Full Length Research Paper
ABSTRACT
Blends of maize-soyabean complementary foods were fortified with foods rich in calcium, iron, zinc and vitamin A. Crayfish, bonga fish, and carrot that were processed into flours separately and blended in ratios (2:1:1 w/w) to produce food fortificant. Maize flour, soyabean flour and fortificant were mixed in the ratio of 70:30:0, 60:30:10 and 50:30:20 (% w/w dry basis), respectively to obtain three blends of complementary food. Standard chemical methods were used to determine the blends’ proximate composition, mineral, vitamin A and anti-nutrientional factors. The unfortified blend contents of calcium, iron, zinc and vitamin A were at level of 417.37, 9.15, 6.20 mg/100 g and 387.67 μg RE/100 g, respectively but increased in the fortified blends to the range of 560.45 to 620.12 mg/100 g, 12.43 to 14.25 mg/100 g, 8.72 to 10.67 mg/100 g, and 550.13 to 710.25 μg RE/100 g. Fortified blends formula had micronutrients significantly higher (p<0.05) than the proprietary formula, Cerelac. The blends had 4.03 kCal/g average energy content, 12-19% protein-energy ratio and 25-28% energy from lipids. The proposed fortification levels are based on a daily ration size of 65 g for infants aged 9 to 11 and children aged 12 to 23 months. Feeding 65 g of the diets to infants aged 6 to 11 months will meet the 200, 300 kCal/day and 350 μg RE/day Recommended Nutrient Intakes (RNI) for energy and vitamin A and will be adequate for the minerals (calcium and zinc) requirement of children aged 12 to 23 months.
Key words: Complementary foods, maize, soybean, crayfish, carrot, bonga fish.
INTRODUCTION
Complementary feeding has been recommended to start at 6 to 23 months of infants’ age when they are fed breast milk, other soluble foods and liquids that are enough to meet their nutritional requirements, while continuing breast feeding beyond two years (Campoy, 2018). Balanced nutrition administered to infants and children will definitely give rise to the development of each child’s full human potential. Inadequate nutrition at early stages results in long term growth and health impairment. Early undernutrition or nutritional defects during the first 2 years of life are causes of impairment of infants and children’s intellectual capabilities, stunting and wasting (Srivastava and Chaturvedi, 2020). Available records had it that stunting affected 149 million children under 5 worldwide (Dulal et al., 2017). Also 6.7% of children under 5, or 45.4 million are affected by wasting which is the most destructive form of malnutrition (WHO/UNICEF, 2021). During the breastfeeding and complementary feeding period, essential nutrients should be fed to children to reach their full physical and cognitive potentials, with the attendant benefits that last into adulthood (Arikpo et al., 2018; Owais et al., 2017). Panjwani et al. (2017) reported that 45% of deaths in children under 5 are consequences of micronutrient malnutrition which is the major cause of ill-health in this age group.
Iron and vitamin A deficiencies present major nutritional deficiencies in the world today of greatest significance in public health management. Undernourishment affects more than 800 million people in the world and it had been reported that between 1.5 and 2 billion people have chronic and severe micronutrient deficiencies (MND), mostly deficiencies in the minerals iron, selenium, calcium, zinc and vitamins A and folate (Beal et al., 2017; FAO, 2020). The instruments of fortification, supplementation, food-based approaches with dietary diversification have been used to resolve issues associated MND. Supplementation failed to address the root cause of the MND despite its cost effectiveness. Food-based approaches have therefore recently been suggested to offer over a long term, more practical and cheaper means to address MND, given its capacity in providing the opportunity to target a larger segment of poorer population (Regan et al., 2015).
