Full Length Research Paper
ABSTRACT
Platform tests are usually simple and rapid quality control tests which serve as a basis for accepting, setting aside or rejecting raw milk. Some of the rapid antibiotic residues test kits available in the Kenyan market include: Delvo test Fast BL, Delvo Sulphadiazine Penicillin No Tablet (SPNT), Mtusbio Beta-lactam BLQ Rapid Test Kit and Ringbio beta-lactam, tetracycline, sulfa drugs, BTS 3 in 1 TriTest S. Ndungu Antibiotic residues (NAR) is a colour comparison test that is simple, rapid and does not require electric power in its analysis procedure. This study compared these four rapid antibiotics tests with novel NAR test method in terms of sensitivity and specificity. A total of 28 samples were prepared; 8 milk samples without residues were spiked with milk known to have beta-lactam residues while 10 had no residues and 10 had known beta-lactam (benzyl penicillin) residues. The four analysis methods were carried out as described in their technical bulletins and manuals. For the NAR test, 50 mg of its active ingredient was mixed with 3 ml of milk and colour change observations made. The sensitivity and specificity of the NAR test was found to be 66.7 and 100% respectively while for all the other four tests, sensitivity and specificity was established to be 100% for each. The Kappa coefficient was 0.5882 which indicates moderate agreement, between NAR test method and the other test methods, according to Landis-Koch scale. The odds ratio exhibited positive association between the NAR test and the four methods. NAR test is best applicable at the milk collection routes or farm level before bulking for transportation.
Key words: Antibiotic residues, rapid test kit, beta-lactam, beta-lactamase, NAR test.
INTRODUCTION
Antibiotics are used in management of herd health, mainly prevention and control of diseases affecting dairy cattle. Such diseases include mastitis and reproductive tract infections which are readily treated and potentially controlled by antibiotics (Asredie and Engdaw, 2015). Globally, the most commonly used antibiotics can be grouped into five major classes and include: the beta-lactam group (36.54%), tetracyclines (14.01%), fluoroquinones (13.46%), sulfonamides (12.64%) and aminoglycosides (10.44%) (Sachi et al., 2019). The widespread use of antibiotics has created potential residue problems in dairy products making them unfit for human consumption (FAO and WHO, 2018). In developing countries, Kenya included, risks associated with milk residues are higher due to inadequate detection methods and insufficient enforcement by the regulatory bodies (Kebede et al., 2014).
Differences exist in the methods applied in antibiotic residues testing including, the type of the test, susceptibility of test groups of antibiotics, the determination procedures and financial demands (Gondova et al., 2014). Ideal testing of antibiotic residues is mostly through sophisticated methods, such as mass spectroscopy, liquid chromatography, vibrational spectroscopy, microbiological methods and immunological assays. These methods have high accuracy and resolution but require expertise and are expensive (Salois et al., 2015). Others take long time to give results and are therefore not applicable in milk acceptance as platform tests. The high cost of the available testing kits and low or lack of consumer demand for antibiotic free dairy products makes the situation worse. To curb this vice, a need to sensitize farmers on withdrawal periods should be enhanced since this is a critical control point (Ndungu et al., 2016). Milk processing companies, cooperatives societies, formal and informal milk marketers should prioritize antibiotic residues testing before accepting milk. This will instill discipline to the value chain actors and enhance consumer safety.
The use of commercially available rapid antibiotic residue screening tests has played a large role in preventing the sale and consumption of antibiotic contaminated milk and its products. These rapid tests are designed to produce on-farm results and facilitate milk grading. They are mostly manufactured using the lateral flow technology which detects biomolecules in complex samples. The sensitivity of these assays depends on the components used, how the components and sample are treated, and the properties of the nano scale reporter particles that generate the diagnostic signal (Sajid et al., 2014). This technology has been verified to be efficient but its sustainability is questionable, considering the way milk collection is carried out in Kenya; 6-10 farmers bulking in a 50 kg milk can. Also, these tests require power source in their testing procedure, which is not readily available in the milk collection routes.
