Full Length Research Paper
ABSTRACT
In this study, chitosan-coatings were prepared with acetic, lactic, propionic, gallic and caffeic acids and used for coating chicken and quail eggs to understand their effect on the quality and shelf-life of chicken and quail eggs. Shelf-life study of (weight loss, Haugh unit, yolk index, albumen pH, mineral levels and shell breaking strength) the coating formulations were investigated for 4 weeks. All chitosan coated chicken and quail egg samples showed greater interior (weight loss, Haugh unit, yolk index, albumen pH) and exterior quality (shell breaking strength) than non-coated samples (p<0.05).
Key words: Chitosan, phenolic substances, chicken egg, quail egg, shelf-life.
INTRODUCTION
For the past decades, in food packaging, the manufacturing and use of plastic films have grown quickly resulting in serious environmental concerns as resistance to degradation (Muscat et al., 2012). Consumers preferred biodegradable materials in terms of reducing the environmental difficulties related with food packaging. Biopolymer polymers as a raw material for food packaging and preservation have been the subject of research (Persin et al., 2011). Because of its ability to minimize moisture and scent loss, solute movement, water absorption in the food matrix, and oxygen penetration, edible and biodegradable films could be applied as a substitute for synthetic packaging materials (Aider, 2010).
Researches and developments in active food packaging have been focused on bio-based functionalpackaging materials incorporating natural active compounds and ingredients (Leceta et al., 2013; van den Broek et al., 2015; Madureira et al., 2015). Chitosan is a cationic linear polysaccharide with a variable degree of N-acetylation derived from chitin and is widely used in agriculture, food, biomedicine and environmental industry due to the positive charges on its amino groups and the presence of other multiple functional groups (Ding et al., 2014; Junter et al., 2016). Chitosan is the second most abundant biopolymer in nature. It has been determined to have significant film-forming abilities as well as other advantageous properties such as biodegradability, biocompatibility, low oxygen permeability coefficients, good mechanical properties, mucoadhesiveness, and derivability from low-cost biomass (Wu et al., 2013; Szymanska and Winnicka, 2015). In addition, chitosan has a great potential to be used as film material due to its non-toxicity, low permeability to oxygen, biocompatibility and excellent film forming ability under acidic conditions (Bonilla et al., 2014), also antimicrobial and antifungal properties against various groups of pathogenic and spoilage microorganisms (Derelio?lu and Turgay, 2019; Tan et al., 2015).
The chicken egg has almost complete balance of essential nutrients with protein, vitamins, minerals and fatty acids with great biological value with the lowest cost for the low-income population (Menezes et al., 2012). The loss of moisture and carbon dioxide via the shell pores causes quality changes in albumen and yolk as well as weight loss of eggs during storage (Wardy et al., 2011). Film coatings can be inhibited moisture, gas, and aroma transfer from the shell pores. For example, chitosan coating can be preserved the internal quality of eggs without affecting consumer acceptance (Kim et al., 2009).
In this study we aimed to investigate the synergistic effect of chitosan coating prepared with acetic, lactic, propionic, gallic and caffeic acids, on the shelf life of chicken and quail eggs. The objective was to study the effects of different formulations of coatings on the physical, structural properties of chicken and quail eggs.
MATERIALS AND METHODS
In this study, chicken eggs were obtained from Kahramanmara? Sütçü Imam University Agricultural Faculty Research and Application Farm, Turkey and quail eggs were obtained from a local producer. All the eggs were obtained daily. The study was conducted in food engineering laboratory of Kahramanmara? Sütçü Imam University, Turkey.