Experts recently agreed that greater emphasis should be directed to the food approach and consumption of large quantities of micronutrient-rich foods (Guamuch et al., 2014). Therefore, one of the major aims of this study is to use blends of pre-treated maize-soybean flours with good balance of nutrients and fortification of the blends with foods (crayfish, bonga fish and carrot flours) rich in the nutrients to enhance the densities of vitamin A, calcium, zinc and iron that would meet the recommended daily allowance (RDA) for infants and young children. Also part of the study is to compare the nutrients composition of the complementary food blends with Cerelac - a product of Nestle (Nigeria) PLC so as to assess potentials of the complementary foods in addressing issues of micronutrient deficiencies (MND) prevalent in third world countries especially Nigeria.
MATERIALS AND METHODS
Maize (Zea mays L.), soyabeans (Glycine max), carrot (Daucus carota), bonga fish (Ethmalosa fimbriata) and crayfish (Macrobrachium species) were purchased from Ogbate market in Enugu, Nigeria. The foodstuffs were used to formulate three complementary foods composite blends in this study. Also, Cerelac (a product of Nestle Nigeria PLC), was purchased from the same market and used for comparison purposes (Table 2).
Preparation of germinated maize and soybean grains
Germination was done by the modified method of Ren et al. (2017). Maize and soybean seeds were disinfected by soaking in 70% ethanol solution for 15 min at room temperature after cleaning by hand to remove foreign materials. The ethanol was washed out of the seeds with tap water and distilled water and later soaked in distilled water (1:5, w/v) for 12 h at room temperature (~30°C). The seeds were placed between thick layers of jute bag after the water was drained off and germination lasted for 4 days in the dark. Fresh distilled water was used every day to moisten the seeds. The germinated seeds coats were removed. The decoated-germinated beans were subjected to 10 min steaming and subsequently cooled to room temperature and dried at 50°C in a Gallenkamp oven (ModelIH-150; Gallenkamp, England) and later milled into flour using a Bentall attrition mill (Model 200 L090). The flour was stored in polyethylene bags at 4°C for further analysis.
Preparation of carrot, bonga fish and crayfish flours
Tap water was used to wash the carrots which were air dried for 48 h after processing with kitchen grater. Crayfish and bonga fish (bony fish) were milled and sun-dried on a hot day and high breeze for 12 h.
Preparation of composite flours
The formulations of three complementary foods composite flours and fortificants were as follows:
Blend 1: Maize: Soyabean: food fortificant (70:30:0 %w/w)
Blend 2: Maize: Soyabean: food fortificant (60:30:10 %w/w)
Blend 3: Maize: Soyabean: food fortificant (50:30:20 %w/w)
Food fortificants: Crayfish: Bonga fish: Carrot. 2:1:1 (w/w) ratio
Each of the three complementary foods composite flours mixtures was separately milled into a homogenous powder in a 2 L mistral grinder and stored in containers that are airtight awaiting analyses. The Nestle Cerelac was used as control in comparing the nutrient levels of the composite blends against quantities of nutrients present in the estimated 65 g (dry weight) daily intake size of local weaning food by 6 to 11 month old infants. Thereafter, the blends nutrient densities were compared with Recommended Nutrient Intakes (RNIs) to assess the local diets compliance with the nutrient recommendations (Solomon, 2005).
Analysis
The proximate composition (moisture, crude protein, fat, crude fibre and ash) of the fortified maize-soyabean blends were determined by AOAC (2009) methods. Triplicate analysis was carried out to obtain a mean value for each nutrient. Carbohydrate content was determined by subtracting the sum of moisture, protein, fat, ash and crude fibre percentage from 100.
The energy value was determined by Atwater conversion factor (Nyahabeh et al., 2020) as follows:

The oxalate contents of the fortified complementary food blends were analysed using the spectrophotometric (Genway 6305, England) method of Fassat (1973) as modified by Mishra et al. (2017). The method of Price et al. (1980) as described by Abidemi (2013) was used to analyse the tannin content. Also, the analysis of phytate was according to the method of Latta and Eskin (1980) as modified by Sivakumaran and Kothalawala (2018). The vitamin A content in the fortified complementary food blends was assayed according to the method of Arroyave et al. (1982). Minerals’ (iron, zinc and calcium) content was assayed using Atomic Absorption Spectrometer (AAS) (model 210 VPG) according to the method described by the AOAC (2009).
