Full Length Research Paper
ABSTRACT
Aflatoxins are poisonous, mutagenic, and carcinogenic compounds produced by Aspergillus fungi that contaminate various agricultural produce and products including peanut butter. Peanut butter is among the most consumed recipe in The Gambia. Thus, a cross-sectional assessment was conducted to evaluate the levels of aflatoxin contamination in processed peanut butter, sold and consumed locally in the country. In total, 85 peanut butter samples of approximately 2.0 kg each were bought at random within the six administrative regions across the country. All the samples were analyzed for aflatoxin contamination using thin layer chromatography (TLC) technique. Aflatoxin B1 was detected in 8 (9.4%) of the samples and only one (1.20%) of the samples exceeded both the Codex Alimentarius Commission and FAO/WHO Food Standards Program of 15 μg kg-1. Likewise, only 5 of 85 samples representing 5.90% exceeded the European Union maximum limits for total aflatoxin of 4 μg kg-1 in peanut and processed products intended for direct human consumption. The remaining samples (77 of 85) representing 90.6% were negative or without any detectable aflatoxins. The analyzed samples therefore indicate that majority of peanut butter especially homemade is safe for human consumption.
Key words: Aflatoxin B1, peanut butter, thin layer chromatography (TLC), The Gambia.
INTRODUCTION
Several agricultural produce are susceptible to fungal attack that produces toxic metabolites referred to as mycotoxins. Aflatoxin is a food or grain-borne toxic secondary metabolites produced by the fungi Aspergillus flavus and Aspergillus parasiticus. These fungi can infect a wide variety of crops such as corn, groundnut, cottonseed, tree nuts, etc., either in the field or during post-harvest (Horn, 2005; Miningou et al., 2021). Spores if not atoxigenic of A. flavus are saprophytic in nature and once they become pathogenic, they are known to produce an array of toxic secondary metabolites including aflatoxins (Nallathambi and Umamaheswari, 2009).
Infestation by the fungus can occur on these produce even during processing, handling, storage or transportation (Nakai et al., 2008). Both raw and processed products are highly susceptible to mycotoxin contamination including milk and vegetables (Giryn and Szteke, 1995).
Aflatoxins have become a concern in agriculture, trade, animal and human health on a global scale (Bennett and Klich, 2003). Aflatoxin B1 (AFB1) is known as a potent hepatocarcinogen and can play a synergic action with hepatitis B or C viral infections leading to hepatocellular carcinoma (
Aflatoxin B1 is strongly linked to immune-system suppression, increased susceptibility to diseases, and growth retardation, notably stunting (Henry et al., 1999; Gong et al., 2002; Turner et al., 2003; Williams et al., 2004). Reports have shown that high exposure to aflatoxin causes infertility, abortions, and delayed onset of egg production in birds (Oladele, 2014). Furthermore, loss of appetite, skin discoloration, and even yellowish pigmentation on skin can be observed in fish. This hazardous toxin can be transformed to aflatoxin M1 when feeds of livestock are contaminated (Carvajal et al., 2003; Mohammadi, 2011). In The Gambia, groundnut is the main cash crop of the country and peanut butter is among the most consumed product in the country. Aflatoxin impact on international trade resulting from price losses and rejected exports is detrimental to the economy of the country. It has been reported by Joseph Ndenn, Iris Consulting (2018) that an average price loss per annum sums to US$1.5 M (2000-2014) and an average annual loss from rejected exports adds US$62,854 (2012-2015).
The Gambia is a tropical country with a sahelian climate; defined by a long dry season (November- May) and a short wet season (June-October). The country has an average monthly temperature range of 18 to 30°C during the dry season and 23 to 33°C during the wet season. The average monthly relative humidity varies from 68 to 70% during the dry and wet season, respectively, and the average rainfall ranges from 800 to 1200 mm (GOTG, 2020). These climatic conditions provide the optimum environments for the growth of the Aspergillus fungus. Improper agricultural practice like continuous cropping, late weeding, poor drying and storage of nuts with mechanical damage, coupled with low humidity, drought, insect, crop genotype, and soil condition can increase crop susceptibly to aflatoxin (Leszczynska et al., 2000).