Shrimp shell chitosan was obtained from the (deacetylation grade was 75%) Sigma (C3646). Chitosan was dissolved by using acetic acid (Sigma, 320099), lactic acid (Sigma, 69775) and propionic acid (Sigma, 402907). Gallic acid (Merck, 1.01347.0500) and caffeic acid (Acros, 114930250) were used in the coating formulations as phenolic materials. Chitosan solutions were prepared by dissolving 3 g of chitosan in 100 ml distilled water that containing equally added as 1% of organic acids (AA: acetic acid, LA: lactic acid, PA: propionic acid) and 1 g/chitosan phenolic compounds gallic acid (AA+GA: acetic acid with gallic acid, LA+GA: lactic acid with gallic acid, PA+GA: propionic acid with gallic acid), caffeic acid (AA+CA: acetic acid with caffeic acid, LA+CA: lactic acid with caffeic acid, PA+CA: propionic acid with caffeic acid). The solution was heated (40°C) and agitated constantly for 45 min. Finally, polyethylene glycol added to the solution for elasticity (0.25 ml/g chitosan) and agitated 15 min (No et al., 2002).
Coating treatment and storage of eggs
Eggs were coated in chitosan solution by dipping and allowed to dry. Coating treatment was made two times. After coating, the eggs were allowed to dry before being placed on cardboard egg racks with the little end down and stored at room temperature (24±2°C) (Kim et al., 2009). For determination of weight loss, Haugh unit, yolk index, albumen pH, shell strength and albumen viscosity ten replicates per each treatment were taken weekly for up to 4 weeks at 24±2°C.
Determination of weight loss, Haugh unit, yolk index, albumen pH, shell strength and albumen viscosity
Weight loss (%) of the eggs was calculated according to Alleoni and Antunes (2004). The height of albumen and yolk was measured with a tripod micrometer (Model S-6428, B.C. Ames Inc., Melrose, MA, USA). The yolk width was measured with a digital calliper (General Tools & Instruments, New York, NY, USA). The Haugh unit was calculated according to described by Alleoni and Antunes (2004). The yolk index was calculated as yolk height/yolk width (Bhale et al., 2003). After measurement of Haugh unit and yolk index, the albumen was separated from the yolk. The thin and thick albumen were mixed thoroughly prior to measuring pH with a pH meter. For each coating group, 20 chicken eggs and 60 quail eggs were broken and the part of the albumen was separated from the yolk and placed in the viscometer tube. Measurements were carried out at 20°C at a speed of 30 rpm using the L62 heading. The first measurement was recorded as 20 s and the second measurement was recorded as millipascal seconds from 10 s rotation of the head. Torque was applied between 10-100%.
Mineral analyses
Mineral analyses of the samples were done according to EPA method (1994).
Shell breaking strength
Shell breaking resistance analysis, the strength of the egg, which proved to be a criterion is resistance against breaking. Measured with a texture analyzer (STM-1, Santam Co., Tehran, Iran), kg/m2 is expressed as (Ezazi et al., 2021).
Statistical analysis
For internal and external quality of chicken and quail eggs, mean ± standard deviation values were reported based on ten measurements (five eggs/replicate) per treatment. One-way analysis of variance of data was carried out using the IBM SPSS Statistics software (v.24). The difference between pairs of means was resolved by means of confidence intervals using Duncan’s tests; the level of significance was set at P < 0.05.
RESULTS AND DISCUSSION
Weight loss
The weight loss of the control (non-coated) and coated of chicken eggs with chitosan during 4-week of storage at 24±2°C was shown in Table 1. The weight loss of chicken eggs was significantly (P<0.05) increased with storage periods. Non-coated chicken eggs showed more weight loss than all coated ones during of storage. This might be due to the evaporation of water and carbon dioxide from pores (Kumari et al., 2020).
