ABSTRACT
Approximately 38% of Tanzanian children have vitamin A deficiency (VAD), and the majority of them do not have access to vitamin A-fortified foods. Orange-fleshed sweet potato (OFSP), a new crop in Tanzania, is rich in β-carotene, and could be a cheaper solution for VAD. The objectives of this study were to develop a type of Ugali (stiff maize porridge) fortified with OFSP, to correlate its β-carotene content (using colour measurement), and to assess its proximate composition and consumer acceptability. Ugali was prepared using maize flour with various amounts of added OFSP (0, 30, 50, 70 and 100%). Samples of Ugali with more OFSP had higher colour values (a* and b*) that imply the increase in β-carotene as the OFSP amount increased. The proximate compositions of Ugali with different amounts of OFSP were different (P<0.05). All samples that were made with the mixture of OFSP and maize flour have shown to have higher sensory scores than those with 100% maize or 100% OFSP; Ugali with 50% OFSP was most favourably rated by Tanzanian consumers. This sample was selected as a potential possibility for everyday consumption since it was shown to potentially supply more than 50% of the RDA of provitamin A for a specific age group. This supplementation method may be simple, affordable, and effective in reducing VAD in Tanzania.
Key words: β-carotene, colour parameters, proximate composition, total carotenoids.
Micronutrient deficiencies are one of the major growing health problems in the world (Magee and McCann, 2019); more than two billion people are at risk of vitamin A, iron, and iodine deficiencies (Ramakrishnan, 2002). At least half of children aged 6 months to 5 years worldwide suffer from one or more micronutrient deficiencies (CDC, 2014). Low serum retinol concentration (<0.70 μmol.L-1), an indication of vitamin A deficiency (VAD), affects an estimated 190 million preschool-age children and 19.1 million pregnant women globally (WHO, 2009). This problem is more pronounced in developing countries especially in Africa, Asia and in low-income populations in Latin America, the Caribbean (López de Romaña et al., 2015), and Europe (Darmon et al., 2002).
In Tanzania, vitamin and mineral deficiencies significantly contribute to more than 27,000 infant and 1600 maternal deaths annually (World Bank, 2012)with hidden hunger index of 35 (Ritchie and Roser, 2017)in a score of 100. According to the 2010 Tanzania Demographic and Health Survey, about 38% of children between 6 and 59 months had VAD (NBS and ICF, 2011)and a demographic health survey of 2015 showed that only 41% of the children aged 6-59 months has received vitamin A supplementation 6 months before the survey (Ministry of Health et al., 2016). Tanzania and other sub Saharan Africa have shown a decline in poverty condition. However, in 2018 about 26% (14 million) of the Tanzanian population lived below the poverty line (equivalent to about US $1.9-2 a day) (World Bank, 2020). Of these, more than 80% resided in rural areas, and could not afford a quality diet (UNICEF, 2020). According to Lyana and Manimbulu (2014), maize (white corn) is the major staple food crop in many parts of Tanzania. Maize flour is used for Ugali (stiff porridge) preparation in about 91% of households in Tanzania, and Ugali is the most frequently consumed food in Tanzania (Muhihi et al., 2013). Maize comprises 41% of the weekly calorie intake of households in Tanzania (NBS and ICF, 2011). It is a carbohydrate-rich food (25.6%) and is one of the major Tanzanian staple foods (Lukmanji et al., 2008). Vitamin A in food is found as retinol (preformed vitamin A) or as provitamin A carotenoids. Preformed vitamin A is found exclusively in animal foods, including eggs and milk, fish and fish oils, and animal livers (Ross et al., 2020). These foods are too expensive for poor communities (van den Berg et al., 2000); hence, the primary source of provitamin A in these communities is -carotene (or other provitamin A carotenoids) from plant sources. Micronutrient supplements are often given to populations with severe micronutrient deficiencies, but the intervention is expensive, and for it to be effective, it must be repeated every six months (WHO, 1998). Therefore, the success of using these supplements is limited in developing countries. A successful program for the iodification of table salt in Tanzania has been accomplished, but fortification programs for other foods (wheat, cooking oil, and maize flour) have only started in recent years (NBS and ICF, 2011). Large proportions of the population cannot afford and/or get fortified food. One way to reduce the prevalence of VAD in Tanzania and other developing counties could be to add -carotene from locally available food sources to improve the nutritional quality of flour used to prepare Ugali, a common staple food (De-Regil et al., 2011). For instance, orange-fleshed sweet potato (OFSP), which is rich in provitamin A, could be an affordable choice for fortification since it has proven to improve total body vitamin A stores as effectively as supplementation (Gannon et al., 2014). This product could thus reach a greater population and help to eliminate micronutrient deficiencies (Low et al., 2017). Colour parameter methods, from objective colour measurements (that is, CIE L* a*b*, hue, and chroma), have been successfully used for estimating the amount of coloring compounds, such as lycopene and other carotenoids, in many plants. For instance, the lycopene content in tomatoes from HPLC analyses correlates well with colour parameters in linear regression (R2 = 96%) (Arias et al., 2000). Other researchers explored the relationship between carotenoid content and the colour of winter-type squash flowers and flesh (Fransis, 1962; Seroczy?ska et al., 2006); these revealed a weak relationship, indicating that the a* parameter was the strongest (Seroczy?ska et al., 2006). Another study correlating specific carotenoid contents in pumpkins and squash (Cucurbita species) flesh (by HPLC) and colour parameters revealed strong correlations between a* and total carotenoids and b* and lutein. These researchers concluded that this technique (colour parameter measurement) can be used as an “easy-to-use and inexpensive method” for estimating carotenoid contents in large numbers of samples from breeding programs (Itle and Kabelka, 2009). Simonne et al. (1993)correlated colour parameters and specific carotenoid contents (from HPLC data) in sweet potato and found that β-carotene content was highly correlated with hue angle, especially in sweet potatoes with yellow to deep orange (r =-0.99) and conclude that colour parameters can be used for the estimation of β-carotene. Since most of the laboratories in developing countries have limited access to advanced technologies for -carotene analysis (that is, HPLC), this study utilized a simple method of colour measurement to estimate -carotene (from sweet potato) content in Ugali. Orange-fleshed sweet potato is among the bio-fortified staples bred for high provitamin A carotenoid content (CIP, 2006). It has emerged as one of the most promising plant source of β-carotene for reducing vitamin A deficiency prevalence (Hagenimana et al., 1999; Tumwegamire et al., 2004; Low et al., 2017; Bao and Fweja, 2020); OFSP can supply about 50% per 100 g of the daily vitamin A requirement (Low et al., 2001)(Recommended daily allowance is 400-1300 g.day-1 RAE depending on age, sex and health status). In this study, formulated mixtures of maize flour and boiled OFSP were mixed in different amounts to obtain a ratio that provided the optimum level of provitamin A in Ugali. The objectives of this study were (i) to develop fortified Ugali using locally available raw food materials that are rich in provitamin A, that is, OFSP; (ii) to utilize colour measurement as a tool to estimate ß-carotene levels in mixture; and (iii) to assess the nutritional composition and evaluate the chemical and physical properties and consumer acceptability of this Ugali.