Groundnut and its product, peanut butter are considered nutritious, as they contain proteins, oils, fatty acids, carbohydrates, and minerals (Settaluri, 2012). This, ironically, makes them a rich medium for fungal growth and aflatoxin contamination (Barberis et al., 2012). A 100 g roasted peanut is said to constitute 1.55 g of water, 21.51 g of carbohydrates, 8.0 g of fiber, 9.66 g of lipids (fats), 23.68 g of proteins and a total calories of 2448 kJ (585 kcal) (USDA, 2011). Peanut and its additives could provide such a nutritious diet to satisfy WHO recommended average requirement of 0.66 g of protein per kg of ideal body weight, and a “safe level” of 0.86 g/kg of body weight (Food and Nutrition Board, 2002).
Peanut butter locally referred to as “De gay”, is made from roasted groundnut at high temperature (160°C) (Siwela et al., 2011), blanched, deskinned and ground to paste after salt being added as a stabilizer (Peanut Institute, 2012). “De gay” is the primary recipe for a popular soup called “domoda” across all socioeconomic or demographic status in The Gambia. The stew is consumed nationwide due to its relative affordability and organoleptic properties.
Frequent deaths especially in children are repeatedly reported in many sub-Saharan countries due to malnutrition. Groundnut, a rich source of protein containing essential amino acids, can help in preventing malnutrition (Sanghvi and Murray, 1997).
Schroder et al. (2004) reported that people more adherent to a traditional Mediterranean diet, which includes nuts, had statistically lower body mass index (BMI). And a US food survey data revealed that peanut eaters have lower BMIs than non nut and peanut eaters (Sabate, 2003; Griel et al., 2004).
Peanut butter contains beneficial mono and poly unsaturated fats and is rich in antioxidants, vitamin E and the polyphenol, p-coumaric acid and help to lower blood cholesterol levels, reduce risk of heart disease by 50% (Talcott et al., 2005). The β-Sitosterol (phytosterol) is an anti-cancer compound found in peanut and peanut butter (Lee et al., 2004).
Virtually no scientific data exists on aflatoxin contamination of peanut butter in The Gambia. Considering the wide consumption rate of “domoda” and the need to ensure food and public health safety, the study is designed to probe the occurrence and prevalence of aflatoxin in peanut butter nationwide.
MATERIALS AND METHODS
Peanut butter sampling
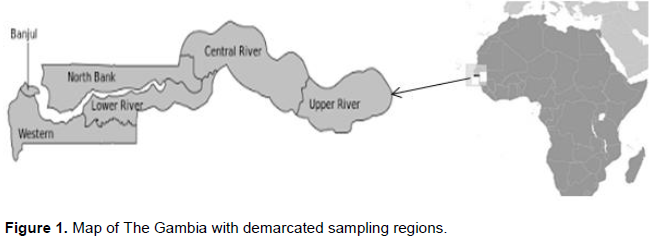
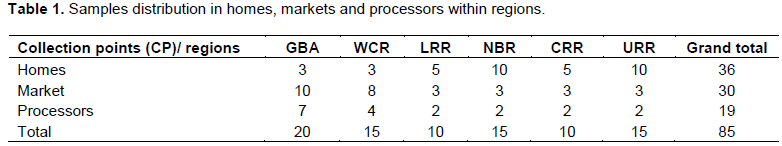
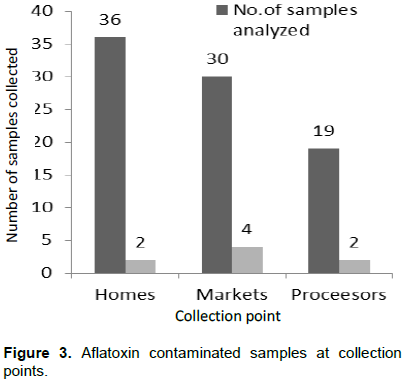
About 2 kg of 85 samples of peanut butter were incrementally collected using multistage sampling method for markets and homes, while purposive sampling for production areas or processors within the six administrative regions in The Gambia (Figure 1). A total of 20 samples from the Greater Banjul Area (GBA) which include the Kanifing Municipality, 15 samples from West Coast Region (WCR), North Bank Region (NBR) and Upper River Region (URR), while 10 samples from Lower River Region (LRR) and Central River Region (CRR) (Table 1). With respect to sample Collection Points (CP), 36 samples were taken from homes, 30 market samples and 19 samples from processors (Table 1). All the samples were kept in zip bags, and kept in a quart cooler then transported to the Food Chemistry Laboratory at the National Agricultural Research Institute (NARI) for aflatoxin contamination analysis.