Statistical analysis
Each experiment was performed in triplicate. Statistical Package for Social Science (SPSS) version 17 was used for the Analysis of Variance. Significantly different means were separated using Least Significant Difference test (LSD) at p<0.05.
RESULTS AND DISCUSSION
Composition of maize-soybean complementary blends
Table 1 shows the proximate composition of maize-soyabean complementary food (CF) blends. The blends energy content ranged from 400.68 to 408.71 kCal/100 g, with average of 4.04 kCal/g energy density. Codex Alimentarius Guidelines (FAO/WHO, 2017) for formulated supplementary foods recommended energy density of at least 400 kCal/100 g of dry food for older infants and young children. Significant difference (p<0.05) was not observed between the energy content of the fortified blends and non fortified blends. However, for age groups 6-8 months, 9-11 months and 12-23 months, the total energy requirements are 615, 686, and 894 kCal/day, respectively. Also, for infants in developing countries that lack adequate breast milk intake, the energy need from CFs was expected to increase from 200 to 300 kCal/day and 550 kCal/day at 6-8 months, 9-11 and 12-23 months (Table 3), respectively (WHO/UNICEF, 1998; Dewey and Brown, 2003).
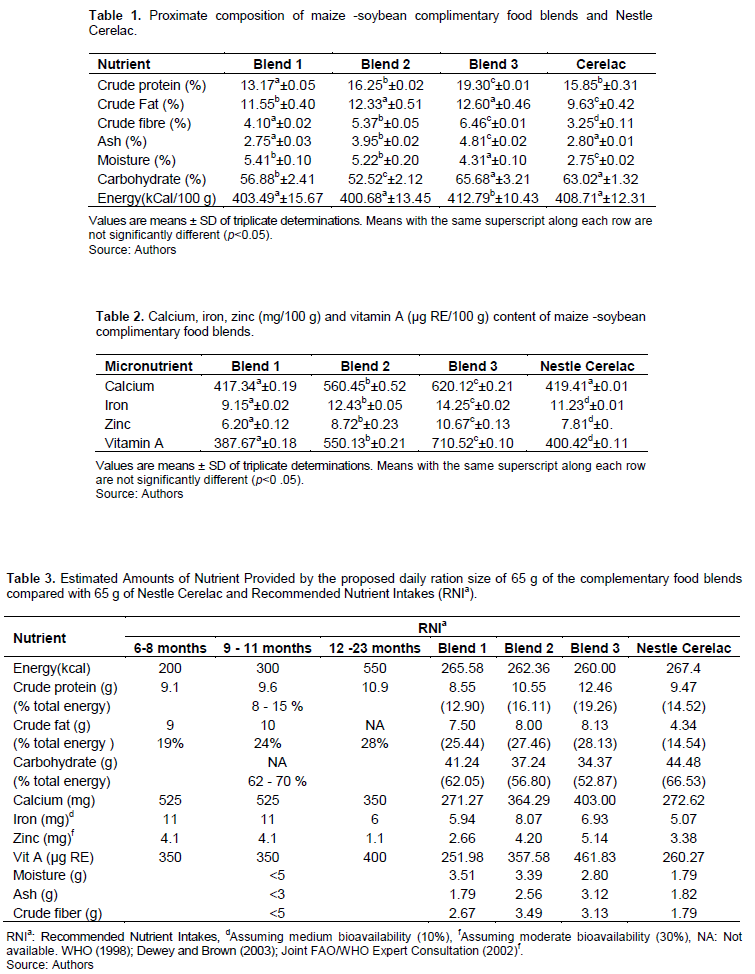
A proposal of fortification levels based on a single daily ration size of 65 g (Table 3) for the age groups was made. It follows that the consumption of 262 kCal/day (65 g of dry product of blend 2) by infants, 9-11 months, and children 12-23 months will amount to 88.5 and 48% of their energy intake from complementary foods. Also, the observed inverse relationship between the age and proportion of energy requirements that would be met is attributed to the fact that a greater proportion of complementary foods provided from the family diet would be consumed gradually by the child (Abeshu et al., 2016). Infants have only limited gastric capacity to consume adequate quantity of food, hence the recommendation by the World Health Organisation that they be fed with high nutrient density diets (Stephenson et al., 2017).