Initial work to optimize analytical methods (colour and β-carotene, moisture), and Ugali preparation was accomplished at the University of Florida Food Safety and Quality Research Laboratory (UF-FSQL) in Gainesville, Florida, USA. Most other subsequent work (including sample preparation, chemical analysis, sensory evaluation, and texture analysis) was conducted at the Department of Food Technology, Nutrition and Consumer Sciences laboratory at the Sokoine University of Agriculture (SUA), Morogoro, Tanzania. Colour measurements were conducted at the International Institute of Tropical Agriculture (IITA) in Mikocheni, Dar es Salaam, Tanzania. The consumption study to estimate Ugali intake was conducted in Morogoro town with five volunteer consumer households (N =15) near the SUA campus in Morogoro.
Orange-fleshed sweet potato (Ipomea batatas Lam) [OFSP] used in the initial study to optimize analytical methods (colour and β-carotene, moisture) and in the Ugali preparation were purchased from local grocery stores in Gainesville, Florida. While the variety information was not available, the supply was uniform enough for the purpose of the initial study. The average root weight was 514.7 g before peeling and 408.6 g after peeling (79.4% recovery). OFSP (Jewel variety) for the subsequent studies were purchased locally in Tabora, Tanzania in three batches of 100, 100, and 300 kg. OFSP of similar maturities were transported to a lab at SUA in Morogoro, Tanzania. At the SUA laboratory, the OFSP were sorted according to the USDA grading system (USDA, 2005). OFSP of U.S. Extra No. 1 grade were selected and average sweet potato root weight was about 181 g.
Maize flour (8 kg, Maseka Instant Corn Masa mix) for the preliminary study at UF-FSQL was purchased from a local grocery store in Gainesville, FL. Maize flour (about 20 kg, Ndaiga Super Sembe, Ndaiga Milling Morogoro-Tanzania) for the actual study at SUA was purchased from a local grocery store at the SUA campus and sieved in a stainless steel testing sieve with an aperture of 1 mm and a wire diameter of 0.56 mm (Tokyo Screen Co. Ltd, Japan).
All chemicals were of analytical grade and were purchased from the Techno Net Scientific Ltd. store, Mwenge, Dar es Salaam, Tanzania. Acetone, petroleum ether, sulphuric and hydrochloric acid, sodium hydroxide, diethyl ether, and boric acid (Carlo Ebra Reagents, Val De Ruil-France), anhydrous sodium sulfate (Uni-Chem Chemical Reagent, Belgrade-Serbia), sodium chloride (Nentech Ltd., Brixworth, Northants-UK), protein analysis catalysts (copper sulphate: potassium sulphate: selenium = 10: 10: 1) and indicators (Bromocresol Green/Methyl Red, mixed indicator solution; Sigma-Aldrich, Louis USA), β-carotene standard [Fulka BioChemika (22040 standard with purity of >97.0% HPLC), Buchs-Switzerland].
Preparation of OFSP for Ugali fortification
The OFSP for sensory evaluation as well as for Ugali preparation were prepared from sorted samples one day after reaching the laboratory. The OFSP were washed with tap water, rinsed with distilled water, peeled with a kitchen knife, and cut into uniform cubes (1 × 1 × 1 cm). The sweet potato cubes (about 2 kg average for each batch) were then fully immersed in boiling water (1: 2 OFSP: water (w/v) at 100°C for 15 min in a 5 L stainless steel pot. The boiling time of 15 min under this condition was previously determined at the University of Florida, following a boiling time study at 100°C. Boiling time was recorded after water with sweet potato reached 100°C (about 7 min). Water was drained off, and the pot with boiled OFSP was kept in a water bath filled with tap water at room temperature (26°C) to cool for about 10 min. The cooled OFSP was blended with a mixer blender (Pigeon Appliances Pvt Ltd., India) at setting number 2 for 1 min. For raw OFSP, cubes were blended with this blender at this condition and used for colour, texture and proximate analysis. The boiled and blended OFSP was then ready for either sensory evaluation, mixing with maize flour for preparing Ugali, or for chemical analysis.
Preparation of Ugali and Ugali with OFSP
Ugali was prepared according to Nyotu et al. (1986)with minor modifications as to the composition of Ugali (to add OFSP) and the cooking time. Water was boiled (100°C) then maize flour was added to the boiling water (1: 2.8 w/v maize flour: water) while stirring. This mixture was stirred for 5 to 10 min until a uniform consistency and stiffness was obtained. Ugali with OFSP was prepared by adding boiled OFSP to boiling water together with maize flour while stirring. The amount of OFSP, maize flour, and water was adjusted based on the moisture ratio of boiled OFSP and maize to obtain five combinations with increments of 0, 30, 50, 70 and 100% OFSP that were coded as A, B, C, D and E, respectively. These ratios together with cooking times were formulated through the preliminary studies done at UF-FSQL.