This study highlights some rapid tests for beta-lactam antibiotics available in the Kenyan market; Delvo test Fast BL (Delvo BL), Delvo Sulphadiazine Penicillin No Tablet (Delvo SPNT), Mtusbio Beta-lactam BLQ Rapid Test Kit (Mtusbio) and Ringbio beta-lactam, tetracycline, sulfa drugs, BTS 3 in 1 TriTest S (Ringbio). The sensitivity and specificity of these rapid tests will be compared with a novel beta-lactam detection test [Ndungu Antibiotic Residues (NAR) test method], which is a colour comparison test; hence it is qualitative, simple, rapid and does not require electric power in its analysis procedure.
MATERIALS AND METHODS
Study site
This research was carried out at Olenguruone Dairy Farmers Cooperative Society Milk Quality Control Laboratory as well as Happy Cow Limited laboratory.
Experimentation
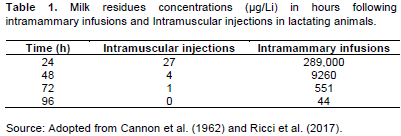
The sampling procedure was as per ISO 707; IDF 50, 2008 without modification. The beta-lactam antibiotic positive milk samples were obtained from cows which have been treated with beta-lactam. Table 1 indicates the depletion of concentration of milk residues in hours following treatment for both intramammary infusions and intramuscular injections. A total of 28 different samples with an approximate volume of 50 ml were prepared for use in the analysis. The beta-lactam (penicillin) antibiotic positive samples were 18 including 8 antibiotic free milk samples spiked with 1, 5, 10, 15, 20, 25, 50 and 75% of known beta-lactam residues positive milk samples. The known antibiotic free milk samples (negative) were 10. All samples were numerically coded for randomization before the experiment. The samples were then analyzed immediately after sample preparation using five different test methods earlier mentioned. The analysis procedures as described on their technical bulletins and as per their manufacturer’s instructions were followed.
Antibiotic residue detection methods
Delvo Sulphadiazine Penicillin No Tablet (SPNT) (Delvo SPNT)
The method of analysis applied was AOAC 982.18 and is as described in the Delvo test technical bulletin. It is a microbiology broad spectrum inhibitor test and therefore able to detect antibiotic residues and any other inhibitory substances in milk. The microorganism involved is Bacillus stearothermophilus and is used because of its high sensitivity to the majority of antibiotics (Abebew et al., 2014). In addition, the test ampoules have selected nutrients, and pH indicator bromocresol purple. After adding 0.1 ml milk sample directly to the ampoules, an incubation step was conducted for 3 h at 64 ± 0.5°C. During incubation, microbial metabolism resulted in a change in pH and the ampoule agar colour from purple to yellow in absence of inhibitory substances. By contrast, if the sample contained sufficiently high concentrations of inhibitory substances, the colour would remain purple (Stead et al., 2008). Test results interpretations were performed according to the manufacturer’s instructions.
Delvotest Fast BL (Delvo BL)
This is a one-step receptor assay using lateral flow technology that enables the detection of beta-lactam residues of antibiotics in raw cow milk. The method of analysis applied was as described in the Delvo test technical bulletin for this particular test. The test method is sensitive to all drugs containing a beta-lactam ring, including: Penams (penicillins) extended and narrow spectrum, Penams beta-lactamase resistant and beta-lactamase sensitive, Cephalosporines of 1st, 2nd, 3rd, 4th, and 5th generation. It detects positive samples with a confidence of at least at 95% in raw milk samples with Penicillin. The levels of detection have been indicated to be 4, 3 and 3 µg/L for amoxicillin, ampicillin and penicillin, respectively. The test kit is made of ampoules, disposable pipettes and test strips which are to be used together in the analysis. It takes approximately 7 min to carry out the analysis and read the results.
