Full Length Research Paper
ABSTRACT
Chronic non-communicable diseases are responsible for a large majority of deaths worldwide. These include cardiovascular and respiratory diseases, diabetes and cancer. Inflammation has been reported to be involved in the initiation or progression of these conditions. Populations in developing or low to middle- income countries often rely on traditional medicine using locally available herbs and plants for their medical care. This study examined the anti-inflammatory potential of aqueous extracts of twelve medicinal plants used in Nigeria. The antioxidant activity was estimated using the total radical-trapping antioxidant parameter (TRAP) and ferric reducing ability (FRAP) assays. The abilities to inhibit nuclear factor kappa light chain enhancer of activated B cells (NfkB), a key regulator of the inflammatory response, and to activate nuclear factorE2 related factor 2 (Nrf2), a transcription factor that regulates cellular antioxidant defense systems, were determined using in vitro cell based assays. Extracts of Erythrina senegalensis (leaves), Sclerocarya birrea (bark), Boswellia dalzielli (leaves and bark), Pseudrocedrila kotschyi (bark), Sterculia setigera (stem bark), and Sarcocephalus esculentus (bark) contained the highest levels of antioxidant activity. Extracts that showed the greatest inhibition of NfkB were S. esculentus (bark), 91.8%; E. senegalensis (leaves), 81.4%; S. birrea (stem bark), 77.5%; and S. setigera (stem bark), 75.5%. B. dalzielli (leaves) and Xylopia aethiopica (leaves) gave 7.4 and 7.7 fold activation of Nrf2, respectively. These were comparable to activation by sulphorophane.
Key words: NfkB inhibition, Nrf2 activation, antioxidant activity, inflammation, medicinal plants, Nigeria.
INTRODUCTION
According to the World Health Organization, more than 70% of deaths worldwide can be attributed to chronic non-communicable diseases. These include cardiovascular diseases, cancer, respiratory diseases, and diabetes (WHO, 2018; Unwin and Albert, 2006). The majority of these deaths occur in developing and low- to middle-income countries where people rely on traditional medicine for their everyday health care (WHO, 2019). Inflammation is reported to play a role in the initiation and/or progression of many of these conditions (Kosmas et al., 2019; Christodoulidis et al., 2014; Kundu and Surh, 2008; Fernandes et al., 2015; Wang et al., 2018; Shaikh et al., 2019; Tsalamandris et al., 2019;Baker et al., 2011).
Inflammation is initiated by the innate immune system in response to infection or tissue injury. Activated macrophages and neutrophils release reactive oxygen species (ROS) such as superoxide, hydrogen peroxide, nitric oxide, and pro-inflammatory cytokines that include TNFα (Abbas et al., 2012). Both ROS and TNFα activate NFκB, a proinflammatory transcription factor that is a critical regulator of the inflammatory response (Liu et al., 2017; Kunnumakkara et al., 2020).NFκB regulates a large number of genes involved in processes of the immune and inflammatory responses as well as the expression of enzymes involved in the generation of ROS including NADPH oxidase (Anrather et al., 2006), NO synthase (Li et al., 2007), cyclooxygenase (Deng et al., 2003), xanthine oxidase (Xu et al., 1996), and phospholipase A2 (Schutze et al., 1992; Szymczak-Pajor et al., 2020).
Although mounting an immune response to infection or injury is essential to life, unregulated production of ROS may result in chronic inflammation. The generation of ROS beyond cellular antioxidant capacity can damage DNA, proteins, lipids and cell membranes (Farber, 1994) and result in the initiation of various chronic diseases. Balancing the production of ROS with their removal is attained by activating cellular antioxidant defense systems. Nrf2 is the primary transcription factor that regulates the synthesis of various detoxification enzymes and proteins such as glutathione-S transferases, NADPH: quinone oxidoreductaase, gamma-glutamylcysteine synthase, ferritin, and heme oxygenase (Chen and Kunsch, 2004). Inhibiting NFκB and increasing Nrf2 activity in the occurrence of excessive ROS production may provide a therapeutic approach to treating chronic inflammation (Chen and Kunsch, 2004; Gupta et al., 2010; Zhang et al., 2017; Sivandzade et al., 2019).
Although steroids and other non-steroidal drugs are commonly used for treating inflammatory disorders, populations in many low- income and developing countries rely on traditional medicine for their health care because conventional pharmaceuticals are costly or may not be accessible (WHO, 2019; Oyebode et al., 2016; James et al., 2018). Traditional medicine practitioners commonly use infusions or decoctions made from plants available in their area (Gurib-Fakim, 2006). Plant extracts contain antioxidants and other phytochemicals that have been demonstrated to affect various transcription factors and cytokines involved in the inflammatory process (Spelman et al., 2006; Talhouk et al, 2007; Orlando et al., 2010; Ghosh et al., 2016; Qin and Hou, 2016).
It is estimated that more than 80% of Nigerians rely on traditional healers and herbal remedies made from whole plants or plant parts such as leaves, bark, and roots (Odugbemi, 2006). The present study aimed to determine the antioxidant capacity of aqueous extracts of 12 plants commonly used by traditional medical practitioners in Nigeria. The ability of these preparations to inhibit NFκB and activate Nrf2 activities in cell-based assays was also determined.