Extraction of aflatoxin
A 100 g representative sub-sample was obtained from each 2 kg sample and blended with 300 ml of distilled H2O into slurry. A 100 g slurry was blended for 3 min with 250 ml methanol, 100 ml hexane and 2 g of NaCl, filtered using a Whatman filter paper of 32 cm and 50 ml collected. Exactly 150 ml of distilled water was poured in a separating funnel then the 50 ml filtrate and 25 ml of chloroform were respectively added and the separating funnels topped then slightly shaken. When separation settled, elutes were collected in a small beaker containing 2 anti-bumping granules for calm boiling then heat to dryness in a steam bath. The dried beaker was allowed to cool and about 5 to 8 ml chloroform pipetted to it, a dropper was used to rinse its wall to cleanse off any aflatoxin then the chloroform was subsequently filled in a vial containing 2 anti-bumping granules. The vials were evaporated to dryness using an electric vial-rack heater. After dryness and the vials cooled, a 0.25 ml of benzeneacetonitrile (98:2 v/v) solution was pipetted into them and vortexed.
Reading of TLC plates
Samples were spotted using an assipettor-fix for (5-10-40 µl), respectively on the TLC silica gel coated glass plates of 20 cm × 20 cm and thickness layer of 0.25 mm against the standard (1-3-7-10-15-20 µl) labeled using a pencil on top of the plate. The TLC plate is gently lowered in an already loaded tank with diether: methanol: water (94:4.5:1.5 v/v) positioned on a flat surface and covered with the lid. After about 45 to 60 min, when the plate in the tank had absorbed the solvent and at three-quarter length of the plate, the plates were removed and allowed to dry up for about a minute then illuminated below the TLC machine or lamb and viewed under long-wave of UV 366 nm in the dark room. The fluorescence intensities of aflatoxin B1 spots in samples were compared to the respective spots of standard in terms of color and retention factor (Rf). Aflatoxin B2, G1, and G2 spots were compared by the same procedure. Both preparation of the standards and the calculation of aflatoxin B1 concentration were done as stated by Jallow et al. (2019) in part per billions.
Statistical analysis
Data were statistically analyzed using SigmaPlot 12, applying t-test (P<0.05) for pairwise comparisons.
RESULTS
The data indicated that aflatoxin contamination in peanut butter is generally low in the country. There was no significant differences (p>0.05) among regions (Table 2) not at collection points (Table 3). Aflatoxin was not detected in 77 (90.59%) of the 85 samples analyzed (Table 2). Furthermore, there were no detectable aflatoxin in all the peanut butter samples collected from the GBA and NBR, therefore those regions are regarded as aflatoxin contamination free zones under this study (Figure 2).