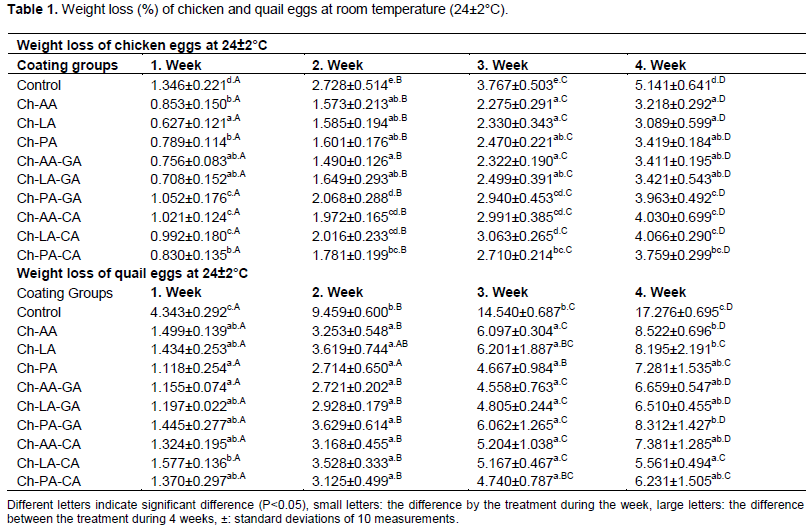
Overall, storage periods progressively increased the weight loss; however, the extent of coated quail eggs was lesser than non-coated quail eggs (Table 1). For all coated quail eggs, weight loss gradually with increase in the storage periods. Significant variations of weight loss existed coated and non-coated quail eggs (P<0.05).
Without exception, all quail eggs coated with Ch-LA-CA had less weight loss than non-coated quail eggs throughout 4 weeks of storage (P<0.05).
Weight loss of eggs during storage is caused by the evaporation of water and loss of carbon dioxide from the albumen through the shell. According to FAO (2003), a weight loss of 2-3% is common in marketing eggs and is hardly noticeable to consumers. This study demonstrated that chitosan coating may be offered a protective barrier against transfer carbon dioxide and moisture through the eggshell even when stored at 24±2°C, thus minimizing weight loss and extending the shelf life of eggs. Torrico et al. (2011), stated that mineral oil coating significantly reduced the weight loss (0.72 to 1.20%) of coated chicken eggs, compared to (4.17%) non-coated chicken eggs, after 5 weeks of storage at 4°C. Bhale et al. (2003) reported that chicken eggs coated with chitosan storage at 25°C showed a lower weight loss (6.8%) compared with that of non-coated chicken eggs (7.84%). Eggs held at 25°C for 8 weeks lost 14.50% of their weight, according to Ezazi et al. (2021).
Haugh unit
The gelatinous structure of thick albumen ultimately deteriorates during egg storage, ending in thin albumen. The Haugh unit is a measurement of albumen quality that is based on the weight of the egg and the thickness of the thick albumen. The greater Haugh unit value, the better the albumen quality of eggs. Changes in the Haugh unit of non-coated and coated chicken eggs during 4 weeks of storage at 24±2°C were presented in Table 2. Compared with non-coated chicken eggs, coated chicken eggs had significantly greater Haugh units (P<0.05) throughout 4 weeks of storage. Generally, the Haugh unit decreased with increasing storage periods. Non-coated chicken eggs and coated with Ch-AA-GA and Ch-PA-GA chicken eggs had no significant differences at first week (P>0.05). However, at the other weeks there were significant differences between coated and non-coated chicken eggs. During storage Haugh unit of coated chicken eggs were higher than non-coated chicken eggs.
