ABSTRACT
The aim of this study was to evaluate the effect of storage methods on the nutritional qualities of African Catfish (Clarias gariepinus). Fresh fish samples were obtained from Johnybeth Farm, Ilesa, Nigeria. Fish specimens were divided into two equal parts. One part was stored fresh in a freezer at -6°C the other was smoked at 65 ± 5°C until equilibrium moisture content was attained, and was further divided into two parts. One part of the smoked fish was stored in continuous heated environment at 40 ± 4°C while the second part was packaged in polyethylene bags and stored at -6°C. Samples were analyzed over a period of six weeks. Nutrients (protein, lipid and carbohydrate), non-nutritional (ash, fibre) and trace element (Ca, Fe, Zn,) content and spoilage parameters (peroxide, pH, TVN, PV, TBA and FFA) were determined using standard methods. The crude protein ranged between 40.53 and 74.00%, lipid 5.46 and 21.71%, ash 2.59 and 8.57%, fibre 0.0 and 2.36%, carbohydrate 0.15 and 38.86%. Peroxide value ranged between 4.60 and 16.02 meq, O2/kg FFA 0.17 and 1.92%, TVN 4.10 and 23.97 mgN/100g, TBA 0.18 and 1.42 mg MDA/g and pH 6.52 and 7.80. Ca ranged between 0.03 and 1.62, Fe 0.28 and 2.27, Zn 0.30 and 2.59, mgkg 1 respectively. There was significant reduction in the nutrient, non-nutrients and trace elements while spoilage parameters increased during the six weeks storage period at (P<0.05). However smoked fish stored in heated environment has less reduction in quality.
Key words: Freezing, smoking, nutrients, quality indices, Clarias gariepinus
Fish is a potential source of animal protein available in the tropics and has been widely accepted as a good source of protein and essential nutrients for the maintenance of a healthy body (Fawole et al., 2007). Fish compared to other human dietary items, are excellent sources of highly digestible essential nutrients considering considering their amino acid (Louka et al., 2004). In addition, fish provides a good source of vitamins, minerals (Bashir et al., 2012). Minerals are important for vital body functions such as acid, base and water balance. Fish is highly perishable commodity recording considerable losses in quality before consumption; their susceptibility to deterioration has been the main obstacle in preservation. Locally fish spoilage has been known to be influenced to a large extent by high ambient temperatures, and inadequate infrastructure for post-harvest processing and landing. According to Adesehinwa et al. (2005), captured fisheries which provides over 60% of total domestic production per annum, have been hampered largely due to post harvest losses estimated at 30-50% of total catches. In Africa postharvest loses, are around 5% of the total artisanal productions while for the West African Region at between 10 and 20% (Ward and Jefferies, 2000). Hence concerted effort in adoption and improvement of preservation such as refrigeration, freezing, salting, brining (wet salting), icing, smoking, glazing, drying, frying to reduce or avoid losses due to quality deterioration and spoilage (Tairu et al., 2017). The successful application of this techniques results in the conservation
of desirable qualities in stabilized fish products.
Most processing methods serve not only to conserve the fish but also to alter their nutrient levels either positively or negatively. Reports exist in the agro industry that smoking is not only a conservation method but also a flavour, aroma and coloration improving method which are attributes sought by consumers. Smoking is the most popular method of fish preservation in many developing countries (Kumolu-Johnson et al., 2010; Emere and Dibal, 2013). Most consumers of fish in Nigeria consume smoked fish. It is relished for its taste and aroma as well as longer shelf life as a result of the combined effects of dehydration, antimicrobial and antioxidant activities of several smoke constituents mainly: formaldehyde, carboxylic acids and phenols (Serkan et al., 2010). Thus, making it is an important ingredient in the Nigerian traditional diet (Foline et al., 2011; Kiin-Kabiri et al., 2011; Akise et al., 2013).