The crude protein contents of fortified CF blends vary from 13.17 to 19.30 mg/100 g. Results showed significantly (p<0.05) higher values than unfortified blend. The increase in protein content followed with incremental addition of fortificants. This may be due to protein contributions from bonga fish and crayfish in the fortificant (FAO/WHO, 2017).
The percentage total energy of 12.90, 16.11 and 14.52% (blends 1, 2 and cerelac) (Table 3) contribution from protein in 65 g CF agreed with the 8-15% recommendation (Alvisi et al., 2015). The reported Protein-Energy (PE) ratios of our CF blends are therefore adequate.
The fortified blends fat content (12.33-12.60 g/100 g) were 6.75 to 9.10% more than the fat in unfortified blend. The observed high values were probably contributions from fortificants. The fat contents in 65 g of the formulated CF (7.5-8.13 g) were comparatively higher than that from commercial formular, Cerelac (4.34 g). The addition of oil-dense soya beans in the local blends could be responsible for the higher values. The percentage of energy as fat from the proposed 65 g formulated CFs is 25.44 to 28.13% (Table 3). This is in agreement with recommendation of 24 and 28% energy sourced from fat for infants and children aged 6 to 11 months, and 12 to 23 months, respectively (WHO/ UNICEF, 1998; Dewey and Brown, 2003). The energy, essential fatty acids, and fat soluble vitamins (A, D, E, and K) for infants and young children are derived from dietary fats (Abeshu et al., 2016).
The fortified blends fibre content (5.37 to 6.46 g/100 g) was 31.0 to 57.6% higher when compared with the unfortified blend. The fiber content increases with level of fortificants added. This may be due to carrot flour which contains relatively large amount of cellulose as fibre. Bioavailability of micronutrients during infancy is encouraged by the reduction of use of large quantities of pulses, whole grain cereals and nuts since they could cause a low-energy diet (Caballero et al., 2005). The level of fibre obtained in this study is within the standard which must not exceed 5 g/100 g of food. The low fibre content could result from the use of dehulled raw materials in the formulation.
The fortified blends carbohydrate content varied from 52.52 to 56.88 mg/100 g (Table 1). The values were 10.8 to 20.1% lower than unfortified blend. The carbohydrate content in the proprietary formula (44.8 g) is higher than the CF blends (34.37- 41.24 g). This could be as a result of increases in protein, fat, ash and fiber contents resulting from CF blends fortification.
The fortified blends ash content varies from 3.95 to 4.81 g/100 g and 43.63 to 70.90% higher when compared with the unfortified blend. The increase which is probably an indication of presence of high quantity of minerals, especially the macro minerals might be attributed to the fortification effect. The ash content (Table 3) in the proposed 65 g CF blends (2.56-3.12 g) is relatively higher than that of the proprietary formula, cerelac (1.82 g). Reported ash contents in complementary foods by other workers that included crayfish in their respective formulations were similar to the results obtained in this study (Abbey and Nkanga, 1998; Solomon, 2005).
The low moisture content of the samples (4.31-5.41 g/100 g) could be attributed to the hydrolysis of starch and protein macromolecules during seed sprouting that resulted in loss of water holding capacity.
Mineral and vitamin A contents of maize-soyabean complementary food blends
Table 2 shows the minerals (calcium, iron, zinc) and vitamin A contents of maize-soyabean blends.