MATERIALS AND METHODS
Specimen collection
Plants used for medicinal purposes were collected in the Babale Ward in the Jos North Local Government area of Plateau State, Nigeria. Plants were identified and authenticated by Dr. M. Adul-Kareem from the Department of Horticulture at the Federal College of Forestry, Plateau State, Nigeria. Voucher specimens were deposited at the Herbarium of the Federal College of Forestry at the same location.
After removing dirt and debris by rinsing with water, the plants were in the shade. The plants were separated into parts (leaves, stem bark, bark). Some specimens were analyzed in the whole plant form. Samples were transported to Albuquerque, NM, USA for analyses. Prior to analyses, specimens were ground to a fine powder in a stainless steel grinder and dried in a vacuum desiccator to constant weight. A list of the plant materials collected for analyses and some of their common uses are presented in Table 1.
Preparation of aqueous extracts
Aqueous plant extracts were prepared by adding 10 ml of distilled water to 0.5 g of sample in a 16 × 150 mm Pyrex glass tube. After vortexing, samples were heated at 80°C for 30 min. The samples were then centrifuged (Fisherbrand 614 series centrifuge, Fisher Scientific, Waltham, MA, USA)at 1200 g for 10 min to clarify the extract. The clarified samples were filter-sterilized and stored at 4°C in the dark until analyzed or at -70° for longer term storage.
Antioxidant capacity determination
The term antioxidant includes substances with different mechanisms of action. Since no single assay can recognize all the different types of antioxidants, the antioxidant capacity of the aqueous extracts was determined by two different methods (Schleiser et al., 2002; Huang et al., 2005): the total radical-trapping antioxidant parameter assay (TRAP) (Re et al., 1999) and the ferric reducing ability assay (FRAP) (Benzie and Strain, 1999). The TRAP assay measures the ability of antioxidants in the sample to interfere with the reaction between peroxyl radicals and a target probe. It is useful for determining the content of non-enzymatic antioxidants, such as glutathione and ascorbic acid. The FRAP assay reflects the electron reducing power of antioxidants (Moon and Shibamoto, 2009).
TRAP assay
For the TRAP assay, 2, 2’-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) (Sigma Aldrich, St. Louis. MO, USA) was reacted with potassium persulfate in the dark, overnight, to generate the colored ABTS. + radical cation, which has an absorption maximum at 734 nm. The activity of the plant preparations was determined by their ability to quench the color of the radical cation. Duplicate 10 µl aliquots of the extracts were used for analysis. Dilutions of the sample extracts were madcorrespond to the standard curve generated using Trolox (6-hydroxy-2, 5, 7, 8 tetramethylchroman-2-carboxylic acid (Sigma Aldrich, St. Louis, MO, USA) as the reference material. Duplicate measurements were made and given as the average. Results are expressed as the mmol Trolox equivalents/ml aqueous extract.
FRAP assay
For the FRAP assay, the ferric complex of 2,4,6–tripyridyl-s-triazine was prepared at acidic pH, and the anti-oxidant activities of the plant preparations were determined by their abilities to reduce the ferric complex to the ferrous complex, monitored by formation of the ferrous complex at 593 nm. Results are expressed as the mmol
Trolox equivalents/ml aqueous extract.
Inhibition of NFκB activation
An NFkB reporter stable cell line derived from human 293T embryonic kidney cells (293T/NFκB-luc) (Panomics, Inc. Redwood City, CA) was grown in a humidified atmosphere at 37°C in 5% CO2/95% air. The cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM-high glucose containing 4 mM glutamine) supplemented with 10% fetal bovine serum (FBS), 1 mM sodium pyruvate, 100 units/ml penicillin, 100 µg/ml streptomycin and 100 µg/ml hygromycin (Gibco/Invitrogen, Carlsbad, CA) to maintain cell selection. One day before treatment with the plant extracts, the 293T/NFkB-luc cells were plated into 24-well cell culture plates (Costar, Cambridge, MA) at approximately 70% confluency in the above media without hygromycin. The following day cells were fed fresh media 1 h prior to treatment. Media, with or without recombinant tumor necrosis factor (TNFα, 2ng/ml) (R&D Biosciences/clontech, Palo Alto, CA), was then applied to the cells followed by immediate addition of 50µL plant extracts. The cells were then placed in a humidified atmosphere in 5% CO2/95% air for 7 h. Plate wells were gently washed with 1x passive lysis buffer (Promega, Madison, WI). The subsequent lysates were analyzed using the Luciferase Assay System (Promega) utilizing a TD-20/20 luminometer (Turner Designs, Sunnyvale, CA). The firefly luciferase relative light units were normalized to protein (mg/ml) with BCA Protein Assay Kit (Pierce, Rockford, Il) and standardized to percent of control (TNFα control). Results (percent of control) are given as the average of duplicate analyses.
Activation of Nrf2
As previously described (Deck et al., 2017), a Nrf2-ARE reporter-Hep G2 stable cell line (BPS Bioscience, San Diego, CA, USA) was grown in a humidified atmosphere at 37°C in CO2 (5%)/air (95%). The cells were maintained in MEM medium with Earles balanced salts and L-glutamine (Gibco, Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS), 1 mM sodium pyruvate, 1% non-essential amino acids, penicillin (100 units/ml), streptomycin (100 µg/ml), and Geneticin (400 µg/ml) to maintain cell selection.