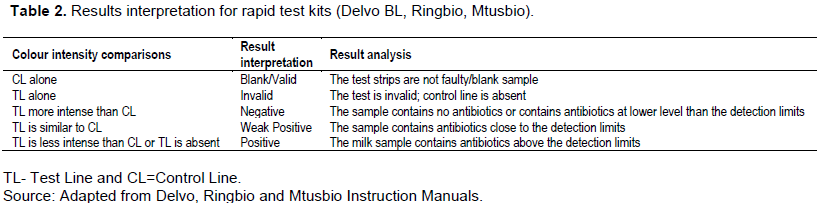
After adding 0.15 ml of raw milk in the ampoule, the contents were shaken until they completely dissolved. The ampoule was then incubated at 64°C±2°C for 2 min and then test strip inserted and incubated for 3 more minutes. After 7 min, the results were read and interpreted as described in Table 2.
Mtusbio Beta-lactam BLQ Rapid Test Kit (Mtusbio)
The kit is a competitive colloidal gold immunoassay method. The test strip is used for detecting beta-lactam family in raw cow milk above or below tolerance levels. The test kit detection limit is 4 µg/L for penicillin, ampicillin and amoxicillin, respectively.
During the analysis, 200 µl of milk sample was added into the ampoule with reagent. These were then thoroughly dissolved for 5 min until the milk turned pink. The test strip was then inserted into the ampoule and the results were read after 5 min. The results interpretation was as described in Table 2.
Ringbio beta-lactam, tetracycline, sulfa drugs, BTS 3 in 1 TriTest S (Ringbio)
This test is used to detect beta-lactam, tetracycline and sulfonamides antibiotic residues in milk. It is a receptor assay that utilizes high affinity antibodies and capture proteins against the antibiotics, which can easily identify the residues without any instrument. This test is ready to use, does not require incubation and results are obtained within 6 min. Its limit of detection is 4 µg/L for penicillin, amoxicillin and ampicillin.
During the analysis, 200 µl of the milk sample was added into the micro well, then repeatedly absorbed up and down for 5 times. This ensured complete mixing of the sample with the reagent in the wells until it turned pink. The mixture was incubated for 5min at room temperature, and then the test strip inserted into the well. Another incubation for 5 min at room temperature was done and the strip was taken out for results reading. Interpretation of the results was carried out as described in Table 2.
Ndungu antibiotic residues (NAR) test
This is an acidometric method which links the hydrolysis of beta-lactam ring by beta-lactamase enzyme resulting to a drop in pH due to production of penicilloic acid. Consequently, a colour change is exhibited by phenol red which is an acid-base pH indicator. This test method is simple, rapid and should be carried out at room temperature. The NAR test method uses test ampoules in the analysis. For a negative sample result, the colour expected is fuchsia purple while for a positive sample, the colour is peach or pink (Ndungu et al., 2021).
The procedure involved mixing 3 ml of raw milk sample in one ampoule. The contents were then shaken until they completely dissolved. The colour change observations were immediate and the results were recorded accordingly. Figure 1 shows the colours expected for a positive and a negative test.

Statistical analysis
The true positive and negative results obtained were tallied and data analyzed using the diagt command of STATA version 12 to determine the sensitivity, specificity, positive predictive value and negative predictive values of the new method as compared to the other test methods. Positive predictive value (PPV) was the proportion of milk samples with positive test which indeed were positive and the negative predictive value (NPV) was the proportion of milk samples with a negative test result which indeed were negative. In addition, the Cohen’s kappa coefficient (k) was used to find out the agreement between the methods tested while odds ratio was used to evaluate the association amongst the methods.
Research permit and ethical approval
This study was approved by Egerton University Board of Post Graduate Studies. Ethical clearance approval was obtained from Egerton University Ethics Review Committee (EUREC) (approval number; EUREC/APP/097/2020) and the research license obtained from National Commission for Science, Technology and Innovation (NACOSTI) (license number; NACOSTI/P/20/5861).
The aim and procedures of the study were explained to the study participants who were required to give written informed consent prior to their voluntary participation in the study. Confidentiality of research information and data was observed and maintained through forms issued and signed before research commenced, password protected computers and observing good professional conduct.