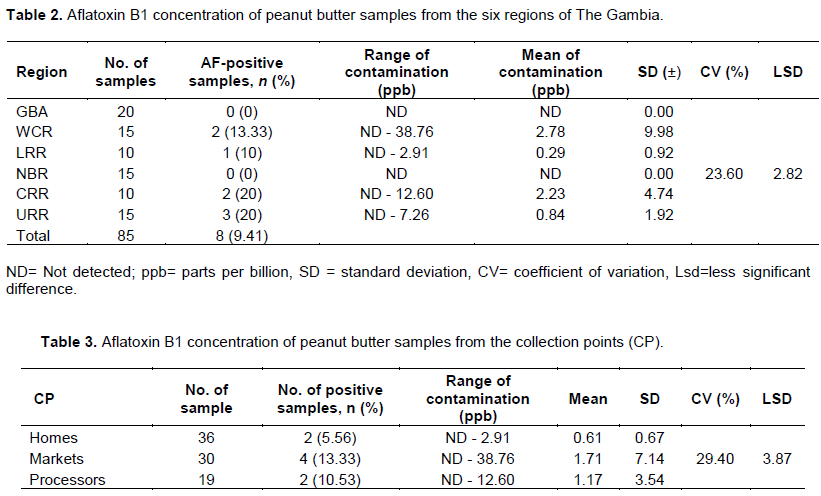
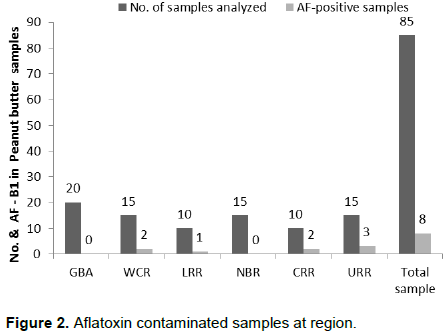
Out of the 15 samples from WCR, only two (2) samples (13.33%) were contaminated with aflatoxin by 2.91 and 38.76 ppb of aflatoxin. While from the 10 samples of LRR, only one (1) sample (10%) was found to have been contaminated with aflatoxin with 2.91 ppb of aflatoxin. CRR and URR both registered 20% of aflatoxin contamination of the 10 and 15 samples analyzed, respectively. The two (2) contaminated samples from CRR recorded 12.60 and 9.65 ppb, while 2.91, 6.74 and 7.26 ppb from URR. Out of the total samples (85) analyzed, only eight (8) samples (9.4%) were found to have contaminated aflatoxin B1. Only 1.20% (1 of 85) of samples in WCR at the Collection Point “Homes” Table 3 with 38.76 ppb exceeded both the Codex Alimentarius Commission, Joint FAO/WHO Food Standards Program of 15 µg/kg (ppb) intended for further processing (Codex Alimentarius Commission, 2001). Furthermore, only 5.90% (5 of 85) samples exceeded the EU maximum limit for total aflatoxins of 4 µg/kg (ppb) for groundnut, nuts, and processed products intended for direct human consumption or use as an ingredient in foodstuffs (EU, 2010). Only 1.20% (1 of 85) of samples exceeded all international acceptable limits with 38.76 ppb (Table 2).
A 42.35% were collected from homes, 35.29% from markets and 22.35 from processors (Table 3), two (2) came out positive for both homes and processors 5.56 and 10.53% and 4 samples (13.33%) from the markets (Figure 3).
DISCUSSION
These finding reports the level of aflatoxin contamination in peanut butter samples processed and consumed in The Gambia. The negative results of the peanut butter in the GBA which is mostly supplied from NBR could be as a result of the frequent and quick completion of peanut butter consignments sale due to the high demand of the densely population of the area (about 20% of the country’s population). In the same light, a 5 km ocean separates GBA from NBR, a region known as the most peanut grown hub. Consequently, the farmers in this agro-ecological zone (sudano-sahelian) are more experienced in good agronomical practices that mitigate aflatoxin in groundnut and practice proper post-harvest like drying, sorting and screening, thus the negative results. And unlike GBA, most consumed peanut butter is homemade in NBR.