One day prior to treatment, the Nrf2-ARE cells were plated into 24-well cell culture plates (costar, Cambridge, MA, USA) at approximately 30% confluency in the above media without Geneticin. The following day, fresh media with or without sulforaphane (15 µM) was added to appropriate wells as controls. Plant extracts (50 µl) were then applied to the sample wells. The cells were again placed in a humidified atmosphere at 37°C, CO2 (5%), air (95%) for 24 h. Cells were washed with phosphate buffered saline (PBS), pH 7.4, and lysed with 1x passive lysis buffer (Biotium, Hayward, CA, USA). The lysates were analyzed using the Luciferase Assay System (Biotium, Hayward, CA, USA) and a E-5311 GloMax 20/20 luminometer (Promega, Sunnyvale CA, USA). The firefly luciferase relative light units were normalized to protein (mg/ml) determined using the BCA Protein Assay Kit (Pierce, Rockford, IL, USA) and then standardized to percent of control activation.
Plant extract toxicity
Plants commonly used in traditional medicine are assumed to be safe of acute toxicity based on their long-term usage. However, some recent studies have shown that many plants may contain toxic, mutagenic, or carcinogenic compounds (Letsyo et al., 2017; Mounanga et al., 2015; Valdivia-Correa et al., 2016). For determination of cell viability in the presence of the plant extracts, 293T/NFκB-luc cells were prepared as described above and then plated at approximately 5,000 cells/well in a 96-well plate. Triplicate plant extracts, 10 µl in 100 µl media, were then added to the wells. Appropriate dilutions of the plant extracts were made to equal the amount used in the NFκB inhibition analyses. After incubating for 6 h, 10 µl WST-1 Cell Proliferation Reagent (Roche Applied Science, Indianapolis, IN, USA) was added and the cells were incubated as described above for 45 min. The WST-1 dye is reduced by metabolically active cells and was quantified 450 nm using a Spectromax plate reader (Molecular Devices Co., Sunnyvale CA, USA). Results are expressed as the percent absorption of control (untreated) cells
RESULTS
Antioxidant activity
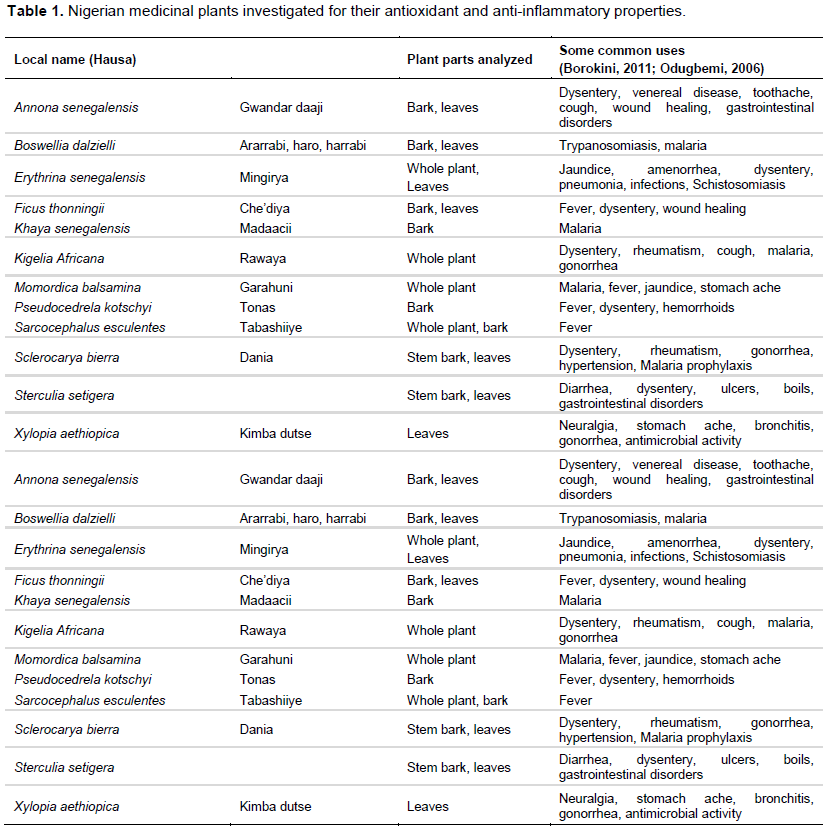
In the TRAP assay, Erythrina senegalensis (leaves) and Sclerocarya birrea (bark) exhibited antioxidant activities approximately five times higher than the following two highest plants: Boswellia dalzielli (both leaves and bark) and Pseudocedrela kotschyi (bark) (Figure 1). Sterculia setigera (stem bark) and Sarcocephalus esculentus (bark) had the next highest activities.