RESULTS
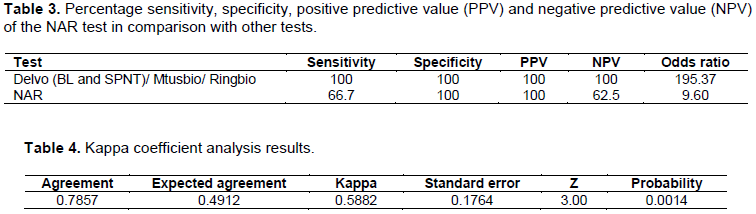
The results in Table 3 show sensitivity and specificity for all the five test methods. For NAR test method, sensitivity and specificity of 66.7 and 100%, respectively. The sensitivity and specificity of Mtusbio, Ringbio, Delvo BL and Delvo SPNT tests was 100%. These results indicate that the four test methods are more sensitive than the NAR test. The NAR test method was able to detect 10 samples that were indeed negative (antibiotic free), and 10 samples that were indeed beta-lactam positive. It however failed to detect presence of residues in 6 samples spiked with beta-lactam positive milk and 2 samples indicated partial positivity. The samples that indicated partial positivity had a mixture of positive and negative milk at a ratio of 1:1 and 3:1, respectively. The specificity, which also explains the reproducibility, for the NAR test method was 100%. This implies that test method gave the same results for all the replications. The positive predictive value indicates the tests that are indeed positive (have beta-lactam residues) while the negative predictive value indicates the negative samples that are indeed negative (antibiotic free). The positive predictive value for the NAR test was 100% while the negative predictive value was 62.5%.
The odds ratio for the NAR test was 9.60 while for all the other tests it was 195.37. Since the odds ratio was greater than one for all the test methods, then there is a positive association among the test methods with NAR test method. Meaning NAR method was 9.60 more likely to give an accurate result compared to 195.37 likelihood for the other methods.
In Table 4, if each method (NAR test method vs any of the others) had made its determination randomly, we would expect the two methods to agree on 49.12% of the test samples. In this case, they agreed on 78.57% of the samples. The amount of agreement indicates that we can reject the hypothesis that the methods are making their determinations randomly. The Kappa coefficient in this case was 0.5882 which lies between 0.40 and 0.60, according Landis-Koch scale. This indicates that there was a moderate agreement between NAR test method and the other test methods. However, and for all the other tests, the agreement was 100% as they were able to detect all test samples as expected. The analysis therefore concludes that there was significant agreement (P ≤ 0.05) between the NAR test method and the other test methods.
DISCUSSION
In every analysis for the other four test methods used in comparison with NAR test method, all antibiotic free milk samples tested negative and all milk samples with beta-lactam residues tested positive. These results provide evidence that these rapid screening tests are potentially useful tools for monitoring raw milk products for the presence of antibiotic residues. Besides these methods, other methods based on inhibition of microorganisms have been used. For instance, the five-plate test, called Screening Test for Antibiotic Residues (STAR), was used on 10 different groups of antibiotics: macrolides, aminoglycosides, cephalosporins, penicillins, quinolones, tetracyclines, sulphonamides, lincosamides, phenicolated and miscellaneous drugs (Gaudin et al., 2004). Khaskheli et al. (2008) screened for β-lactam using a microbial screening test; B. subtilis field disc assay. Nevertheless, despite their usefulness, they have been reported to be less useful in the farm set up due to the complexity of the tests, the resources demanded and the duration of time taken before decision is made (Ahlberg et al., 2016).
Kits based on some of these tests have also been employed in the detection and quantification of antibiotic residues. Yamaki et al. (2004) used Delvotest SP test for β-Lactam and sulfonamide, Kurwijila et al. (2006) used the Charm-AIM screening test kit to detect β-lactam, tetracyclines, aminoglycosides, macrolides, and sulfonamides while Ahlberg et al. (2016) used the Delvotest screening test and the Trisensor test to detect β-lactam, sulfonamides and tetracyclines. Nevertheless, despite their usefulness in the determination of antibiotic residues, these tests and developed methods may have inefficiencies. While analyzing the presence of antibiotic residues in milk, Ahlberg et al. (2016) used two kits: Delvotest screening test and the Trisensor test. Results indicated the presence of antibiotics in milk. However, further analysis with HPLC indicated no antibiotics. In addition, in their research, 76% of the Delvotest positive samples were negative in the Trisensor test. In another study, while using a microbial inhibition assay, Delvotest SP-NT test kit and liquid chromatography coupled to mass spectrometry (LC-MS/MS) was used (Layada et al., 2016).