Sensory evaluation
Ugali was evaluated by a consumer panel (n=181) at the food science sensory evaluation lab at SUA. Participants were recruited in accordance with the University of Florida Institutional Review Board for Human Subjects (#2015-U-1123). All panelists were students of SUA with average age of 23 years old (the maximum age was 37 and the minimum was 19). Each panelist was given five samples (5 g each; samples were served at 26°C and coded with 3-digit random numbers) of Ugali prepared with different amounts of OFSP (0 to 100%), as described previously. Panelists were asked to evaluate each sample based on appearance, texture, flavor, and overall preference, using a 9-point hedonic scale where 9 was anchored with “like extremely” and 1 was anchored with “dislike extremely” (Ihekoronye and Ngoddy, 1985). In addition, the panelists were asked to rank the samples (1-5) based on their preference. Sensory evaluation was conducted on three different days with 40, 40, and 101 panelists. Samples from the same batch that was used for the sensory evaluation were also used for chemical and physical analyses. The samples for chemical and physical analyses were sealed in minimum-oxygen freezer bags that were wrapped with aluminium foil and stored at -18°C at the Food Technology, Nutrition and Consumer Sciences Department laboratory of SUA until the time of experiments (not more than a week).
Consumption study to estimate β-Carotene intake from fortified Ugali
In order to estimate amounts of β-carotene intake from OFSP-fortified Ugali during a normal meal, 5 consumer households were recruited to participate in a consumption study. None of the members of these households were involved with any sensory evaluation described previously. These consumer households (with a combined 15 total members) were randomly chosen from a pool of volunteers who were willing to participate. The Ugali with 50% OFSP was selected for the consumption study, based on the results of the sensory evaluation. During a week-long period selected families were provided with the fortified Ugali (1.2 kg.family-1.meal-1) three times. The members of the family were to consume the Ugali along with typical meal accompaniments (e.g. fried fish, roasted beans, boiled amaranth, boiled sweet potato leaves or roasted meat) in their home. The serving sizes were based on the amount each person needed to reach satiety (without restriction) that was weighed by top scale balance (Citizen Bench Scale, 12R988, India).
According to Burri (2011), bioaccessible -carotene from OFSP can be calculated as follows:
The bioaccessibility of β-carotene in blended foods containing OFSP flour has been measured using in vitro digestion. The in vitro bioaccessibility of β-carotene from heat-processed OFSP was observed and accessible all-trans-β-carotene was 24 and 41% without fat (Bengtsson et al., 2009). Porridge with maize: soybean: OFSP flours (30: 35: 35) had a bioaccessibility of 16% all-trans carotenoids and 30.3% 13-cis carotenoids. Boiled puree of OFSP had a bioaccessibility of 9.9% for all-trans carotenoids and 43.5 for 13-cis carotenoids (Bechoff et al., 2011). Burri (2011)stated that the bioaccessibility of b-carotene is 25%. The retinol equivalency ratio was estimated to be 12 μg b-carotene: 1-μg retinol for women and children with good Vitamin A status (West et al., 2002)as shown in Equation 2 while the Recommended Daily allowance is as shown in Equation 3.

For this study, an additional introduction letter from SUA and a permit to conduct research with households was needed for each household, in addition to the UF-IRB.
????-Carotene analysis
The methodology for β-carotene analysis at SUA was optimized based on the preliminary experiments that were done at the University of Florida Food Safety and Quality Research Laboratory. Based on these preliminary experiments, β-carotene in sweet potato and sweet potato-fortified Ugali was analyzed according to a method described by Kimura et al. (2007), with minor modifications to the amount of solvents used (acetone and petroleum ether). Approximately 2 g of samples were weighed (electronic balance, Contech Instrument Ltd.) into glass tubes and 5 mL of cold acetone (refrigerated at 4°C for about 2 h) was added. The mixture was then homogenized with a homogenizer (Polytron, Omni mixer Homogenizer, Special model, Omni International Inc., USA) for 1 min at 3600 rpm. Extractions (with acetone) were done five times until a colorless residue was obtained and the final total volume of the extract was 25 mL. The supernatant (acetone extract) was pipetted into a 250-mL separatory funnel (containing 50 mL of petroleum ether) for partitioning. The mixture in the separatory funnel was allowed to separate for approximately 3 min and the lower aqueous phase was discarded. The petroleum ether phase was washed 3 to 4 times with 20 mL of distilled water (Wagtech International Ltd Berkshire UK). To prevent emulsion, washing was done slowly along the walls of the funnel without shaking, and when emulsion occurred, saturated sodium chloride (NaCl) solution was added to prevent formation of an emulsion. The petroleum ether phase was collected in 25, 50, or 100 mL volumetric flasks, depending on expected b-carotene amount in the solvent phase. Residual water was removed by passing the extract through a small funnel with glass wool containing about 15 g anhydrous sodium sulfate. The volumes of carotenoid solution were then adjusted to 25, 50, or 100 mL (raw OFSP) with petroleum ether, and absorbance readings were taken using a UV-VIS Spectrophotometer (Biomate 6 UV VIS Thermo Scientific) at 450 nm. The total carotenoid content (b-carotene) of the stock solution was verified according to Davies (1976), using the absorption A1%1 cm of b-carotene in petroleum ether (that is, 2592) and found to be 98%. The total carotenoid was calculated as all trans-b-carotene since 80-90% of the carotenoid in OFSP is b-carotene (Bengtsson et al., 2008).