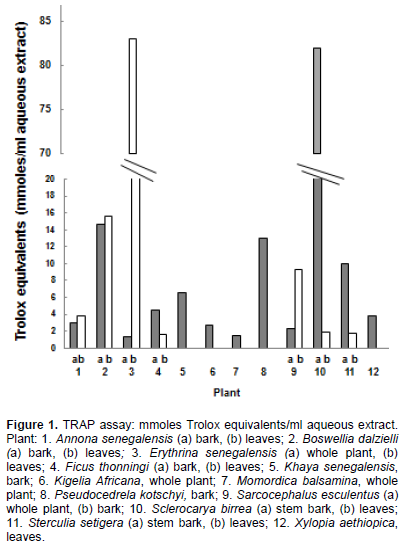
In the FRAP assay, E. senegalensis (leaves), P. kotschyi (bark), and S. birrea (bark) had the highest activity, followed by B. dalzielli (bark and leaves) (Figure 2).
NFkB inhibition
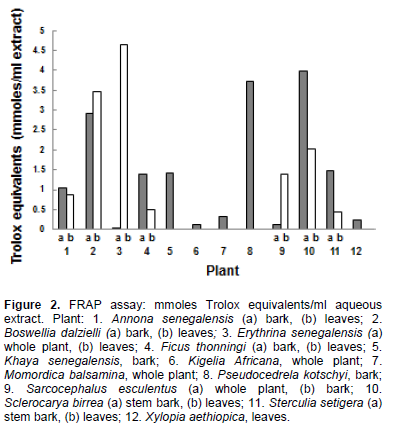
The inhibition of NFκB activation by the plant extracts in the presence of TNFα is shown in Figure 3. Plant extracts that inhibited the activation by more than 50% are: S. esculentus (bark) 91.8%; E. senegalensis (leavesl) 81.4%; S. bierra (stem bark) 77.5%; S. setigera (stem bark) 75.5%; Khaya senegalensis (bark) 62.4%; and Annona senegalensis (bark) 53.1%. The remainder of the plant extracts gave less than 50% inhibition.
.png)
Activation of Nrf2
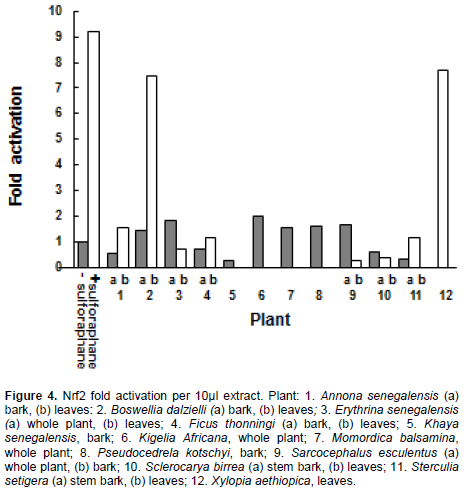
Two plant preparations gave activation of Nrf2 comparable to sulphorophane, a known promoter of Nrf2 activity (Keum, 2011) (Figure 4). Extracts of B. dalzielli (leaves) and Xylopia aethiopica (leaves) demonstrated a 7.4 and 7.7 fold increase in Nrf2 over baseline (no sulphorophane), respectively. These activities were equal to 83 and 81% of the sulphorophane activation.
Correlation of antioxidant capacity and NFκB inhibition
The antioxidant capacity as determined by the TRAP assay was significantly correlated with NFkB inhibition (p=0.002, r =-0.64). A significant relation was also observed between antioxidant capacity determined by the FRAP assay and NFκB inhibition (p=0.008, r= -0.569). Correlation between the FRAP and TRAP antioxidant activities was also found (p=0.001, r=0.89).
Plant extract toxicity
293TN/NFκB cells treated with plant extracts had 58 to 100% viability compared to control (untreated cells): S. birrea (stem bark) 58%; S. esculentus (bark) 65%; E. senegalensis (leaves) 68%; Annona senegalensis (bark) 70%; Pseudocedrela kotschyi (bark) 71%; Annona senegalensis (leaves) 76%; Khaya senegalensis (bark) 80%; S. setigera (stem bark) 76%; Khaya senegalensis (bark) 80%; Ficus thonningii (bark) 89%; B. dalzielli (bark) 84%; S.birrea (leaves) 90%; X. aethiopica (leaves) 92%; B.dalzielli (leaves) 98%; S. setigera (leaves) 100%; Momordica balsamina (whole plant) 100%; Kigelia africana (whole plant) 100%; Ficus thonningii (leaves) 100%; E. senegalensis (whole plant ) 100%; S. esculentus (whole plant) 100%.
DISCUSSION
Aqueous extracts of several of the Nigerian medicinal plants examined in this study, namely E. senegalensis (leaves), S. birrea (stem bark), S. esculentus (bark), S. setigera (stem bark), B. dalzielli (bark), and X. aethiopica (leaves) inhibited the pro-inflammatory NFκB signaling pathway or activated the antioxidant NrF2 signaling pathway.