Inefficiencies in methods of testing for antibiotic residues can potentially result in consumption of milk contaminated with antibiotic residues despite testing. To assure food safety and reduce processing losses, testing of residues in raw milk should be used as a parameter in milk acceptance. The sensitivity of NAR test method is based on the presence or absence of the beta-lactamase enzyme in raw milk. This means that the test detects beta-lactams antibiotics that are in milk endogenically and hence intentionally added antibiotics as adulterants cannot be detected. The more available the enzyme is, the more easily it can be detected.
The β-lactam (penicillin, monobactams, carbapenems, and cephalosporins) have a β-lactam ring structure that gives them their antibacterial activity. According to Kivirand et al. (2015), all β-lactam antibiotics have a common element in their molecular structure: a four atom ring known as a β-lactam ring. They comprise the 4-membered β-lactam ring, the 6-membered ring (penicillins, carbapenems, and monobactams) and the 7-membered ring–structure (cephamycins and cephalosporins) antibiotics (Pandey and Cascella, 2020). Beta-lactam antibiotics act by inhibiting the bacterial cell wall biosynthesis, consequently leading to cell lysis and death. They specifically bind and acylate active site of penicillin-binding protein (PBP), the enzyme essential for the biosynthesis of bacteria cell wall.
To counteract bactericidal effect of β-lactam antibiotics, bacteria have been reported to show resistance (King et al., 2017). Bacterial resistance may occur if the bacteria can produce β-lactamase enzyme that is able to destroy β-lactam antibiotics. Beta-lactamase (BLs; Enzyme commission (EC) number 3.5.2.6) is an enzyme first identified in E. coli and has been described as penicillinase. Most enzymes are reported to act exclusively against penicillins or cephalosporins (Shaheen, 2013; Munita and Arias, 2016). BLs specifically hydrolyzes β-lactam rings present in antibiotics such as penicillin, cephalosporins, monobactam, and carbapenem, and confer resistance against these antibiotics. However, Bush (2018) reported poor hydrolysis on cephalosporins, carbapenems, or monobactams contrary to benzyl penicillin.
During the analysis, to establish the sensitivity of the test, beta-lactam residues free milk samples were spiked with beta-lactam positive milk samples. This influenced the availability of the beta-lactamase enzyme to initiate a reaction due to the dilution effect which reduced the concentration of the enzyme and consequently reduced the sensitivity of NAR test method. Moreover, reduced enzyme concentration decreased the formation of penicilloic acid which resulted to inadequate effect on the colour change. Differentiating between a known beta-lactam positive and negative raw milk sample became a challenge. However, NAR test can be applied in the milk collection routes to detect milk supplied by the unscrupulous farmers. This study agrees with Livermore and Brown (2005), who indicated that there could be slow hydrolysis reaction when beta-lactamase in milk sample is minimal. Li et al. (2014) also indicated that the dosage of beta-lactamase influences the production of penicilloic acid.
The NAR test can accurately detect a negative sample as well as a positive test when there is no dilution effect. Because of this, NAR test is most suitable at the farm level or milk collection routes before bulking for transportation. If applied, testing will instill discipline at the farm level and minimize entry of residues at the best critical control point as is required (Ndungu et al., 2016) and this will assure consumer safety and reduce processing costs. The cost of analyzing antibiotic residues where a large number of samples are involved plays a key role. The smallholder supply chain in Kenya involves over 1.8 million dairy farmers (KDB, strategic plan). It is therefore necessary to use less expensive methods (Layada et al., 2016) to cope with the numbers and become economically sustainable. According to Mwagore et al. (2019), rapid antibiotic residues detection kits available in Kenya are expensive and therefore unsustainable. Hence, there is a need to develop cheaper test methods to promote raw milk testing for the presence of beta lactam antibiotic residues. NAR test method is easy to carry out, does not require incubation, the results are immediate and therefore less time is required during testing.