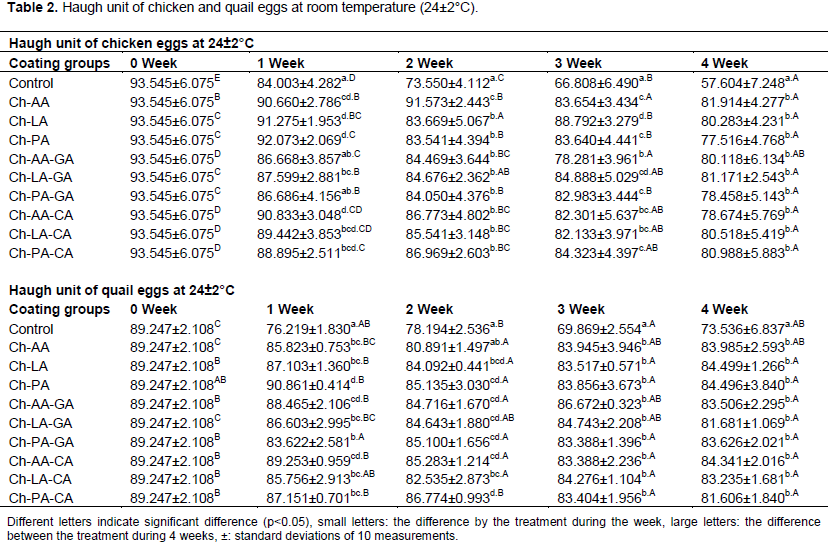
The utmost drop in Haugh unit occurred during the second week of storage for all coated quail eggs. Significant differences were observed (P<0.05) between coated and non-coated quail eggs, except first week. The Haugh unit of Ch-LA after 4 weeks of storage was at the highest level (84.499± 1.266). Haugh unit of coated quail eggs main effects including storage period and initial albumen quality were significant (P<0.05) but in the third and fourth week there were no significant differences between coated groups of quail eggs (P>0.05). Throughout storage of eggs, changes in albumen quality may be occurred primarily due to storage conditions such as time and temperature.
This study shows that all chitosan coating formulations were effective in preserving the albumen quality of chicken and quail eggs. Alleoni and Antunes (2004), reported that chicken eggs coated with whey protein the quality of Haugh unit (A> 55 HU) and non-coated chicken eggs the quality of Haugh unit C (C <30 HU) during storage for 4 weeks.
Several studies have shown that HU decreased as storage duration increases, and that the usage of coatings delayed this decline (Pires et al., 2020). Whey protein isolate, sodium montmorillonite nanoparticles, and sodium metabisulfite were used by Soares et al. (2021). The results demonstrated an 18.33% difference in HU between non-coated and coated eggs after the first week of storage. Between the beginning (7 days) and the end (35 days) of the experimental period, Oliveira et al. (2020) found that HU variation in pectin-coated eggs (86.84) was lower than in uncoated eggs (83.01).
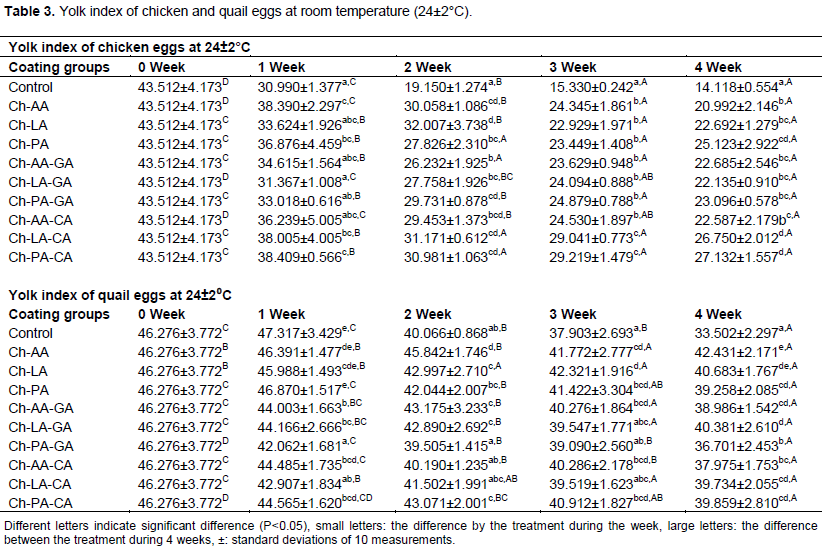
Yolk index
A yolk index value is an indication of freshness of eggs and calculated as yolk height/yolk width. A decrease in a yolk index value during storage indicated a progressive weakening of the vitelline membranes and liquefaction of the yolk caused mainly by diffusion of water from the albumen. Table 3 showed that the changes in yolk index of control and chitosan coated chicken eggs during 4 weeks of storage at 24±2°C. Overall, the yolk index decreased with increasing storage period. Compared with the non-coated chicken eggs, chitosan coated eggs irrespective of formulation of coating, showed significantly higher yolk index during storage. When compared to uncoated eggs, Pires et al. (2019), found that eggs coated with RPC + propolis had the highest yolk indexes (0.37) at the end of 6 weeks of storage (0.33). When Yüceer and Caner (2020), tested that several coatings based on chitosan, lysozyme, and ozone on eggs stored for 6 weeks, they found that coated eggs had higher YI values than untreated eggs. As a result, it is certain that the coatings enhanced in the preservation of the yolk's integrity while storage.