-carotene standard was used to prepare the stock solution of 100 g.mL-1. -carotene of the samples was calculated from a standard curve obtained from 0.4, 0.8, 1.7, 3.3, 6.7 and 13.3 g.mL-1. -carotene concentration was expressed on a fresh weight basis to synchronize the results with sensory panelists’ assessment of the product as a wet product. Precautionary measures to prevent artifact formation and losses of carotenoids during analysis were rigorously followed such as completion of the analysis within the shortest possible time, protection from direct light, high temperatures was avoided, exclusion of oxygen and the use of high-purity solvents (Kimura et al., 2007).
Determination of proximate composition of samples
Proximate composition [moisture (AOAC, 2000), ash (AOAC, 2000)#942.05, crude fiber (AOAC, 2000)#978.10, fat (AOAC, 2000)#920.390, protein (Thiex et al., 2002)and carbohydrate (calculated by difference)] analysis was carried out in duplicate on the raw OFSP, boiled OFSP, and Ugali samples. Slight modification was done on protein analysis by the use of slight different ratio of the catalyst mixture as shown in the chemical supplies list.
Colour
Colour measurement in the preliminary study at UF-FSQL was done using a Minolta Chroma meter (Konica CR- 400 Tokyo Japan), while the subsequent colour analysis was done at IITA with a PCM+ Chroma meter with settings at Ist LAB C10 CIELAB 1:1:1. Approximately 20 g of each sample from three batches, each with one boiled OFSP, one raw OFSP, and four Ugali with different levels of OFSP, were analyzed. Samples were wrapped in very low-density polyethylene transparent material, and the measurements (L*, a* and b*) coordinates were read and recorded in ten different locations (Simonne et al., 1993).
Statistical analysis
The statistical comparison of data was performed by one-way analysis of variance (ANOVA) using R software in R Studio (Version 3.5.0 © 2018 RStudio, Inc) to determine differences among samples; ANOVA assumptions were validated by normality and homogeneity of the variance by Shapiro-Wilk normality test, Bartlett test of homogeneity of variances, respectively while independence of variance by residuals against fitted values plots. Independent variables were five samples (at three replicates) of Ugali, while dependent variables were colour parameters (ten measurements (L*, a*, b* coordinates and Chroma*), -carotene (ug.g-1), proximate composition (moisture, ash, crude fiber, fat, protein, and carbohydrate) and firmness. Sensory scores (appearance, texture, flavor, and overall preference) were analyzed by two-way analysis of variance, where panelists (n = 181) and attributes were independent factors. Ranking scores were analyzed by Friedman’s test. The correlation coefficients and their probability levels were obtained from linear regression analysis. The means of sensory scores from different attributes (appearance, texture, flavor, and overall preference), regardless of the sample to which they belonged, were ranked from 1st to 20th; overall Rank Sum Index (ORSI) for the samples was calculated by adding the ranks for each attribute from a given sample (Simonne et al., 1999). Determination of significance of differences (mean separation) among chemical, physical and sensory scores was obtained by Tukey's HSD multiple ranks test. P values of 0.05 were considered significant; p values less than 10-8 were expressed as ≤ 0.001.
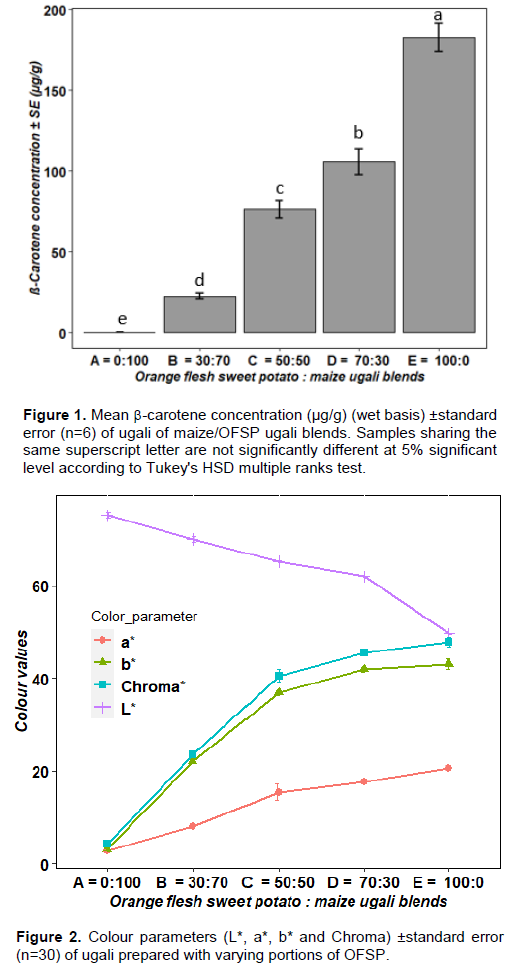
Addition of boiled OFSP in the samples significantly increased the -carotene concentration in the Ugali samples (p ≤ 0.001) (Figure 1). A similar trend was observed in other OFSP fortified products, such as flatbread (Tadesse et al., 2015), chapatti, mandazi, and buns (Hagenimana et al., 1998)and yellow maize ogi porridge (Ukom et al., 2019). Sample D (70% OFSP: 30% maize) has a similar β-carotene concentration to that of the raw OFSP (112.3±2.24 g/g).
The higher β-carotene content of boiled OFSP may result from higher extractability of β-carotene from the OFSP tissues. It has been found that processed OFSP has significantly higher (P< 0.05) bioaccessible β-carotene, as compared to the raw forms. Bioaccessibility varies with processing treatments in this order: raw <baked < steamed/boiled < deep fried (Tumuhimbise et al., 2009). As early as 1948, it was reported that homogenization improves the bioavailability of β-carotene of carrots for humans (Van Zeben and Hendriks, 1947).