The fortified blends calcium content ranged from 560.45 to 620.12 mg/100 g. The increase over the unfortified sample was 34.29 to 48.58% and could be derived probably from high calcium content in bonga fish, crayfish and carrot. The proposed daily ration size of 65 g would provide 271 to 403 mg of calcium from the formulated complementary foods for infants 6-8, and children 9-11 and 12-23 months (Table 3). This amount is inadequate considering the World Health Organization recommendation of 525 g for ages 6-8, and 9-11 and 350 g for age 12-23 months, respectively (WHO, 1998). However, increasing the frequency of CFs meal consumption to 2 times per day would meet 72% of calcium needed from complementary foods.
The iron content of the fortified samples varies from 12.43 to14.25 mg/100 g. The significant (p< 0.05) increase in iron content after fortification varied from 32.31 to 60.10%. This could be as a result of addition of bonga fish and crayfish which are known sources of iron and enhancer of its bioavailability in the formulation. The 65 g proposed daily ration size would provide 5.94 to 8.07 mg of iron from complementary foods for infants 6-8 and children 9-11 and 12-23 months old (Table 3). This range is inadequate considering phytate restrictive effect on iron bioavailability. To overcome the constraint, administration of the ration size two times per day to infants would help meet the 97% of iron needed from CFs for 9-11 age groups and prevent iron deficiency (Dewey, 2013). Fewtrell et al. (2017) recommended 11 g/day for infants 6-11 months and 6 g/day iron for children, 12-23 months, respectively.
The fortified samples zinc content ranged from 8.27 to 10.67 mg/100 g. The observed increase follows same pattern as iron. The increase when compared with the unfortified blend varied from 2.52 to 4.47 g/100 g and could be attributed to the contributions from bonga fish and crayfish. The earlier proposed 65 g daily ration size would provide 2.66 to 5.14 mg of zinc from the formulated complementary foods for infants 6-8 and children 9-11 and 12-23 months (Table 3). Masters et al. (2017) reported that 4 to 5 mg of zinc should be provided by the daily ration of a fortified complementary food. Also, a recommendation of 4.1 mg zinc for ages 6-8 and 9-11 months, and 1.1 mg for age 12-23 months was made by the Joint FAO/WHO Expert Consultation (2002). The complementary food (blend 3) that provides 5.14 g zinc could be adequate to meet the zinc RDA for the age groups and 86% of zinc needed from complementary foods (Dewey, 2013). Breast milk intake partially covered calcium requirements; however, most iron and zinc RNIs need to be obtained from complementary foods (Ortenzi and Beal, 2021).
The vitamin A content of the samples ranged from 387.67 to 710.52 μg RE/100 g. Results show that fortified samples had values ranging from 162.46 to 322.85 μg RE/100 g and were significantly higher (p<0.05) than the unfortified sample and may be due to the retinol content in bonga fish and crayfish and high carotenoids contents as reported in carrots (Simon and Wolf, 1987). Noccolo et al. (2003) reported that carotenoids main physiological function is as precursor of vitamin A. The proposed daily ration size of 65 g would provide 251 to 461 μg RE/100 g of Vitamin A from the formulated complementary food blends for infants 6 to 8 and children 9 to 11 and 12 to 23 months old (Table 3). The Joint FAO/WHO Expert Consultation (2002) recommended 400 μg RE/100 g vitamin A Daily Intake for both age groups. However, 350 and 400 μg RE/100 g daily vitamin A intake were variously recommended by World Health Organization (WHO, 1998) for infants and children 6-11 and 12-23 months old. According to Ortenzi and Beal (2021), breast milk provides largely vitamin A requirements, with only 20% needed from complementary foods.