Albumen pH
The albumen pH can also be used as an indicator of the albumen quality of eggs (Wardy et al., 2011). Freshly laid eggs have an albumen pH value of 7.6 to 8.7 (Waimaleongora-Ek et al., 2009). During storage, carbon dioxide escapes via eggshell pores, resulting in thinning of the thick albumen and an increased albumen pH value up to 9.6-9.7 (Kemps et al., 2007).
In this study, albumen values of chicken eggs coated chitosan groups were significantly (P<0.05) lower than those of non-coated chicken eggs throughout 4 weeks of storage at 24±2°C (Table 4). There were no significant differences (P>0.05) in albumen pH among Ch-PA-GA and Ch-AA-CA coated chicken eggs during 4 weeks of storage.
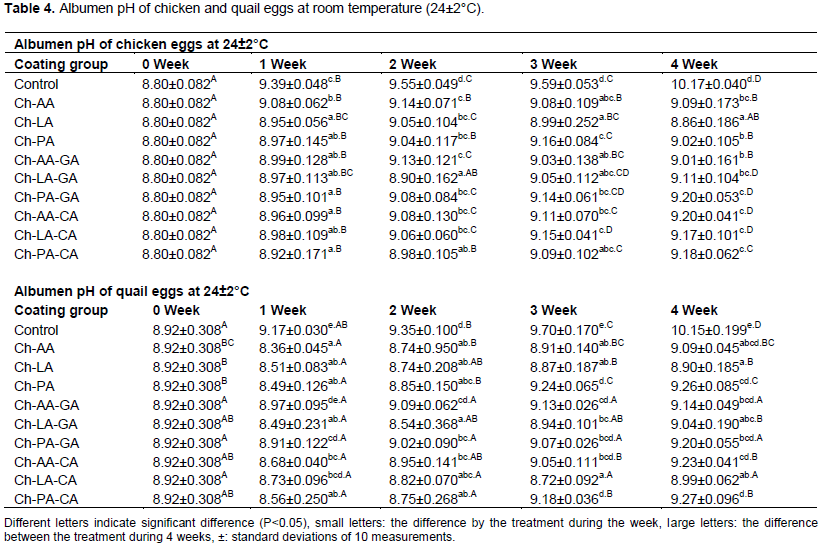
Table 4 showed that the albumen pH of non -coated quail eggs rapidly increased from initial value of 8.92 to 10.15 after 4 weeks of storage at 24±2°C. However, the albumen pH of quail eggs of coated with chitosan formulations the pH gradually increased from 8.92 to 9.27 (Ch-PA-CA). This implied that chitosan coated groups could be retarded loss of carbon dioxide through eggshell pores by acting as a gas barrier.
In the fifth week of storage at 25°C, Soares et al. (2021) found that albumen pH values above 9 for uncoated eggs. Uncoated eggs had an albumen pH above 9 after 21 days of storage at 25°C, according to Lima et al. (2020).
The release of CO2 into the environment through the eggshell pores changes the albumen pH over time (Soares et al., 2021). Coatings are effective at delaying this reaction because they operate as a physical barrier, reducing gas exchange between the internal and exterior environments.