Similar results were reported by Azer and Cooper (1991) who analyzed 73 samples of peanut and peanut butter and found the contamination in only one (1) sample equal to 61 μg/kg. Again in Turkey, where only one peanut butter sample from a total of 85 peanut and peanut product samples had 2.0 ppb (Ozay, 1989). A 91% (10 of 11) peanut butter aflatoxin contamination with a mean 75.66 ng/g was found in Zimbabwe (Mupunga, 2014) and 27% (3 of 11) exceeds the EU maximum limits of 4 ppb. In Sudan, a range of 26.6 to 853 μg/kg in peanut butter was reported with 90% exceeding the EU maximum limit (Elzupir et al., 2011). This two African countries are known to be prone to drought and erratic rainfall. A survey conducted in Taiwan showed aflatoxins were detected in 10 out of 21 peanut butter samples, but the highest level of AFB1 was only 2.59 μg/kg (Ying-Chun et al., 2013). Also, higher total aflatoxin level was reported in Nepal with 42.5% (43 of 101) of aflatoxin contamination (Koirala et al., 2005). This might be due to high rainfall, hot and humid conditions that are prevalent in the Asian subcontinent.
Though consumption of aflatoxin susceptive crops most specifically peanut and it products has been identified as a strong risk factor for hepatocellular carcinoma (HCC). A report by PACA (2018d) revealed 285 liver cancer cases in 4 years in The Gambia.
Although, no established correlation was made to relate the cancer cases to the consumption of peanut and or its products or as a result of aflatoxin contamination. Other natural, environmental and habitual factors like, genetic makeup, exposure to hazardous chemical, smoking, alcohol, and underlying medical history may also be a factor to consider for the cancer cases. It is estimated that the risk of developing aflatoxin-induced liver cancer in The Gambia is 8.3 cases per 100,000 people. This is because of the HBV prevalence (15%) of the population (Gambia Aflatoxin Control, 2015). In The Gambia, HBV, HCV and a?atoxin exposure are known aetiologies of HCC with HBV accounting for a majority of cases. In a study, Bah et al. (2001) and Kirk et al. (2004) showed 10% cases of cancer were as a result of smoking. A Similar report indicates 57% of liver cancer cases in The Gambia are attributable to chronic hepatitis B infection (Kirk et al., 2006).
The low aflatoxin sequence of the CPs (Table 3) could be correlated to the shelf life of the product which might be as a result of the oil in it during production. Secondly, in the presence of phytoalexins, an inhibiting protein in seed for fungal colonization (Jallow et al., 2018) and time of sampling. Homemade peanut butter as expected, recorded the lowest contamination level. This is certainly because more precautionary measures like sorting the black and moldy and all foreign materials are done with precisions and much care is also given in its storage, since is purposely for home and family consumption. The product is transformed to soap if it loses one of its organoleptic features. Processors serve as the first and transitional points of the product, fewer and lower contamination are as a result of less time spent at this point before entering its finally stage. The market recorded the highest aflatoxin level which could be due to contrary practices to homemade and possible adulterations of the peanut butter to increase its volume for profit.
When proper food safety practices are maintained, this study confidently shows that the peanut butter consumed in The Gambia is safe, and therefore encourages the continuation of the consumption of this delicious and cultural stew “domoda” in order to gain the maximum health benefits. As stated by Kelly and Sabaté (2006), 37% lower from cardiovascular disease (CVD) and stroke for regular consumers of the product than those who do not at all. Furthermore, a water soluble vitamin (B9) known as folate or folic acid found in peanuts could help in human growth and aid the normal functioning of nerves and brain and may also help protect against cancers of the lung, colon, and cervix (Fishman et al., 2000).
Again these findings are substantiated with the attestation of a popular US TV talkshow called The Drs (2013), where a dietitian described this Gambian stew as a factor that may lower cancer risk. As a result the country is having one of the lowest cancer cases in the world. Epidemiological studies have confirmed that consuming peanut and its products, and snack food, at least four to five times per week may contribute to protect against, type two diabetes and gallbladder disease (Hu et al., 1998; Jiang et al., 2002; Tsai et al., 2004), weight management (Jennette, 2005), no positive correlation to increase body mass index (BMI) (Hu and Stampfer, 1999). The high protein and high unsaturated fat nature of peanuts may also contribute to the lack of weight gain associated with peanut consumption (Johnston, 2005).