Anti-nutrientional contents of maize-soyabean complementary blends
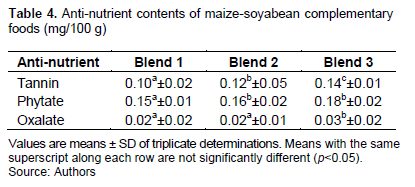
The levels of anti-nutrient properties of CFs produced from dehulled, germinated and cooked maize-soybean are shown in Table 4. The tannin, phytate and oxalate contents range from 0.10-0.14, 0.15-0.18 and 0.02-0.03 mg/100 g for blends 1, 2 and 3, respectively. The results showed that the anti-nutrient contents were generally low due to the processing methods employed. The levels however increased by the increased addition of carrots in fortificants. The increase in tannin, phytic and oxalate contents of fortified samples was 20-40, 6-20 and 0-50% when compared with the unfortified sample and was significant (p<0.05). Soaking, cooking and germination have been reported by many investigators to cause reduction in phytic acid content in millets and chickpeas (Sarita and Singh, 2016; Shi et al., 2018). The decrease in phytic acid content by the pre-treatment processes may be due to phytase action and leaching of this compound in water (Sinha and Khare, 2017). Handa et al. (2017) also reported that germination removed heat stable compounds in cereals and legumes such as tannins and hydrates. The observed values of tannin, phytate and oxalate were safe and could not result in adverse physiological effects when consumed since they were lower than the safe levels of 2.0, 5.0 and 2.2 g/100 g, respectively (Monri and Bassir, 1969).
CONCLUSION
Home-made complementary foods in sub-Sahara Africa are mostly produced from cereals and legumes and are frequently part of family foods such as porridges and gruels. The diets therefore have high energy content and consequently very low in nutrient density of micro-nutrients, such as iron, calcium and zinc, recorgnized as “problem nutrients” by World Health Organization (WHO). The complementary food formulated during this investigation using dehulled germinated maize-soybean flours fortified with bonga fish, crayfish, and carrot had shown to contain higher levels of protein, calcium, iron, zinc compared to cerelac the commercial complementary food. Therefore, household level production of the fortified complementary foods is recommended. This is feasible since the crops employed in the formulation are produced in large quantity in Nigeria. Also, the technologies which are easily accessible to both the rural and urban poor with less expensive equipment are evidently available at household level. When this is done, infants would be fed diets that will promote good health and prevent micronutrient deficiency diseases and protein energy malnutrition. Blend comprising 50% maize, 30% soyabean and 20% fortificant flours which contained the highest macro and micronutrients when compared with the others is considered the best in terms of nutritional content.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Abbey BW, Nkanga UE (1988). Production of High Quality Weaning Product Maize-cowpea-crayfish mixtures. Nutrition Report International 37(5):951-957. |
|
|
Abeshu MA, Azeb L, Bekesho G (2016). "Complementary Feeding: Review of Recommendations, Feeding Practices, and Adequacy of Homemade Complementary Food Preparations in Developing Countries- Lessons from Ethiopia." Frontiers in Nutrition 3(October). |
|
|
Abidemi OO (2013). Phytochemicals and Spectrophotometric Determination of Metals in Various Medicinal Plants in Nigeria International Journal of Engineering Science Invention 2(5):51-54. |
|
|
Alvisi P, Brusa S, Alboresi S, Amarri S, Bottau P, Cavagni G (2015) Recommendations on complementary feeding for healthy, full-term infants. Italian Journal of Pediatrics 41:36. |
|
|
Arikpo D, Edet ES, Chibuzor MT, Odey F, Caldwell DM (2018). Educational interventions for improving primary caregiver complementary feeding practices for children aged 24 months and under. Cochrane Database System Review 5: p. CD011768. |