E. senegalensis (leaves) had the highest antioxidant activity of the plants tested and was also a promising inhibitor of pro-inflammatory NFkB activation (81.4%). A spiny tree found in west tropical Africa, it is commonly used as an ornamental shrub (Tropical Plants Database, 2020). Decoctions of E. senegalensis leaves are traditionally used for treating wounds, various fevers, gastrointestinal disorders, and infertility in women (Togola et al., 2005). An aqueous leaf extract was reported to provide a protective effect against oxidative liver damage in rats exposed to CCl4 (Wakawa and Hauwa, 2013). In an in vivo study using the egg- albumin induced acute edema model, an aqueous extract of the stem bark demonstrated significant analgesic and anti-inflammatory activity in rats (Saidu et al., 2000). An ethanol extract of Erythrina senegalensis leaves was also reported to have wound healing activity in albino rats (Ilodigwe et al., 2014). Various phytochemicals have been isolated from the plant and may provide some of the anti-inflammatory and antioxidant activities observed (Kone et al., 2011).
S. birrea is a medium –size deciduous tree found in the semi-arid and savannah regions of sub-Saharan Africa (National Research Council, 2008) and its fruit is a traditional African food. Preparations from various parts of the tree are widely used throughout Africa for treating dysentery, diarrhea, indigestion, fungal infections, snake bite, and diabetes (Ojewole et al., 2010). In the present study, the aqueous bark extract had antioxidant levels comparable to Erythrina senegalensis and was also a good inhibitor of NFκB activation (77.5%). In an in vivo test of anti-inflammatory activity, both aqueous and methanol extracts of the stem bark were reported to reduce rat paw edema induced by egg albumin in Wistar rats (Ojewole, 2003; Fotio et al., 2009). Analyses of the stem bark and leaves of S. birrea demonstrated the presence of various phytochemicals such as phenols, flavonoids and flavonols that could contribute to the antioxidant and anti- inflammatory properties (Tanih and Ndip, 2012; Braca et al., 2003).
The bark of the S. esculentus, widely found throughout West Africa (Dalziel, 1937, a) showed the most promising inhibition of NFκB activation of the extracts investigated in this study (91.8%). Bark preparations are used for fevers, indigestion related halitosis, vomiting, and toothache. Otimenyin and co-workers (Otimenyin et al., 2008) reported that aqueous extracts of the roots of S. esculentus inhibited egg-albumin induced rat paw edema and acetic acid-induced writhing in mice in a dose-dependent manner.
The two plants in this study that exhibited activation of Nrf2 comparable to sulforophane were B. dalzielli (bark) and X. aethiopica (leaves). B. dalzielli, found in northern Nigeria and the West African savannah (Dalziel, 1937b), is a species of the frankincense tree. Extracts of the leaf are used for diarrhea and extracts of the stem bark are commonly used for fever, rheumatism, and gastrointestinal disorders. (Mbiantcha et al., 2018) reported that a methanol extract of the stem bark had an anti-inflammatory effect in several models of rat paw edema including the carrageenan, arachidonic acid, histamine and bradykinin models (Mbiancha, 20018). The extract also significantly reduced the production and release of ROS from isolated human polymorphonuclear cells and mouse peritoneal macrophages and decreased the production of the inflammatory cytokines TNFα and IL-1b. In another study of the anti-inflammatory properties of the B. dalzielli stem bark, Kafuti et al. (2019) reported that the methanol and aqueous fractions of the extract had significant antioxidant activity measured by both a radical scavenging assay and the FRAP assay. These results are in agreement with those obtained in our study using an aqueous preparation.
X. aethiopica, an aromatic evergreen plant that is widespread in West Central and Southern Africa, is used for the treatment of fever, cough, dyspepsia, skin infections and muscular and rheumatic pain (Dalziel, 1937). An ethanol/water extract of the leaves was reported to decrease TNFα and IL-6 levels in LPS activated THP-1 derived macrophages, suggesting its usefulness as an anti-inflammatory agent (Macedo et al., 2020). Moukette et al., (2015) tested ethanol and ethanol/water extracts of the X. aethiopica bark in liver homogenates and found that both extracts exhibited antioxidant activity in both free radical scavenging and the FRAP assays. The present study used the unfractionated aqueous extract of the plants and did not identify individual components. A variety of compounds may be present in plant extracts depending on the part of the plant used and the solvent used for preparing the extract. Constituents may include various vitamins (e.g., vitamin C, vitamin E, carotenoids) and other phytochemicals such as polyphenols. This may allow synergistic effects of multiple components to contribute to the antioxidant and anti-inflammatory effects observed (Gilbert and Alves, 2003).
When using plant preparations for treatment, several factors need to be considered: the bioavailability of the active components, the co-extraction of toxic compounds, and interference in the efficacy of conventional pharmaceuticals when used simultaneously with the herbal preparations. Polyphenols occur in multiple forms including glycosides and polymers. Depending on the particular polyphenol, the compounds must be acted on by intestinal enzymes prior to absorption by enterocytes and then modified by the liver prior to export into the circulation. The rate of absorption has been shown to be dependent on the structure of the polyphenol (Kawabata et al., 2019; Silberberg et al., 2006). Herbal preparations may also interfere with the effectiveness of conventional pharmaceuticals by causing alterations in gastrointestinal function affecting absorption, inhibiting or inducing enzymes involved in metabolism, or affecting the renal excretion of drugs or their metabolites (Fasinu et al., 2012).