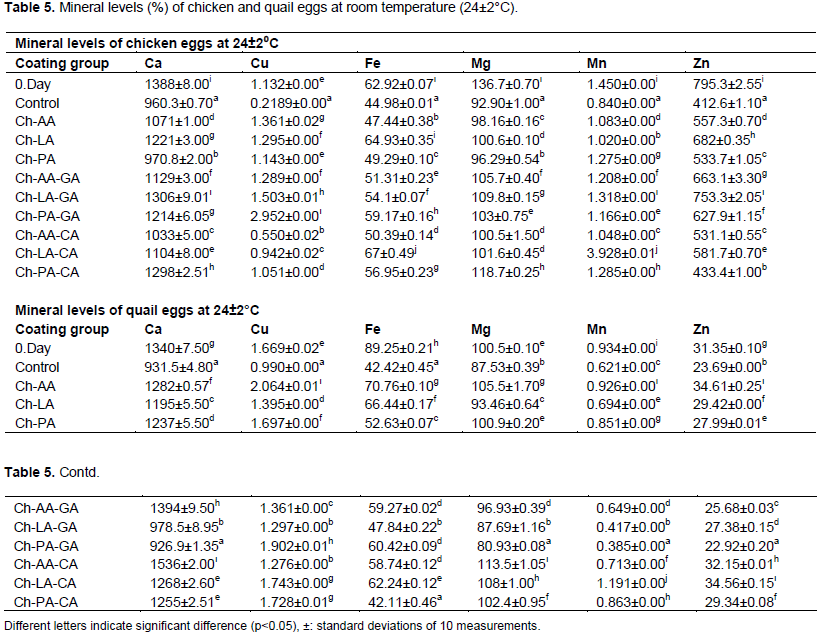
Mineral levels
According to Table 5 the coated eggs mineral elements values of mineral elements were higher than the control group. The differences between the values of mineral elements that have been used in the initial experiment thought to be caused by the difference of mineral levels of chicken eggs. Higher values of mineral matter in the coated groups can be explained by the barrier formation properties of the coating material and the reduction of losses.
Shell breaking strength
Analysis of the fracture of the shell that repeated every week for the duration of 4 weeks storage the control group had the lowest value (p<0.05). Addition of phenolic compounds caused higher fracture resistance (Table 6). According to Table 6, Ch-AA, Ch-LA, Ch-PA, CH-AA-GA, Ch-LA-GA, Ch-PA-GA and CH-LA-CA shell coating caused the fracture resistance (p<0.05).
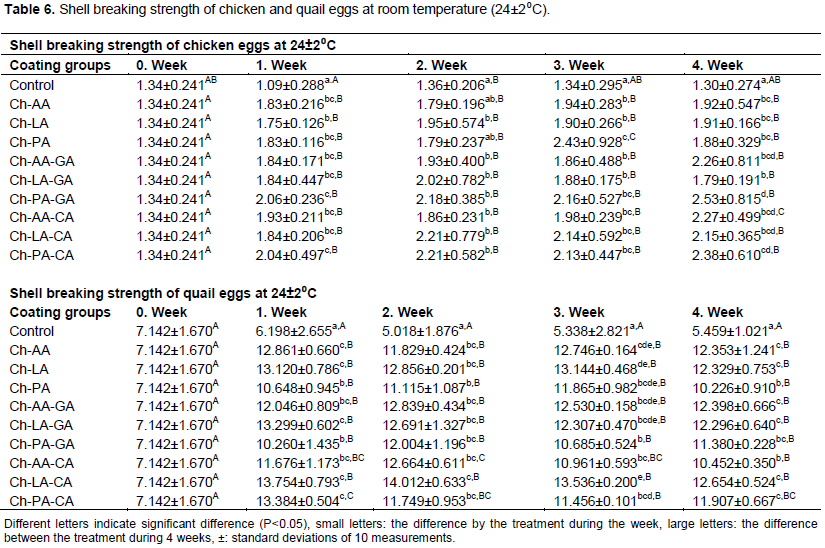
In the poultry industry, shell breaking has generally been associated with significant financial loss. The resistance of eggshells coated with RPC plus propolis was not improved, according to Pires et al. (2019) (5 or 1%).