|
|
Arroyave G, Chichester CO, Hernando F, Glover J, Mejia LA, Olson JA (1982). Biochemical Methodology for the Assessment of Vitamin A Status. The Nutrition Foundation, DC pp. 24-30. |
|
|
Association of Official Analytical Chemists (AOAC) (2009). Official methods of Analysis of the Association of Official Analytical Chemists International, 17th edition, Arlington U.S.A. Official Methods 945.1. |
|
|
Beal T, Massiot E, Arsenault JE, Smith MR, Hijmans RJ (2017). Global trends in dietary micronutrient supplies and estimated prevalence of inadequate intakes. PLoS One 12 (4):e0175554. |
|
|
Caballero B, Allen L, Prentice A (2005). Encyclopedia of Human Nutrition. 2nd ed. Oxford: Elsevier Academic Press. |
|
|
Campoy C, Campos D, Cerdó T, Diéguez E, García-Santos JE (2018). Complementary Feeding in Developed Countries: The 3 Ws (When, What, and Why?). Annual Nutrition Metabolism 7(1):27-36. |
|
|
Dewey KG (2013). "The Challenge of Meeting Nutrient Needs of Infants and Young Children during the Period of Complementary Feeding: An Evolutionary Perspective." The Journal of Nutrition 143(12):2050-2054. |
|
|
Dewey KG, Brown K H (2003). Update on technical issues concerning complementary feeding of young children in developing countries and implications for intervention programs. Food Nutrition and Bulletin 24(1):5-28. |
|
|
Dulal B, Mundy G, Sawal R, Rana PP, Cunningham K (2017). Homestead Food Production and Maternal and Child Dietary Diversity in Nepal: Variations in Association by Season and Agroecological Zone Food and Nutrition Bulletin 38(3):338-353. |
|
|
FAO (2020). Food and Agriculture Organization of the United Nations. Rome, Italy: FAOSTAT database. Accessed May 15, 2020. |
|
|
FAO/WHO (2017) Codex standard for processed cereal-based foods (including guidelines on formulated supplementary foods for older infants and young children). World Health Organization, Geneva, Switzerland. |
|
|
FAO/WHO (2002). Joint Expert Consultation. Vitamin and mineral requirements in human nutrition. Geneva: World Health Organization. |
|
|
Fasset D W (1973). Oxalates, in Toxicants Occurring Naturally in Foods, ed. by National Research Council. National Academy of Science, Washington DC. pp. 346-362. |
|
|
Fewtrell M, Bronsky J, Campoy C, Domellof M, Embleton N, Fidler MN (2017). Complementary feeding: a position paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. Journal of Pediatrics Gastroenterol Nutrition 64:119-132. |
|
|
Guamuch M, Dary O, Rambelson Z, Cruz V, Villalpando S, Tom C, Afidra R, Makhumula P (2014) . Model for estimating nutrient addition contents to staple foods fortified simultaneously: Mexico and Kampala data. Annual New York Academy of Science Journal 1312:76-90. |
|
|
Handa V , Kumar V, Panghal A , Suri S, Kaur J (2017). Effect of soaking and germination on physicochemical and functional attributes of horsegram flour. Journal of Food Science and Technology 54(13):4229-4239. |
|
|
Latta M, Eskin MA (1980). Simple and rapid colorimetric method of phytate determination. Journal of Agriculture and Food Chemistry 29:315-317. |
|
|
Masters A, Nene MD, Bell W (2017). Nutrient composition of premixed and packaged complementary foods for sale in low- and middle-income countries: Lack of standards threatens infant growth. Maternal and Child Nutrition 13(4):e12421. |
|
|
Mishra DP, Mishra N, Musale HB, Pinaki P, Mishra S P, Swain DP (2017). Determination of seasonal and developmental variation in oxalate content of Anagallis arvensis plant by titration and spectrophotometric method. The Pharma Innovation Journal 6(6):105-111. |
|
|
Monri A, Bassir O (1969). Oxalate in Nigeria vegetables, West African Journal of Biology, Agriculture and Chemistry 12(1):14-17. |
|
|