In addition to beneficial components, plants may also contain toxic compounds. For example, Letsyo and coworkers analyzed 70 herbal medicine products commonly used in Ghana and other West African countries and reported that 60% of them contained pyrrolizidine alkaloids (Letsyo et al., 2017).These are present as a defense against herbivores and occur predominantly as N-oxides. They show little or no toxicity in that form but are metabolized by the liver to toxic tertiary pyrrolizidine alkaloids. All of the plant extracts examined in this study gave viability of 65% or greater in the cell toxicity tests.
In summary, aqueous extracts of the plants examined in this study showed anti-inflammatory activity by various mechanisms such as the inhibition of the pro-inflammatory NfkB signaling pathway or the activation of the Nrf2 antioxidant signaling pathway in in vitro assays. Further studies should include the identification of active components present in these extracts and the determination of their bioavailability in vivo.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Abbas AK, Lichtman AH, Pillai S (2012). Cellular and Molecular Immunology 7th edition. Elsevier Saunders, Philadelphia. |
|
|
Anrather J, Racchumi G, Iadecola C (2006). NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. Journal of Biological Chemistry 281(9):5657-5667. |
|
|
Baker RG, Hayden MS, Ghosh S (2011). NF-kB, inflammation, and metabolic disease. Cell Metabolism 13(1):11-22. |
|
|
Benzie IF, Strain JJ (1999). Ferric reducing/antioxidant power assay: direct measurement of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods in Enzymology 299:15-27. |
|
|
Borokini T (2011). Conservation and Utilization of Medicinal Plants in Nigeria. Lambert Academic Publishing GMBH & Co.KG; Berlin, 2011. |
|
|
Braca A, Politi M, Sanogo R, Sanou H, Morelli I, Pizza C, de Tommasi N (2003). Chemical composition and antioxidant activity of phenolic compounds from wild and cultivated Sclerocarya birrea (Anacardiaceae) leaves. Journal of Agriculture and Food Chemistry 51(23):6689-6695. |
|
|
Chen XL, Kunsch C (2004). Induction of cytoprotective genes through Nrf2/antioxidant response element pathway: a new therapeutic approach for the treatment of inflammatory diseases. Current Pharmaceutical Design 10(8):879-891. |
|
|
Christodoulidis G, Vittoria TJ, Fudim M, Lerakis S, Kosmas CE (2014). Inflammation in coronary artery disease. Cardiology in Review 22(6):279288. |
|
|
Dalziel JM (1937). The useful plants of West Tropical Africa. The Crown Agents for the Colonies, London pp. 552-560. |
|
|
Deck LM, Whalen LJ, Hunsaker LA, Royer RE, Vander Jagt D (2017). Activation of anti-oxidant Nrf2 signaling by substituted trans stilbenes. Bioorganic and Medicinal Chemistry 25(4):1423-1430. |
|
|
Deng WG, Zhu Y, Wu KK (2003). Up-regulation of p300 binding and p50 acetylation in in tumor necrosis factor-alpha-induced cyclooxygenase-2 promoter activation. Journal of Biological Chemistry 278(7):4770-4777. |
|
|
Farber J (1994). Mechanism of cell injury by activated oxygen. Environmental Health Perspectives 102(suppl 10):17-24. |
|
|
Fasinu PS, Bouic PJ, Rosenkranz B (2012). An overview of the evidence and mechanisms of herb-drug interactions. Frontiers in Pharmacology 3:69. |
|
|
Fernandes JV, Cobucci RN, Jatoba CA, Fernandes TA, de Azevedo JW, de Araujo JM (2015). The role of the mediators of inflammation in cancer development. Pathology &Oncology Research 21(3):527-534. |
|
|
Fotio AL, Dimo T, Nguelefack TB, Dzeufiet PD, Ngo Lemba E, Temdie RJ, Ngueguim F, Olleros ML, Vesin D, Dongo E, Kamtchouing P, Garcia I (2009). Acute and chronic anti-inflammatory properties of the stem bark aqueous and methanol extracts of Sclerocarya birrea. Inflammopharmacology 17(4):229-237. |
|
|
Ghosh N, Ali A, Ghosh R, Das S, Mandal SC, Pal M (2016). Chronic inflammatory diseases: progress and prospect with herbal medicine. Current Pharmaceutical Design 22(2):247-264. |
|
|
Gilbert B, Alves LF (2003).Synergy in plant medicines. Current medicinal chemistry 10(1):13-20. |
|
|
Gupta SC, Sundaram C, Reuter S, Aggarwal BB (2010). Inhibiting NF-kappaB activation by small molecules as a therapeutic strategy. Biochimica Biophysica Acta 1799(10-12):775-787. |
|
|
Gurib-Fakim A (2006). Medicinal plants: traditions of yesterday and drugs of tomorrow. Molecular Aspects of Medicine 27(1):1-93. |
|
|
Huang D, Ou B, Prior RL (2005). The chemistry behind antioxidant capacity assays. Journal of Agricultural Food Chemistry 53(6):1841-1856. |