Several studies have raised concerns about the utility of rapid on-farm antibiotic screening tests because of a high occurrence of false-positive results (Mullen et al., 2017). However, it is safer to create systems that allow for testing as this has been found to instill discipline in farmers regarding observation of the withdrawal periods. NAR test method could play a key role at the farm level to detect the presence of antibiotics in the raw milk and the suspected milk samples can be transported separately to the laboratory for further testing.
CONCLUSIONS
The NAR test method could be used to detect the presence of beta-lactam antibiotics in the raw milk samples at farm. This test may require further development to explore its functionality in detection of the different concentration of beta-lactamase enzyme which influences the production of penicilloic acid and also other associated parameters such as spore concentration, time, LOD, volume of the samples, regulatory requirements and cost. In addition, its application and or modification in detecting milk adulterated with antibiotics as well as detecting other antibiotic residues in milk should be evaluated. It is important to enhance antibiotic testing in milk collection chains to assure food safety and minimize processing losses often incurred.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGEMENTS
This research was carried out with financial support from the Centre of Excellence in Sustainable Agriculture and Agribusiness Management (CESAAM), Egerton University. The authors are thankful to those farmers who kindly provided milk samples and cows treatment data. The authors are also grateful to Olenguruone Dairy Farmers Cooperative Society and Happy Cow Ltd. for providing the laboratory where the study took place and to the laboratory staff who actively participated in the study.
REFERENCES
|
Abebew D, Belihu K, Zewde G (2014). Detection and determination of oxytetracycline and penicillin G antibiotic residue levels in bovine bulk milk from Nazareth dairy farms. Ethiopia. Ethiopian Veterinary Journal 18(1):1-15. |
|
|
Ahlberg S, Korhonen H, Lindfors E, Kang'ethe E (2016). Analysis of antibiotic residues in milk from smallholder farms in Kenya. African Journal of Dairy Farming and Milk Production 3(4):152-158. |
|
|
Asredie T, Engdaw TA (2015). Antimicrobial Residues in Cow Milk and its Public Health Significance. World Journal of Dairy and Food Sciences 10(2):147-153. |
|
|
Bush K (2018). Past and present perspectives on beta-lactamases. Antimicrobial Agents Chemotherapy 62:e01076-18. |
|
|
Cannon RY, Hawkins GE, Wiggins AM (1962). Duration of secretion of bacteriostatic drugs in milk. I. Penicillin, following oral and parenteral administration. Journal of Dairy Science 45(6):769-773. |
|
|
FAO and WHO (2018). Codex Alimentarius, Maximum residue limits (MRLS) and risk management recommendations (RMRS) for residues of veterinary drugs in foods CX/MRL 2-2018. |
|
|
Gaudin V, Maris P, Fuselier R, Ribouchon JL, Cadieu N, Rault A (2004). Validation of a microbiological method: the STAR protocol, a five-plate test, for the screening of antibiotic residues in milk. Food Additives and Contaminants 21(5):422-433. |
|
|
Gondova Z, Kozarova I, Polakova Z, Madarova, M. (2014). Comparison of four microbiological inhibition tests for the screening of antimicrobial residues in the tissues of food-producing animals. Italian Journal of Animal Science 13(4):3521. |
|
|
ISO E (2008). ISO 707: 2008 (IDF 50: 2008) Milk and Milk products -Guidance on sampling. Geneva, Switzerland: International Organization for Standardization. |
|
|
Kebede G, Zenebe T, Disassa H, Tolosa T (2014). Review on detection of antimicrobial residues in raw bulk milk in dairy farms. Africa Journal of Basic and Applied Science 6:87-97. |
|
|