CONCLUSION
The overall results demonstrate that 90% (77 of 85 samples) were not contaminated with aflatoxin. From the contaminated samples, eight (9.41%) sample exceeded the EU limits of 2 µg/kg (ppb) for aflatoxin B1 for direct consumption and only one sample exceeds the Codex Alimentarius maximum limits of 15 ppb. This study reassures the safety of peanut butter consumed in The Gambia, and therefore encourages the frequent consumption of this peanut butter stew in order to gain the maximum health benefits it had in it. And also recommends a national policy and regulatory document with budgetary allocations for aflatoxin management activities should be setup for the nation to redeem herself from the huge aflatoxin economic impact she is facing. A regular awareness creation and analytical surveillance programs be conducted by food control agencies and stakeholders is highly recommended to monitor the incidences of aflatoxin contamination in the country.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Azer M, Cooper C (1991). Determination of aflatoxins in foods using hplc and a commercial elisa system. Journal of Food Protection 54(4):291-294. |
|
|
Bah E, Parkin DM, Hall AJ, Jack AD, Whittle H (2001). Cancer in The Gambia: 1988-97. Britain Journal of Cancer 84(9):1207-1214. |
|
|
Barberis CL, Dalcero AM, Magnoli CE (2012). Evaluation of aflatoxin B1 and ochratoxin A in interacting mixed cultures of Aspergillus sections Flavi and Nigri on peanut grains. Mycotoxin Research 2012. |
|
|
Bennett JW, Klich M (2003). Mycotoxins. Clinical Microbiology Reviews 16(3):497-516. |
|
|
Carvajal M, Rojo F, Mendez I, Bolanos A (2003). Aflatoxin B1 and its interconverting metabolite aflatoxicol in milk: the situation in Mexico. Food Additives and Contaminants 20(11):1077-1086. |
|
|
Codex Alimentarius Commission (2001). Report of the 33rd session ofthe Codex Committee on Food Additives and Contaminants. Codex Alimentarius Commission, Joint FAO/WHO Food Standards Pro-gramme,Rome. |
|
|
Eaton DL, Groopman JD (2013). The toxicology of aflatoxins: human health, veterinary, and agricultural significance. Academic Press, San Diego, CA, USA. |
|
|
Elzupir AO, Salih AOA, Suliman SA, Adam AA, and Elhussein AM (2011). Aflatoxins in peanut butter in Khartoum State, Sudan. Mycotoxin research 27(3):183-186. |
|
|
European Union, EU (2010). Commission Regulation (EC) No 165/2010of 26 February 2010, amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs asregards aflatoxins. Official Journal of the European Union L 50:8-12. |
|
|
Fishman SM, Christian P, West KP (2000). The Role of Vitamins in the Prevention and Control of Anemia. Public Health Nutrition 3(2):125-150. |
|
|
Food and Nutrition Board (2002). Dietary Reference Intakes for Energy, Carbohydrates, Fiber, Fat, Protein and Amino Acids (Macronutrients). The National Academy Press, Washington, |
|
|
Gambia Aflatoxin Control (2015). Strengthening aflatoxin control in the Gambia: Policy Recommendations. |
|
|
Giryn H, Szteke (1995). Estimation of Alternaria mycotoxins in some raw or processed fruit and vegetables. Roczniki Pa?stwowego Zak?adu Higieny 46:129-133. |
|
|
Gong YY, Cardwell K, Hounsa A, Egal S, Turner PC, Hall AJ, Wild CP, (2002). Dietary aflatoxin exposure and impaired growth in young children from Benin and Togo: Cross sectional study. British Medical Journal 325(7354):20-21. |
|
|
Government of The Gambia (GOTG) (2020). Third National Communication under the UNFCCC. Ministry of Environment, Climate Change and Natural Resources (MECCNAR), Banjul 75 p. |
|
|