|
|
Ilodigwe E, Okonkwo BE, Agbata CA, Ajaghaku DL, Eze PM. (2014). Wound healing activity of ethanol leaf extract of Erythrina senegalensis. British Journal of Pharmaceutical Research 4:531-540. |
|
|
James PB, Wardle J, Steel A, Adams J (2018). Traditional, complementary and alternative medicine use in Sub-Saharan Africa: a systematic review. British Medical Journal Global Health 3(5):e000895. |
|
|
Kafuti YS, Alemika TE, Ojerinde OS, Balogun O, Alamika TE, Taba KM, Mpiana PT, Kindombe NM (2019). Antioxidant and antiproliferative activities of the stem bark extract and fractions of Boswellia dalzielii Hutch. International Journal of Pharmacognosy and Phytochemical Research 11(3):177-182. |
|
|
Kawabata K, Yoshioka Y, Terao J (2019). Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules 24(2):370-395. |
|
|
Keum YS (2011). Regulation of the Keap1/Nrf2 system by chemopreventive sulforaphane: implications of posttranslational modifications. Annals of the New York Academies of Science 1229(1):184-189. |
|
|
Kone WM, Solange KN, Dosso M (2011). Assessing Sub-Saharan Erythrina for efficacy: Traditional uses, biological activities and phytochemistry. Pakistan Journal of Biological Science 14(10):560-571. |
|
|
Kosmas CE, Silverio D, Sourlas A, Montan PD, Guzman E, Garcia M (2019). Anti-inflammatory therapy for cardiovascular disease. Annals of Translational Medicine 7(7):147-153. |
|
|
Kundu JK, Surh YJ (2008). Inflammation: gearing the journey to cancer. Mutation Research Review 659(1-2):15-30. |
|
|
Kunnumakkara AB, Shabnam B, Girisa S, Harsha C, Banik K, Devi TB, Choudhury R, Sahu H, ParamaD, Sailo B, Thakur KK, GuptaSC, Aggarwal BB. (2020). Inflammation, NF-kB, and chronic diseases: how are they linked? Critical Review of Immunology 40(1):1-39. |
|
|
Letsyo E, Jerz G, Winterhalter P, Beuerle T (2017). Toxic pyrrolizidine alkaloids in medicines commonly used in Ghana. Journal of Ethnopharmacology 202:154-161. |
|
|
Li Y, Zhao Y, Li G, Wang J, Li W, Lu J (2007). Regulation of neuronal nitric oxide synthase exon 1f gene expression by nuclear factor-kappaB acetylation in human neuroblastoma cells. Journal of Neurochemistry 101(5):1194-1204. |
|
|
Liu T, Zhang L, Joo D, Sun S-C (2017). NF-kB signaling in inflammation. Signal Transduction and Targeted Therapy 2:17023. |
|
|
Macedo T, Ribeiro V, Oliveira AP, Pereira DM, Fernandes F, Gomes NGM, Araujo L, Valentao P, Andrade PB (2020). Anti-inflammatory properties of Xylopia aethiopica leaves: Interference with pro-inflammatory cytokines in THP-1-derived macrophages and flavonoid profiling. Journal of Ethnopharrmacolgy 248:112312. |
|
|
Mbiantcha M, Almas J, Atsamo AD, Ateufack G, Shabana SU, Tatsinkou DF, Yousseu Nana W, Nida D (2018). Anti-inflammatory and anti-arthritic effects of methanol extract of the stem bark of Boswellia dalzielli Hutch (Burseraceae). Inflammopharmacology 26(6):13383-1398. |
|
|
Moon J-K, Shibamoto T (2009). Antioxidant assays for plant and food components. Agriculture and Food Chemistry 57(5):1655-1666. |
|
|
Moukette BM, Pieme CA, Nya Biapa PC, Ngogang JY (2015). In vitro antioxidant and anti-lipoperoxidative activities of bark extracts of Xylopia aethiopica against ion-mediated toxicity on liver homogenates. Journal of Complementary and Integrative Medicine 12(3):195-204. |
|
|
Mounanga MB, Mewono L, Angone SA (2015). Toxicity studies of medicinal plants used in sub-Saharan Africa. Journal of Ethnopharmacology 174:618-627. |
|
|
National Research Council (2008). "Marula" in Lost Crops of Africa: Volume III: Fruits. Lost Crops of Africa 3 Washington, DC. National Academies Press P 117. |
|
|
Odugbemi T (2006). Outlines and Pictures of Medicinal Plants from Nigeria., University of Lagos Press. |
|
|
Ojewole JA, Mawoza T, Chiwororo WD, Owira PM (2010). Sclerocarya birrea (A. Rich) Hochst. ['Marula'] (Anacardiaceae): A review of its phytochemistry, pharmacology and toxicology and its ethnomedicinal uses. Phytotherapy Research 24(5):633-639. |
|
|
Ojewole JAO (2003). Evaluation of the anti-inflammatory properties of Sclerocarya birrea (A.Rich.) Hochst. (family: Anacardiaceae) stem-bark extracts in rats. Journal of Ethnopharmacology 85(2-3):217-220. |
|
|
Orlando RA, Gonzales AM, Hunsaker LA, Franco CR, Royer RE, Vander Jagt DL, VanderJagt DJ (2010). Inhibition of nuclear factor kB activation and cyclooxygenase-2 expression by aqueous extracts of Hispanic medicinal herbs. Journal of Medicinal Food 13(4):888-895. |
|
|