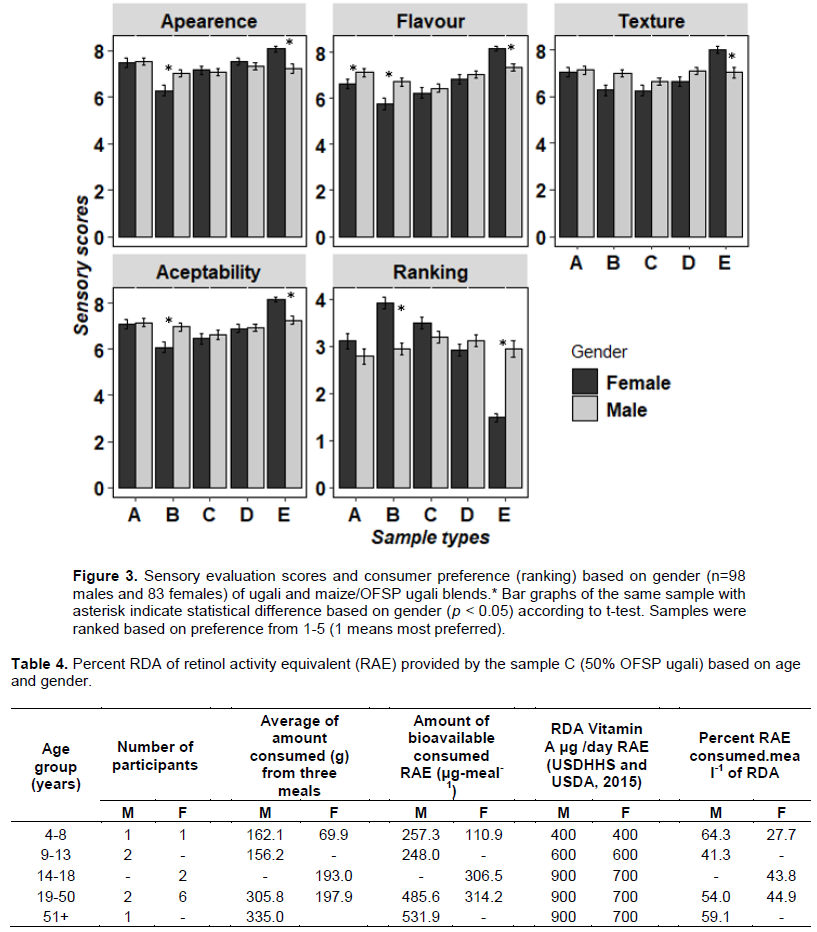
The colour of the OFSP-fortified Ugali samples was measured with a Chroma meter and L*, a* and b* coordinates were recorded. Results showed that L* coordinate (values) differed significantly among all samples (p ≤ 0.001). As the proportion of OFSP increased, the lightness (L*) decreased (Figure 2). This is because maize flour is white in colour, and as the proportion of this flour decreases, the whiteness in the sample decreases. In general, samples with a higher proportion of OFSP had higher a* and b* values.
The results of this study show that as the b-carotene increased, the intensity of the orange colour increased also (Figure 2). Of the sensory attributes that related to the carotenoid content, the most visible to consumers is the colour (orange), and this visible trait can influence marketing and promotion (Tomlins et al., 2010). Regression equations for the prediction of -carotene based on a*, b*, and Chroma*, based on this study, are shown in Table . The results of this study are consistent with previous studies on correlation of colour parameters and carotenoid contents in sweet potatoes (Simonne et al., 1993; Ameny and Wilson, 1997).
This study shows a very high negative correlation between b-carotene and L* due to varying proportions of maize flour in the Ugali samples (Table 1). The correlation correlation between b-carotene and b* was lower compared to a*; however, the relationship between b-carotene and a* or b* in this study was low when compared with the study done by Bengtsson et al. (2009)(r = 0.96 b-carotene and a*). Yet the correlation is higher when compared with Takahata et al. (1993)(r = 0.897 b-carotene and a*) and Simonne et al. (1993)(r = 0.73 b-carotene and a*, r = 0.57 b-carotene and b*).
The result of the proximate composition of Ugali from maize: OFSP blends are shown in Table 2. Results show that crude fiber content increased as the amount of OFSP increased (p ≤ 0.001); a similar trend was observed by Zegeye et al. (2015). Very low amount of crude fat (0.04-0.16%) was found in the samples, this might be a result of using maize flour from deshelled grains, negligible fat content in OFSP and exclusion of addition fat/oil in preparation of Ugali.
The presence of % ash content (0.2-0.9%) in the samples is an indication of the presence of an important element for human nutrition. Percent carbohydrate differed significantly in all the samples (p ≤ 0.001); the presence of a high amount of carbohydrate indicates that the samples prepared can provide energy to the body when consumed. It has been found that OFSP is a good source of energy and can supply energy of 293 to 460 Kj per 100 g (Hagenimana et al., 2001), this amount was comparable to the samples in this study except samples A, B and C. Sample C was found to have a significantly higher value of energy (545 Kj) than other samples due to high carbohydrate content which resulted by its lower moisture content. Lower values of energy in raw OFSP and sample E (377.1 and 272.1 Kj, respectively) resulted from their higher values of moisture content and lower protein content.
A consumer panel (n=181) consisting of SUA students aged 19 to 37 evaluated the Ugali products. The results in Table 3 show that most of the panelists preferred samples with no blending, that is, samples A and E. Sample E (100% OFSP: 0% maize Ugali) was the significantly preferred sample (p < 0.05), based on ORSI. In general, the addition of OFSP resulted in higher preference in the samples studied (excluding sample A). The panelists accepted all samples; this indicates that the developed samples can be introduced at the household level to fight against vitamin A deficiency. Stiff porridge has a bland taste because it is commonly prepared from unseasoned maize meal without any other ingredients or additives (Calvin, 2014).
A moderate positive correlation between ORSI and b-carotene (r = 0.612) was observed because most of the panelists preferred non-fortified samples to the fortified samples. When 100% maize Ugali (traditional) was removed from the regression calculation, the correlation was highly positive (r = 0.958).