Griel AE, Eissenstat B, Juturu V, Hsieh G, Kris-Etherton PM (2004). Improved diet quality with peanut consumption. Journal of the American College of Nutrition 23(6):660-668. |
|
|
Groopman JD, Kensler TW, Wild CP (2008). Protective interventions to prevent aflatoxin-induced carcinogenesis in developing countries. Annual Review Public Health 29:187-203. |
|
|
Henry SH, Bosch FX, Troxell TC, Bolger PM (1999). Reducing liver cancer - global control of aflatoxin. Science 286(5449):2453-2454. |
|
|
Horn BW (2005). Ecology and population biology of aflatoxigenic fungi in soil. In: Aflatoxin and Food Safety Edited by: Abbas HK. Boca Raton, CRC Taylor and Francis 95-116. |
|
|
Hu FB, Stampfer MJ, Manson JAE, Rimm EB, Colditz GA, Rosner BA, Speizer FE, Hennekens CH, Willett WC (1998). Frequent nut consumption and risk of coronary heart disease in woman: prospective cohort study. British Medical Journal 317(7169):1341-1345. |
|
|
Hu FB, Stampfer MJ (1999). Nut consumption and risk of coronary heart disease: a review of epidemiologic evidence. Current Atherosclerosis Reports 1(3):204-209. |
|
|
Jallow E AA, Jarju OM, Mendy B, Dumevi R, Mendy W, Cham K, (2019). The trend of aflatoxin contamination level in groundnuts from 2008-2018 in The Gambia, London Journal Press 19(8), Compilation 1.0. |
|
|
Jallow EA, Twumasi P, Mills-Robertson FC, Dumevi R (2018). Assessment of aflatoxin-producing fungi strains and contamination levels of aflatoxin B1 in groundnut, maize, beans and rice. Journal of Agricultural Science and Food Technology 4(4):71-79. |
|
|
Jennette H (2005). The potential role of peanuts in the prevention of obesity. Nutrition and Food Science 35(5):353-358. |
|
|
Jiang R, Manson JE, Stampfer MJ, Liu S, Willett WC, Hu B (2002). A prospective study of nut consumption and risk of type II diabetes in women. Journal of the American Medical Association 288(20):2554-2560. |
|
|
Johnston CS (2005). Strategies for healthy weight loss: from vitamin C to the glycemicresponse. Journal of the American College of Nutrition 24(3):158-165. |
|
|
Joseph Ndenn, Iris Consulting (2018). The economic impact of aflatoxins in West Africa: the case of Gambia, Nigeria and Senegal. |
|
|
Kelly JH Jr, Sabaté J (2006). Nuts and coronary heart disease: an epidemiological perspective. British Journal of Nutrition 96(2):S61-S67. |
|
|
Kirk GD, Bah E, Montesano R (2006). Molecular epidemiology of human liver cancer: insights into etiology, pathogenesis and prevention from The Gambia, West Africa. Carcinogenesis 27(10):2070-2082. |
|
|
Kirk GD, Lesi OA, Mendy M (2004). The Gambia liver cancer study: infection with hepatitis B and C and the risk of hepatocellular carcinoma in West Africa. Hepatology 39(1):211-219. |
|
|
Koirala P, Kumar S, Yadav KB, Premarajan KC (2005). Occurrence of aflatoxin in some of the food and feed in Nepal. Indian Journal of Medical Science 59(8):331-336. |
|
|
Lee SS, Kim MB, Chun JC, Cheong YK, and Lee J (2004). Analysis of trans-resveratrol in peanuts and peanut butters consumed in Korea. Journal of Food Research International 37(3):247-251. |
|
|
Leszczynska J, Kucharska U, Zegota H (2000). Aflatoxins in nuts assayed by immunological methods. European Food Research and Technology 210(3):213-215. |
|
|
McKean C, Tang L, Tang M, Billam, M, Wang Z, Theodorakis CW, (2006). Comparative acute and combinative toxicity of aflatoxin B1 and fumonisin B1 in animals and human cells. Food and Chemical Toxicology 44(6):868-876. |
|
|