Nocolle C, Cardinault N, Aprikian O, Busserolles J, Grolier P, Rock E, Demigne C, Mazur A, Scalbert A, Amouroux P, Remesy C (2003). Effect of carrot intake on cholesterol metabolism and antioxidant status in cholesterol fed rats. European Journal of Nutrition 42(5):254-261. |
|
|
Nyahabeh MA, Wasiu AA, Martha SE, Williams BM, Oguntona EB (2020). Appraisal and composition of some traditional complementary foods for infant nutrition in Sierra Leone. International Journal of the Science of Food and Agriculture 4(1):73-79. |
|
|
Ortenzi F, Beal T (2021). Priority micronutrient density of foods for complementary feeding of young children (6-23 months) in South and Southeast Asia Frontier Nutrition 8:785227. |
|
|
Owais A, Schwartz B, Kleinbaum DG, Suchdev PS, Faruque ASG, Das SK, Rahman S, Stein AD (2017). A Nutrition Education Program in Rural Bangladesh Was Associated with Improved Feeding Practices but Not with Child Growth. Journal of Nutrion 147(5):948-954. |
|
|
Panjwani A., Heidkamp R (2017). Complementary Feeding Interventions Have a Small but Significant Impact on Linear and Ponderal Growth of Children in Low- and Middle-Income Countries: A Systematic Review and Meta- Analysis. Journal of Nutrition 147(11):2169S-2178S. |
|
|
Price ML, Hagerman AE, Butler LG (1980). Tannin content of cowpea, chickpea, pigeon pea and mung bean. Journal of Agriculture and Food Chemistry 28(2):459- 461. |
|
|
Regan L, Bailey KP, West Jr, Robert EB (2015). The epidemiology of global micronutrient deficiencies. Analytical Nutrition and Metabolism 66(2):22-33. |
|
|
Ren Q, Wang J, Iiu S, Wang F, Wang H (2017). Identification and determination of isoflavones in germinated black soybean sprouts by UHPLC−Q-TOF-MS mass spectrometry and HPLC-DAD. International Journal of Food Properties 20(12):877-2887. |
|
|
Sarita ES., Singh E (2016). Potential of millets: nutrients composition and health benefits. Journal of Scientific and Innovative Research 5(2):46-50. |
|
|
Shi L, Arntfield SD, Nickerson M (2018) Changes in levels of phytic acid, lectins and oxalates during soaking and cooking of Canadian pulses. Food Research International 107:660-668. |
|
|
Simon P W., Wolff X Y (1987). Carotene in typical and dark orange carrots. Journal of Agriculture and Food Chemistry 35:1017-102. |
|
|
Sinha K., Khare V (2017). Review on: Antinutritional factors in vegetable crops. Pharmaceutical Innovation 6(12):353-358. |
|
|
Sivakumaran K, Kothalawala S (2018). An overview of the analytical methods for food phytates International Journal of Chemical Studies 6(1):2016-2020. |
|
|
Solomon M (2005). Nutritive value of three potential complementary foods based on cereals and legumes. African Journal of Food Agriculture 5(2):1-15. |
|
|
Srivastava S, Chaturvedi N (2020). Complementary feeding practices: a critical intervention for survival and well-being of children. International Journal of Recent Scientific Research 11(8):39557-39563. |
|
|
Stephenson KB, Sophia EA, Oscar DY, Kenneth MM, Chrissie TM, Isabel IT, Mark JM (2017). Complementary feeding with cowpea reduces growth faltering in rural malawian infants: A blind, randomized controlled clinical trial. The American Journal of Clinical Nutrition 106(6):1500-1507. |
|
|
World Health Organization (WHO) (1998). Complimentary Feeding of Young Children in Developing Countries. A review of current scientific knowledge, 228 p WHO/NUT/98.1. |
|
|
WHO/UNICEF (1998). Complementary feeding of young children in developing countries: a review of current scientific knowledge. Geneva: World Health Organization, WHO/NUT/98.1. |
|
|
WHO/UNICEF (2021). United Nations Children's Fund, World Health Organization, & International Bank for Reconstruction and Development/The World Bank. (2021). Levels and trends in child malnutrition: Key findings of the 2021 edition of the Joint Child Malnutrition Estimates. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0