Females preferred samples with higher amounts of OFSP than males (Figure 3); this may be due to the increased sweetness and visual appearance of OFSP. Tadesse et al (2015)observed a similar trend; the degree of preference increased with the substitution level of OFSP flour in the formulations of flatbread prepared from blends of maize and OFSP. This study also suggested that the trend might be due to the fact that the sweetness increases as OFSP amounts increase. Samples were orange in colour, and brightness increased with the increase in the proportion of OFSP, which could be a reason for the demonstrated preference differences.
In order to estimate β-carotene intake from fortified Ugali as retinol activity equivalent (RAE), five consumer households (n =15 total members) were recruited to consume Ugali with 50% OFSP: 50% Maize at their regular meal. Results showed that adultsbetween19 and 51 years might typically consume servings of Ugali up to 305.8 g for males (n=2) and 197.9 g for females (n=6) (Table 4).
Based on these studies, Table 4shows the summary of calculated retinal equivalent provided by sample C (50% OFSP: 50% Maize) supplied to five households. This sample was estimated to give 54% RAE/meal of RDA/day for males (n=2) and 44.9% for female (n=6) for adults (aged 19-50 years).
Ugali is consumed with different accompaniments that usually contain fat/oil; hence, the absorption of this amount of b-carotene as RAE will be considered to be at its maximum, since fat/oil enhances absorption of b-storage, intracellular location, and the intactness of the cellular matrix. Beta-carotene in sweet potato storage roots is found in lipid droplets or bound to a protein that is released during cooking, thereby enhancing its bioavailability (Hof et al., 2000). It is concluded that absorption of β-carotene is enhanced by fat/oil present in the food (Bechoff et al., 2011; Dimitrov et al., 1988; Hof et al., 2000; West et al., 2002). Ugali does not contain oil/fat by itself, but it is usually consumed with a variety of accompaniments, such as meat, vegetables, beans, and fish, that have fat.
The current study measured b-carotene using a spectrophotometer. Improved technologies such as HPLC should be used in the future to provide more reliable results that can show changes in b-carotene, together with its isomers, in Ugali. Performing a larger consumer level study may give a more reliable estimate of the Retinol Equivalent consumed when eating fortified Ugali. Finally, studies that measure retinol changes in the blood after consumption of the developed fortified Ugali for a recommended time period should also be done in the future.
This is the first study to utilize colour measurement as a tool to correlate ß-carotene levels in OFSP fortified Ugali. Supplementing OFSP in maize Ugali resulted to increase in ????- carotene content. The developed samples of Ugali showed a 20 to 28% carbohydrate content that can be used as a source of energy to the body. Sample with 50% OFSP and 50% maize Ugali was estimated to give at least half (50%) of recommended daily allowable of bioavailable retinol activity equivalent per meal to adults (19 to 50 years). This study provided information on development of fortified Ugali using a new locally (Tanzania) available crop (ORSP) to in crease provitamin A activity. In addition, the results revealed that the final product had acceptable nutritional composition and chemical and physical properties and consumer acceptability.
The authors have not declared any conflict of interests.
This work was made possible through the United States Agency for International Development (USAID)-funded Innovative Agricultural Research Initiative project (iAGRI) (Award No. CA-621-A-00-11-00009-00).
REFERENCES
|
Ameny MA, Wilson, PW (1997). Relationship between hunter colour values and β-carotene contents in white-fleshed African sweetpotatoes (Ipomoea batatas Lam). Journal of the Science of Food and Agriculture 73(3):301-306.
Crossref
|
|
|
|
Association of Official Analytical Chemist (AOAC) (2000). Official methods of analysis (18th ed.).
|
|
|
|
|
Arias R, Lee TC, Logendra L, Janes H (2000). Correlation of lycopene measured by HPLC with the L*, a*, b* colour readings of a hydroponic tomato and the relationship of maturity with colour and lycopene content. Journal of Agricultural and Food Chemistry 48(5):1697-1702.
Crossref
|
|
|
|
|
Bao B, Fweja L (2020). Evaluation of the potential of freshly bred orange-fleshed sweet potato varieties in combating vitamin A deficiency. Tanzania Journal of Science 46(1):1-8.
|
|
|
|
|
Bechoff A, Poulaert M, Tomlins KI, Westby A, Menya G, Young S, Dhuique-Mayer C (2011). Retention and bioaccessibility of β-carotene in blended foods containing orange-fleshed sweet potato flour. Journal of Agricultural and Food Chemistry 59(18):10373-10380.
|
|
|
|
|
Bengtsson A, Namutebi A, Alminger ML, Svanberg U (2008). Effects of various traditional processing methods on the all-trans-β-carotene content of orange-fleshed sweet potato. Journal of Food Composition and Analysis 21(2):134-143.
Crossref
|
|
|
|
|
Bengtsson, Anton, Larsson Alminger M, Svanberg U (2009). Invitro bioaccessibility of β-carotene from heat-processed orange-fleshed sweet potato. Journal of Agricultural and Food Chemistry 57(20):9693-9698.
Crossref
|
|
|
|
|
Burri BJ (2011). Evaluating Sweet potato as an intervention food to prevent vitamin A Deficiency. Comprehensive Reviews in Food Science and Food Safety 10(2):118-130.
Crossref
|
|
|
|
|
Calvin O (2014). Physical properties of dry-milled maize meals and their relationship with the texture of stiff and thin porridge. African Journal of Food Science 8(8):435-443.
Crossref
|
|
|
|
|
CDC (2014). IMMPaCt: Micronutrient Facts | DNPAO | CDC.
View
|
|
|
|
|
International Potato Center (CIP) (2006). Report. "McKnight Foundation CCRP East African Regional Sweetpotato Project Annual Report," International Potato Center, Nairobi, April 2005-March 2006.
|
|
|
|
|
Darmon N, Ferguson EL, Briend A (2002). A cost constraint alone has adverse effects on food selection and nutrient density: An analysis of human diets by linear programming. The Journal of Nutrition 132(12):3764-3771.