Miningou A, Traore SA, Kabre B, Konate SALM (2021). Assessment of sixteen varieties of groundnut in two agro-ecological zones in Burkina Faso for yield and tolerance to aflatoxin. African Journal of Agricultural Research 17(1):66-78. |
|
|
Mohammadi H (2011). A review of aflatoxin M1, milk, and milk products. In Guevara-Gonzalez, R.G. (ed.) Aflatoxins - biochemistry and molecular biology. INTECH Open Access Publisher, Rijeka, Croatia pp. 397-414. |
|
|
Mupunga I, lebelo SL, Mngqawa P, Rheeder JP, Katerere DR (2014). Natural occurrence of aflatoxins in peanuts and peanut butter from Bulawayo, Zimbabwe. Journal of Food Protection 77(10):1814-1818. |
|
|
Nakai VK, Rocha LO, Goncalez E, Fonseca H, Ortega EMM, Correa B (2008). Distribution of fungi and aflatoxins in a stored peanut variety. Food Chemistry 106(1):285e290. |
|
|
Nallathambi P, Umamaheshwari C (2009). Detection of aflatoxins in pomegranate arils infected by Aspergillus species Indian Phytopathtology 62(2):178-182. |
|
|
Oladele D (2014). The effects of aflatoxins on animals, "Partnership for Aflatoxin Control in Africa" (Meridian Institute, Washington, DC), Aflatoxin Partnership Newsletter, Vol. II (Accessed, February 2014), 4. |
|
|
Ozay G, Alperden I (1989). Mycotoxins in Peanuts (Arachis hypogaea L.) grown in Turkey. G?da 14(5):267-273 |
|
|
PACA (2018d). Country-led Aflatoxin and Food Safety Situation Analysis and Action Planning for The Gambia: Final Report, Partnership for Aflatoxin Control in Africa, African Union Commission. |
|
|
Peanut Institute (2012). Peanut products: peanut butter. |
|
|
Sabate J (2003). Nut consumption and body weight. American Journal of Clinical Nutrition 78(3 Suppl):647S-650S. |
|
|
Sanghvi T, Murray J (1997). "Improving Child Health through Nutrition; The Nutrition 1 Minimum Package". |
|
|
Schroder H, Marrugat J, Vila J, Covas MI, Elosua R (2004). Adherence to the traditional mediterranean diet is inversely associated with body mass index and obesity in a Spanish population. Journal of Nutrition 134(12):3355-3361. |
|
|
Settaluri VS, Kandala CVK, Puppala N, Sundaram J (2012). Peanuts and their nutritional aspects-a review. Food Nutrition Science 3(12):1644-1650. |
|
|
Siwela AH, Mukaro KJ, Nziramasanga N (2011). Aflatoxin carryover during large scale peanut butter production. Food Nutrition Science 2:105-108. |
|
|
Talcott ST, Passeretti S, Duncan CE, Gorbet DW (2005). Polyphenolic content and sensory properties of normal and high oleic acid peanuts. Journal of Food Chemistry 90(3):379-388. |
|
|
The Dr (2013). |
|
|
Tsai CJ, Leitzmann MF, Hu FB, Willett WC, Giovannucci EL (2004). Frequent nut consumption and decreased risk of cholecystectomy in women. American Journal of Clinical Nutrition 80(1):76-81. |
|
|
Turner PC, Mendy M, White H, Fortuin M, Hall AJ, Wild CP (2000). Hepatitis B infection and aflatoxin biomarker levels in Gambian children. Tropical Medicine and International Health 5(12):837-841 |
|
|
Turner PC, Moore SE, Hall AJ, Prentice AM, Wild CP (2003). Modification of immune function through exposure to dietary aflatoxin in Gambian children. Environmental Health Perspectives 111(2):217-221. |
|
|
USDA (2011). National Nutrient Database for Standard Reference, Release 24. |
|
|
Williams JH, Phillips TD, Jolly PE, Stiles JK, Jolly CM, Aggarwal D (2004). Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. American Journal of Clinical Nutrition 80(5):1106-1122. |
|
|
Ying-Chun C, Chia-Ding L, Hsu-Yang L, Lih-Ching C, Daniel Yang-Chih S (2013). Survey of aflatoxin contamination in peanut products in Taiwan from 1997 to 2011. Journal of Food and Drug Analysis 21(3):247-252 |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0