While freezing, has been known to preserve the quality for a longer period and also minimum deterioration in product colour, flavor and texture. Most consumers in tropics, lack access to freezers, and subsequently stored smoked fish at room temperature in the kitchen. The aim of freezing of food items is to combine shelf life extension with maintenance of sensory and nutritional characteristics. Previous works by (Flick, 2010) on fish quality recommend that freezing of smoked fish in other to preserve quality. At low temperature (below 3°C), micro-organisms become inactive, enzymatic activity also slows down, thus biochemical activities decreases (Flick, 2010). Consequently, the fish remain free from spoilage for longer duration. The aim of this study was intended to determine patterns and rates of fish quality deterioration in (fresh/freeze = FFF), under recommended (smoke/ freeze= SFF) and under conditions more commonly encountered at homes (smoke/warm =SHF).
Twenty-four pieces of catfish, Clarias gariepinus, each weighing 450 ± 5 g were purchased from Johnny-Beth Fish Farm at Ilesa. Osun State, Nigeria. The fish used for this study were cropped and sorted based on size the same day. Standard Unit (S.U.) Turkey Cold Room at Oba’s market Akure was used for frozen storage. The cold room operates at -6°C and the sample was stored for six weeks. Portable smoking kiln used was obtained from Food Science and Technology Laboratory of the Federal University of Technology, Akure, Nigeria.
Sample preparation
The fish samples were killed immediately after capture, carefully degutted and washed with clean water to remove blood and slime according to the standard method described by Ogbonnaya and Ibrahim (2009). The samples were divided into two equal parts. 12 fresh fish were put in polyethylene nylon and stored in a freezer (FFF); 12 fish were smoked at 65± 5oC for 24 h and was divided into two portions of 6 fish as a batch. One batch of the smoked fish was stored in continuous heated environment (SFH) at 40 ± 4°C, while the second part was packaged in polyethylene nylon and stored in thermo thermocool thermostatic freezer (SFF) preset at -6°C. Storage was carried out for six weeks, while samples were taken on weekly basis for analyses. Each sample was divided into three for replicates.
Proximate analyses
Moisture, crude protein, fat, ash, and crude fibre contents were determined according to the standard method of (AOAC, 2000).
Chemical indices analyses
The samples were grounded into powder with a Kenwood blender. The samples was determined for total volatile base (TVN), peroxide value (PV), thiobarbituric acid value (TBA), free fatty acids (FFA) according to (AOAC, 2005). While the pH was determined using a pH meter (Jenway 3015 model, Cole Pamers Co., USA).
Mineral analyses
The minerals such as calcium, zinc and iron were determined on aliquots of the solutions of ash, by using atomic absorption spectrophotometer (210 VGP Buck Scientific Inc., USA).
Statistical analysis
Statistical analysis was performed on the replicate data by one-way analysis of variance (ANOVA) laid in completely randomised design using SPSS 17.0 (SPSS Inc., USA). Separation of the mean was determined by the Duncan New Multiple Range Test (DNMRT) at p<0.05 level of significance.
Proximate compositions of fish samples
Fresh frozen fish (FFF)
The result of the proximate composition of fresh frozen fish (FFF) is presented in Table 1. From results, at Wk 0 (zero time), the protein, lipid, ash and fibre contents were 74.00, 15.75, 7.40 and 2.36%, respectively. During storage, these contents insignificantly difference at Wk 1, but significantly decreased (P≤0.05) from Wk 2 to Wk 6 of storage. Notably, the fiber content was non-significant different from 3rd to 6th Wk.
Smoked fish stored in heated environment (SFH) at 40 ± 4°C
Freshly smoked fish (Wk 0) had the highest protein content 70.91% which decreased significantly (p<0.05) during storage from 66.42% (Wk 1) to 51.77% (Wk 6). The lipid content was 21.71% at Wk 0. However, the lipid content was not significantly different from Wk 1 to 4th Wk but differed significantly from 5 to 6 Wks. Ash content of 6.34% (Wk 0) was not significantly different from 1st and 2nd Wks but increased significantly to 8.57% in Wk 6. The fibre content significantly (p<0.05) decreased during storage period (0.89 to 0.1%) but 1st to 6th Wk were non-significantly different at (p<0.05) (Table 2).