Crossref
|
|
|
|
|
Davies BH (1976). Carotenoids. In Chemistry and biochemistry of plant pigments (T. W. Goodwin). London: Academic Press.
View
|
|
|
|
|
De-Regil LM, Suchdev PS, Vist GE, Walleser S, Peña-Rosas JP (2011). Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age. In The Cochrane Collaboration (Ed.), Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd.
Crossref
|
|
|
|
|
Dimitrov NV, Meyer C, Ullrey DE, Chenoweth W, Michelakis A, Malone W, Boone C, Fink G (1988). Bioavailability of beta-carotene in humans. The American Journal of Clinical Nutrition 48(2):298-304.
Crossref
|
|
|
|
|
Fransis FJ (1962). Relationship between flesh colour and pigment content in squash. Proceedings of the American Society for Horticultural Science 81(408):14.
|
|
|
|
|
Gannon B, Kaliwile C, Arscott SA, Schmaelzle S, Chileshe J, Kalungwana N, Mosonda M, Pixley K, Masi C, Tanumihardjo SA (2014). Biofortified orange maize is as efficacious as a vitamin A supplement in Zambian children even in the presence of high liver reserves of vitamin A: A community-based, randomized placebo-controlled trial. The American Journal of Clinical Nutrition 100(6):1541-1550.
Crossref
|
|
|
|
|
Hagenimana V, Carey EE, Gichuki ST, Oyunga MA, Imungi JK (1998). Carotenoid contents in fresh, dried and processed sweetpotato products. Ecology of Food and Nutrition 37(5):455-473.
Crossref
|
|
|
|
|
Hagenimana V, Low J, Anyango M, Kurz K, Gichuki ST, Kabira J (2001). Enhancing vitamin a intake in young children in Western Kenya: Orange-fleshed sweet potatoes and women farmers can serve as key entry points. Food and Nutrition Bulletin 22(4):376-387.
Crossref
|
|
|
|
|
Hagenimana V, Oyunga MA, Low J, Njoroge SM, Gichuki ST, Kabira J (1999). The Effects of Women Farmers' Adoption of Orange-Fleshed Sweet Potatoes: Raising Vitamin A Intake in Kenya | ICRW.
View
|
|
|
|
|
Hof KH van het, West CE, Weststrate JA, Hautvast JGAJ. (2000). Dietary Factors That Affect the Bioavailability of Carotenoids. The Journal of Nutrition 130(3):503-506.
Crossref
|
|
|
|
|
Ihekoronye AI, Ngoddy PO (1985). Integrated Food Science and Technology for the Tropics. Macmillan.
|
|
|
|
|
Itle RA, Kabelka EA. (2009). Correlation Between L*a*b* Colour Space Values and Carotenoid Content in Pumpkins and Squash (Cucurbita spp.). HortScience 44(3):633-637.
Crossref
|
|
|
|
|
Kimura M, Kobori CN, Rodriguez-Amaya DB, Nestel P (2007). Screening and HPLC methods for carotenoids in sweetpotato, cassava and maize for plant breeding trials. Food Chemistry 100(4):1734-1746.
Crossref
|
|
|
|
|
López de Romaña D, Olivares M, Brito A (2015). Introduction: Prevalence of micronutrient deficiencies in Latin America and the Caribbean. Food and Nutrition Bulletin 36(2 Suppl):S95-S97.
Crossref
|
|
|
|
|
Low J, Walker T, Hijmans R (2001). The potential impact of orange-fleshed sweet potatoes on vitamin A intake in Sub-Saharan Africa. A Paper Presented at a Regional Workshop on Food Based Approaches to Human Nutritional Deficiencies 9-11.
View
|
|
|
|
|
Low JW, Mwanga ROM, Andrade M, Carey E, Ball AM (2017). Tackling vitamin A deficiency with biofortified sweetpotato in sub-Saharan Africa. Global Food Security 14:23-30.
Crossref
|
|
|
|
|
Lukmanji Z, Hertzmark E, Mlingi N, Assey V, Ndossi G, Fawzi W (2008). Tanzania Food Composition Tables (1st ed.). Muhimbili University of Health and Allied Sciences (MUHAS), Dar es Salaam - Tanzania, Tanzania Food and Nutrition Centre (TFNC), Dar es Salaam - Tanzania and Harvard School of Pubic Health (HSPH), Boston, USA.
View
|
|
|
|
|
Lyana AZ, Manimbulu, N (2014). Culture and Food Habits in Tanzania and Democratic Republic of Congo. Journal of Human Ecology 48(1):9-21.
Crossref
|
|
|
|
|
Magee PJ, McCann MT (2019). Micronutrient deficiencies: Current issues. Proceedings of the Nutrition Society 78(2):147-149.
Crossref
|
|
|
|
|
Ministry of Health, Zanzibar MoH, NBS, Zanzibar OCGS, ICF. (2016). Tanzania Demographic and Health Survey and Malaria Indicator Survey 2015-2016.
View
|
|
|
|
|
Mitra S (2012). Nutritional Status of Orange-Fleshed Sweet Potatoes in Alleviating Vitamin A Malnutrition through a Food-Based Approach. Journal of Nutrition and Food Sciences 02(08).
Crossref
|
|
|
|
|
Muhihi A, Gimbi D, Njelekela M, Shemaghembe E, Mwambene K, Chiwanga F, Malik VS, Wedick NM, Spiegelman D, Hu FB, Willett WC (2013). Consumption and acceptability of whole grain staples for lowering markers of diabetes risk among overweight and obese Tanzanian adults. Globalization and Health 9:26.
