual of systematic bacteriology vol. 2. The Williams and Wilkin Co., Baltimore.
|
|
|
|
Sonia Sethi, Aparna Datta, Lal Gupta B, Saksham Gupta (2013). Optimization of cellulase production from bacteria isolated from soil. Hindawi Publishing Corporation, ISRN Biotechnology, Article ID 985685).
Crossref |
|
|
|
Tomme P, Warren RAJ, Gilkes NR (1995). Cellulose hydrolysis by bacteria and fungi. Adv. Microbial Physiol. 37:1-81.
Crossref |
|
|
|
|
Trinh DK, Quyen DT, Do TT, Ndhiem NM (2013). Optimisation of culture conditions and medium components for carboxymethyl cellulase production by a novel Basidiomycete strain Peniophora sp. NDVNO1. Iran. J. Biotechnol. 11:251-259
|
|
|
|
|
Vipul V, Mrigank SA, Abhishek RG, Monika S, Akhilesh K (2011). Amylase production and purification from bacteria Isolated from a waste potato dumpsite in District Farrukhabad U.P State India. Eur. J. Exp. Biol. 3:107-113
|
|
|
|
Zhang YH, Lynd LR (2004). Toward an aggregated understanding of enzymatic hydrolysis of cellulose: Noncomplexed cellulase systems. Biotechnol. Bioeng. 88:797-824
Crossref |
|