Khaskheli M, Malik RS, Arain MA, Soomro AH, Arain HH (2008). Detection of ß-lactam antibiotic residues in market milk. Pakistan Journal of Nutrition 7(5):682-685. |
|
|
King DT, Sobhanifar S, Strynadka NCJ (2017). The mechanism of resistance to Betalactam antibioitcs. In Gotte M., Matlashewski G., Wainberg M., Sheppard D. (eds) Handbook of Antimicrobial Resistance. Springer, New York, NY |
|
|
Kivirand K, Kagan M, Rinken T (2015). Biosensors for the detection of antibiotic residues in milk. Biosensors-Micro and Nanoscale Applications pp. 425-456. |
|
|
Kurwijila LR, Omore A, Staal S, Mdoe NSY (2006). Investigation of the risk of exposure to antimicrobial residues present in marketed milk in Tanzania. Journal of Food Protection 69(10):2487-2492. |
|
|
Layada S, Benouareth D, Coucke W, Andjelkovic M (2016). Assessment of antibiotic residues in commercial and farm milk collected in the region of Guelma (Algeria). International Journal of Food Contamination 3:19. |
|
|
Li L, Guo C, Ai L, Dou C, Wang G, Sun H (2014). Research on degradation of penicillins in milk by beta-lactamase using ultra-performance liquid chromatography coupled with time-of-flight mass spectrometry. Journal of Dairy Science 97(7):4052-4061. |
|
|
Livermore DM, Brown DF (2005). Detection of beta-lactamase-mediated resistance. Journal of Antimicrobial Chemotherapy 48(1):59-64. |
|
|
Mullen KA, Beasley E, Rizzo JQ, Washburn SP, Baynes RE, Mason SE, Anderson KL (2017). Potential of phytoceuticals to affect antibiotic residue detection tests in cow milk in a randomized trial. Veterinary Record Open 4(1):e000214. |
|
|
Munita JM, Arias CA (2016). Mechanisms of Antibiotic Resistance, Microbiology Spectrum 4(2):10.1128/microbiolspec.VMBF-0016-2015. |
|
|
Mwagore D, Koge J, Ng'ang'a M (2019). Milk quality seminar; workshop proceedings. |
|
|
Ndungu TW, Muliro PS, Omwamba M (2021). A novel platform test to detect beta-lactam residues in raw milk. African Journal of Food Science 15(10):336-344. |
|
|
Ndungu TW, Omwamba M, Muliro PS, Oosterwijk G (2016). Hygienic practices and critical control points along the milk collection chains in smallholder collection and bulking enterprises in Nakuru and NyandaruaCounties, Kenya. African Journal of Food Science 10(11):327-339. |
|
|
Pandey N, Cascella M (2020). Beta lactam antibiotics: In StatPearls [Internet]. StatPearls Publishing. |
|
|
Ricci A, Allende A, Bolton D, Chemaly M, Davies R, Herman L (2017). Risk for the development of Antimicrobial Resistance (AMR) due to feeding of calves with milk containing residues of antibiotics. EFSA Panel on Biological Hazards (BIOHAZ), EFSA Journal 15(1):e04665. |
|
|
Sachi S, Ferdous J, Sikder MH, Hussani SAK (2019). Antibiotic residues in milk: Past, present, and future. Journal of Advanced Veterinary and Animal Research 6(3):315. |
|
|
Sajid M, Kawde AN, Muhammad D (2014), Designs, formats and applications of lateral flowassay: A literature review, Journal of Saudi Chemical Society 19(96):689-705. |
|
|
Salois A, Perez I, Palma E, Goolish E, Griko Y (2015). Evaluation of the Chemical Integrity of beta-lactam antibiotics by iodine-based assay. Journal of Biosciences and Medicines 3(11):91. |
|
|
Shaheen BW, Nayak R, Boothe DM (2013). Emergence of a New Delhi metallo-β-lactamase (NDM-1)-encoding gene in clinical Escherichia coli isolates recovered from companion animals in the United States. Antimicrobial Agents and Chemotherapy 57(6):2902-2903. |
|
|
Stead SL, Ashwin H, Richmond M, Sharman PC, Langeveld JP, Barendse JP, Keely BJ (2008). Evaluation and validation according to international standards of Delvotest SP-NT screening assay for antimicrobial drugs in milk. International Dairy Journal 18(1):3-11. |
|
|
Yamaki M, Berruga, MI, Althaus RL, Molina MP, Molina, A. (2004). Occurrence of antibiotic residues in milk from Manchega ewe dairy farms. Journal of Dairy Science 87(10):3132-3137. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0