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGEMENT
This work was supported by KSU-BAP (2014/2-48D).
REFERENCES
|
Aider M (2010). Chitosan application for active bio-based films production and potential in the food industry: Review. LWT-Food Science and Technology 43(6):837-842. |
|
|
Alleoni AC, Antunes AJ (2004). Internal Quality of Eggs Coated with Whey Protein Concentrate. Science Agriculture 61(3):276-280. |
|
|
Bhale S, No HK, Prinyawiwatkul W, Farr AJ, Meyers SP (2003). Chitosan coating improves shelf life of eggs. Journal of Food Science 68:2378-2383. |
|
|
Bonilla J, Fortunati E, Atares L, Chiralt A, Kenny JM (2014). Physical, structural and antimicrobial properties of poly vinyl alcohol-chitosan biodegradable films. Food Hydrocolloids 35:463-470. |
|
|
Derelio?lu E, Turgay Ö (2019). Effect of chitosan combined coating on chicken and quail eggs for controlling Escherichia coli and Salmonella enteritidis. Journal of Food and Health Technology Innovations 2(6):160-168. |
|
|
Ding F, Deng H, Du Y, Shi X, Wang Q (2014). Emerging chitin and chitosan nanofibrus materials for biomedical applications. Nanoscale 6:9477-9493. |
|
|
EPA (1994). Method 200.7. Determination of Metals and Trace Elements in Water and Wastes by Inductively Coupled Plasma-Atomic Emission Spectrometry. |
|
|
Ezazi A, Javadi A, Jafarizadeh-Malmiri H, Mirzaei H (2021). Development of a chitosan-propolis extract edible coating formulation based on physico-chemical attributes of hens' eggs Optimization and characteristics edible coating of egg using chitosan and propolis. Food Bioscience 40:100894. |
|
|
FAO (2003). Egg marketing. A guide for the production and sale of eggs. In FAO Agricultural Services Bulletin (Volume 150). |
|
|
Junter GA, Thebault P, Lebrun L (2016). Polysaccharide-based antibiofilm surfaces. Acta Biomaterialia 30:13-25. |
|
|
Kemps BJ, De Ketelaere B, Bamelis FR, Mertens K, Decuypere EM, De Baerdemaeker JG, Schwägele F (2007). Albumen freshness assessment by combining visible near-infrared transmission and low-resolution proton nuclear magnetic resonance spectroscopy. Poultry Science 86:752-759. |
|
|
Kim SH, Youn DK, No HK, Choi SW, Prinyawiwatkul W (2009). Effect of chitosan coating and storage position on quality and shelf life of eggs. International Journal of Food Science and Technology 44:1350-1359. |
|
|
Kumari K, Tripathi UK, Maurya V, Kumar M (2020). Internal quality changes in eggs during storage. International Journal of Science, Environment and Technology 9(4):615-624. |
|
|
Leceta I, Guerrero P, dela Caba K (2013). Functional properties of chitosan-based films. Carbohydrate Polymers 93:339-346. |
|
|
Lima VVC, de Oliveira Gomes, B, Pereira M, do Carmo Barros EK, Pereira ALF, Silva DS, Freitas ER, Abreu VKG (2020). Cassava and yam starch coverings in egg conservation. (in Portuguese). Research, Society and Development 9:e23984949. |
|
|
Madureira AR, Pereira A, Pintado M (2015). Current state on the development of nanoparticles for use against bacterial gastrointestinal pathogens. Focus on chitosan nanoparticles loaded with phenolic compounds. Carbohydrate Polymers 130:429-439. |
|
|
Menezes PC, Evilda RL, Juliana PM, Wanessa NKO, Joaquim EN (2012). Egg quality of laying hens in different conditions of storage, ages and housing densities. Revista Brasileira de Zootecnia 41(9):2064-2069. |