Otimenyin SO, Uguru MO, Auta A (2008). Anti-inflammatory and analgesic activities of Cassia goratensis and Sarcocephalus esculentus extracts. Journal of Herbs, Spices and Medicinal Plants 13(2):59-67. |
|
|
Oyebode O, Kandala NB, Chilton PJ, Lilford RJ (2016). Use of traditional medicine in middle-income countries: a WHO-SAGE study. Health Policy Plan 31(8):984-991. |
|
|
Qin S, Hou D-X (2016). Multiple regulations of Keap1/Nrf2 system by dietary phytochemicals. Molecular Nutrition Food Research 60(8):1731-1755. |
|
|
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999). Antioxidant activity applying an improved ABTS radical cation decolonization assay. Free Radical Biology and Medicine 26(9-10):1231-1237. |
|
|
Saidu K, Onah J, Orisadipe A, Olusola A, Wambebe C, Gamaniel K (2000). Antiplasmodial, analgesic, and anti-inflammatory activities of the aqueous extract of the stem bark Erythrina senegalensis. Journal of Ethnopharmacology 71(1-2):275-280. |
|
|
Schleiser K, Harwat M, Bohm V, Bitsch R (2002) Assessment of antioxidant activity by using different in vitro methods. Free Radical Research 36(2):177-187. |
|
|
Schutze S, Machleidt T, Kronke M (1992). Mechanisms of tumor necrosis factor action. Seminars in Oncology 19(2 suppl 4):16-24. |
|
|
Shaikh SB, Prabhu A, Bhandary YP (2019). Interleukin-17A a potential therapeutic target in chronic lung diseases. Endocrine, Metabolic &Immune Disorders- Drug Targets 19(7):921-928. |
|
|
Silberberg M, Morand C, Mathevon T, Besson C, Manach C, Scalbert A, Remesy C (2006). The bioavailability of polyphenols is highly governed by the capacity of the intestine and of the liver to secrete conjugated metabolites. European Journal of Nutrition 45(2):88-96. |
|
|
Sivandzade F, Prasad S, Bhalerao A, Cucullo L (2019). Nrf2 and NF-kB interplay in cerebrovascular and neurodegenerative disorders: Molecular mechanisms and possible therapeutic approaches. Redox Biology 21:101059. |
|
|
Spelman K, Burns J, Nichols D, Winters N, Ottersberg S, Tenborg M (2006). Modulation of cytokine expression by traditional medicines: a review of herbal immunomodulators. Alternative Medicine Review 11(2):128-150. |
|
|
Szymczak-Pajor I, Kleniewska P, Wieczfinska J, Pawliczak R (2020) Wide-range effects of 1,25(OH)2D3 on group 4A phospholipases is related to nuclear factor k-B and phospholipase-A2 activating protein activity in mast cells. International Archives of Allergy and Immunology 181(1):56-70. |
|
|
Talhouk RS, Karam C, Fostok S, El-Jouni W, Barbour EK (2007). Anti-inflammatory bioactivities in plant extracts. Journal of Medicinal Food 10(1):1-10. |
|
|
Tanih NF, Ndip RN (2012). Evaluation of the acetone and aqueous extracts of mature stem bark of Sclerocarya birrea for antioxidant and antimicrobial properties. Evidence-Based Complementary and Alternative Medicine 834156. |
|
|
Togola A, Diallo D, Dembele S, Barsett H, Paulsen BS (2005). Ethnopharmacological survey of different uses of seven medicinal plants from Mali, (West Africa) in the regions Doila, Kolokani and Silby. Journal of Ethnobiology and Ethnomedicine 1:7. |
|
|
Tropical Plants Database (2020). Ken Fern.tropical. the ferns.info. 2020-02-03. |
|
|
Tsalamandris S, Antonopoulos AS, OIkonomou E, Papamikroulis GA, Vogiatzi G, Papaioannoou S, Deftereos S, Tousoulis D (2019). The role of inflammation in diabetes: Current concepts and future perspectives. European Cardiology Review 14(1):50-59. |
|
|
Unwin N, Albert KG. (2006). Chronic non-communicable diseases. Annals of Tropical Medicine and Parasitology 100(5-6):455-64. |
|
|
Valdivia-Correa B, Gomez-Guttierrez C, Uribe M, Mendez-Sanchez N. (2016). Herbal Medicine in Mexico: a cause of hepatotoxicity, A critical review. International Journal of Molecular Sciences 17(2):235-244. |
|
|
Wakawa HY, Hauwa M (2013). Protective effect of Erythrina senegalensis (DC) leaf extract on carbon tetrachloride-induced liver injury in rats. Asian Journal of Biological Science 6(4):234-238 (20130501). |
|
|
Wang Y, Xu J, Meng Y, Adcock IM, Yao X (2018). Role of inflammatory cells in airway remodeling in COPD. International Journal of Chronic Obstructive Pulmonary Disease 13:3341-3348. |
|
|
World Health Organization (WHO) (2018). |
|
|
World Health Organization (WHO) (2019) WHO global Report on traditional and complementary medicine. |
|
|
Xu P, Huecksteadt TP, Hoidal JR (1996). Molecular cloning and characterization of the human xanthine dehydrogenase gene (XDH). Genomics 34:173-180. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0