Crossref
|
|
|
|
|
NBS, ICF (2011). Tanzania Demographic and Health Survey 2010. National Bureau of Statistics (NBS) [Tanzania] and ICF Macro.
|
|
|
|
|
Nyotu HG, Alli I, Paquette G (1986). Soy supplementation of a maize based Kenyan food (ugali). Journal of Food Science 51(5):1204-1207.
Crossref
|
|
|
|
|
Ramakrishnan U (2002). Prevalence of micronutrient malnutrition worldwide. Nutrition Reviews 60(5 Pt 2):S46-S52.
Crossref
|
|
|
|
|
Ritchie H, Roser M (2017). Micronutrient Deficiency. Our World in Data.
View
|
|
|
|
|
Ross AC, Hodges JK, Wei C, Li Y (2020). Vitamin A. In Essential and Toxic Trace Elements and Vitamins in Human Health (pp. 202-214). Elsevier.
Crossref
|
|
|
|
|
Seroczy?ska A, Korzeniewska A, Sztangret-Wi?niewska J, Niemirowicz-Szczytt K, Gajewski M (2006). Relationship between carotenoids content and flower or fruit flesh colour of winter squash (Cucurbita maxima Duch.). Folia Horticulturae 18(1):51-61.
|
|
|
|
|
Simonne AH, Kays SJ, Koehler PE, Eitenmiller RR (1993). Assessment of β-carotene content in sweetpotato breeding lines in relation to dietary requirements. Journal of Food Composition and Analysis 6(4):336-345.
Crossref
|
|
|
|
|
Simonne E, Boozer R, Simonne A (1999). Yield, ear characteristics, and consumer acceptance of selected white sweet corn varieties in the Southeastern United States. HortTechnology 9(2):289-293.
Crossref
|
|
|
|
|
Tadesse TF, Nigusse G, Kurabachew H (2015). Nutritional, microbial and sensory properties of flat-bread (Kitta) prepared from blends of maize (Zea mays L.) and orange-fleshed sweet potato (Ipomoea batatas L.) flours. International Journal of Food Science and Nutrition Engineering 5(1):33-39.
|
|
|
|
|
Takahata Y, Noda T, Nagata T. (1993). HPLC determination of beta-carotene content of sweet potato [Ipomoea batatas] cultivars and its relationship with colour values. Japanese Journal of Breeding (Japan).
Crossref
|
|
|
|
|
Thiex N., Manson H, Anderson S, Persson JÅ (2002, March). Determination of Crude Protein in Animal Feed, Forage, Grain, and Oilseeds by Using Block Digestion with a Copper Catalyst and Steam Distillation into Boric Acid: Collaborative Study [Text].
View
|
|
|
|
|
Tomlins K, Rees D, Coote C, Bechoff A, Okwadi J, Masingue J (2010). Sweet potato utilization, storage, small-scale processing and marketing in Africa. In Sweet potato: Post harvest aspects in food, feed and industry. Nova Science Publishers Inc.
|
|
|
|
|
Tumuhimbise GA, Namutebi A, Muyonga JH (2009). Microstructure and in vitro beta carotene bioaccessibility of heat processed orange fleshed sweet potato. Plant Foods for Human Nutrition 64(4):312-318.
Crossref
|
|
|
|
|
Tumwegamire S, Kapinga R, Zhang D, Crissman C, Agili S (2004). Opportunities for promoting orange-fleshed sweetpotato as a mechanism for combat vitamin-a deficiency in Sub-Saharan Africa. African Crop Science Journal 12(3):241-252.
Crossref
|
|
|
|
|
Ukom AN, Adiegwu EC, Ojimelukwe PC, Okwunodulu IN (2019). Quality and sensory acceptability of yellow maize ogi porridge enriched with orange-fleshed sweet potato and African yam bean seed flours for infants. Scientific African 6:e00194.
Crossref
|
|
|
|
|
UNICEF (2020). Sustainable Development Goals and children in Tanzania: Sustainable Changes start with Children P 164.
View
|
|
|
|
|
USDA (2005). United States Standards for Grades of Sweetpotatoes. United States Department of Agriculture.
View
|
|
|
|
|
USDHHS, USDA (2015). 2015-2020 Dietary Guidelines for Americans (8th ed.).
|
|
|
|
|
Van den Berg H, Faulks R, Granado HF, Hirschberg J, Olmedilla B, Sandmann G, Southon S, Stahl W (2000). The potential for the improvement of carotenoid levels in foods and the likely systemic effects. Journal of the Science of Food and Agriculture 80(7):880-912.
Crossref
|
|
|
|
|
Van Zeben W., Hendriks TF (1947). The absorption of carotene from cooked carrots. Internationale Zeitschrift Fur Vitaminforschung. International Journal of Vitamin Research. Journal International De Vitaminologie 19(3-4):265.
|
|
|
|
|
West CE, Eilander A, van Lieshout M (2002). Consequences of Revised Estimates of Carotenoid Bioefficacy for Dietary Control of Vitamin A Deficiency in Developing Countries. The Journal of Nutrition 132(9):2920S-2926S.
Crossref
|
|
|
|
|
World Health Organization (WHO) (1998). Distribution of vitamin A during national immunization days.
View
|
|
|
|
|
World Health Organization (WHO) (2009). Global prevalence of vitamin A deficiency in populations at risk 1995-2005: WHO global database on vitamin A deficiency.
View
|
|
|
|
|
World Bank (2012). Action plan for the provision of vitamins and minerals to the Tanzanian population through the enrichment of staple foods (No. 69991; pp. 1-57). The World Bank.
View
|
|
|
|
|
World Bank (2020). Sub-Saharan Africa. World bank group. databank.worldbank.org › poverty › Global_POVEQ_SSA
|
|
|
|
|
Zegeye M, Singh P, Challa A, Jemberu Y (2015). Development of maize based orange - fleshed sweet potato flat bread for lactating Mothers at Hawassa Zuria Woreda, SNNPRS, Ethiopia. 2015(55):183-190.
|
|