|
|
Muscat D, Adhikari B, Adhikari R, Chaudhary DS (2012). Comparative study of film forming behaviour of low and high amylose starches using glycerol and xylitol as plasticizers. Journal of Food Engineering 109(2):189-201. |
|
|
No HK, Park NY, Lee SH, Samuel PM (2002). Antibacterial activity of chitosan and chitosan oligomers with different molecular weights. International Journal Food Microbiology 74:65-72. |
|
|
Oliveira GO, Santos VM, Rodrigues JC, Santana AP (2020). Conservation of the internal quality of eggs using a biodegradable coating. Poultry Science 99:7207-7213. |
|
|
Persin Z, Stana-Kleinschek K, Foster TJ, van Dam JEG, Boerio CG, Navard P (2011). Challenges and opportunities in polysaccharides research and technology: The EPNOE views for the next decade in the areas of materials, food and health care. Carbohydrate Polymers 84(1):22-32. |
|
|
Pires PGS, Pires PDS, Cardinal KM, Bavaresco C (2020). The use of coating in eggs - a systematic review. Trends Food Science and Technology 106:312-321. |
|
|
Pires PGS, Pires PDS, Cardinal KM, Leuven AFR, Kindlein L, Andretta I (2019). Effects of rice protein coatings combined or not with propolis on shelf life of eggs. Poultry Science 98(9):4196-4203. |
|
|
Soares RA, Borges SV, Dias MD, Piccoli RH, Fassani EJ, Silva EMC (2021). Impact of whey protein isolate/sodium montmorillonite/sodium metabisulfite coating on the shelf life of fresh eggs during storage. LWT-Food Science and Technology 139:110611. |
|
|
Szymanska E, Winnicka K (2015). Stability of chitosan a challenge for pharmaceutical and biomedical applications. Marine Drugs 13:1819-1846. |
|
|
Tan YM, Lim SH, Tay BY, Lee MW, Thian ES (2015). Functional chitosan-based grapefruit seed extract composite film for applications in food packaging technology. Materials Research Bulletin 69:142-146. |
|
|
Torrico DD, No HK, Prinyawiwatkul W, Janes M, Herrera A, Osorio F (2011). Mineral Oil-Chitosan Emulsion Coatings Affect Quality and Shelf-Life of Coated Eggs during Refrigerated and Room Temperature Storage. Journal of Food Science 76(4):262-268. |
|
|
van den Broek LAM, Knoop RJI, Kappen FHJ, Boeriu CG (2015). Chitosan films and blends for packaging material. Carbohydrate Polymers, 116:237-242. |
|
|
Waimaleongora-Ek P, Garcia K, No HK, Priyawiwatkul W, Ingram D (2009). Selected quality and shelf-life of eggs coated with mineral oil with different viscosities. Journal of Food Science 74:423-429. |
|
|
Wardy W, Torrico DD, Jirangrat W, No HK, Saalia FK, Prinyawiwatkul W (2011). Chitosan-soybean oil emulsion coating affects physico-functional and sensory quality of eggs during storage. LWT-Food Science and Technology 44:2659-2668. |
|
|
Wu J, Zhong F, Li Y, Shoemaker CF, Xia WS (2013). Preparation and characterization of pullulan-chitosan and pullulan-carboxymethyl chitosan blended films. Food Hydrocolloids 30(1):82-91. |
|
|
Yüceer M, Caner C (2020). The effects of ozone, ultrasound and coating with shellac and lysozyme-chitosan on fresh egg during storage at ambient temperature - Part 1: interior quality changes. International Journal of Food Science and Technology 55(1):259-266. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0