Smoked fish stored in a freezer (SFF) at -6°C
The protein content smoked fish stored in freezer decreased during storage periods in the range of 70.91% (Wk 0) to 41.30% (Wk 6) (Table 3). The lipid content at Wk 0 was 21.71% was non-significantly different from 1st to 4th Wks but differed significantly in 5th and 6th Wks. However, lipid decreased during storage in the range of 21.71 (Wk 0) to 17.86% (Wk 6). Ash content decreased insignificantly during storage and ranged from 6.34% (Wk 0) to 5.45% (Wk 6). Fibre content decreased significantly during storage in the range of 0.89% (Wk 0) to 0.01 (Wk 6), however, 1st to 6th Wks were non significantly different from each other and ranged from 0.03 to 0.01%(Table 3).
Changes in quality indices of fresh frozen fish (FFF)
The peroxide value (PV) (meq O2/kg) of fresh fish ranged between from 4.60 (Wk 0) to 7.37 (Wk 6), this increased steadily during storage periods. However, 1st Wk was non significantly different (p>0.05) from 2nd Wk. Also, the same trend occurred between 4 and 5th Wk. In general, PV significantly increased between zero time and 6th Wk (4.60 and 7.37 meq O2/kg). In general, FFA significantly increased during the storage periods (1st - 6th Wks), with exception, non-significant difference between (2nd -3rd Wks) and (5-6th Wks). The free fatty acid (FFA) was 0.17% (Wk 0) increased significantly (p<0.05) during storage to 0.96% (Wk 6). Total volatile nitrogen (TVN) (mgN/100 g) of Wk 0 was 4.10. TVN increased significantly as storage progressed from 4.10 (zero time) to 21.00 (6th Wk). TBA values (mg MDA/g), generally, significantly increased (p<0.05) during storage periods, where were 0.18 (0 Wk) to 1.08 (6th Wk) with exception non-significant difference between (0-1st Wks), (2nd - 3rd Wks) and (4-5th Wks). pH values significantly decreased during storage in the range of 7.80 to 6.52. (Table 4
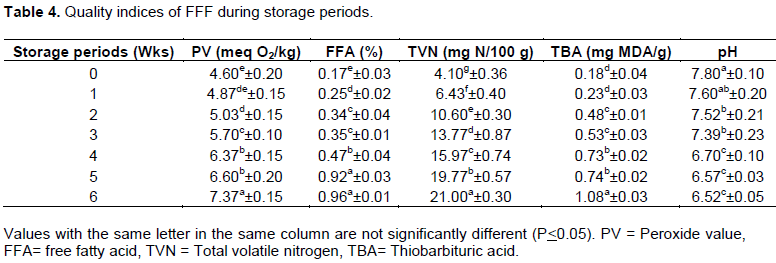
Quality indices changes of smoked fish stored in heated environment (SFH)
Table 5 showed that the PV (meq O2/kg) of fresh fish (Wk
0), increased steadily during storage from 6.30 to 7.55 meq O2/kg (6th Wk). There were non-significant difference in PV between (zero time -1st Wk) and (4th and 5th Wks). FFA (%) of Wk 0 increased during storage from 0.43 to 1.42 (6th Wk). FFA values were non-significant difference during the first three weeks of storage periods. TVN values (mg N/100 g) significantly (p<0.05) increased from 5.30 (Wk 0) to 9.80 (Wk 6). TBA values ranged from 0.25 (Wk 0) to 0.99 (Wk 6). pH decreased during storage in the range of 7.30 (zero time) to 6.45 (6th Wk).
Quality indices changes of smoked fish stored in a freezer (SFF) (-6°C)
The peroxide value (PV) of fresh fish (Wk 0), was not significantly different (p<0.05) from Wk 1 but differed significantly from Wk 6. PV (meq O2/kg) significantly increased during storage period from 6.30 (Wk 0) to 16.02 meq O2/kg (6th Wk) (Table 6). FFA values (%) increased significantly during storage period from 0.43 (Wk 0) to 1.92 (6th Wk). TVN (mg N/100g) increased significantly (p<0.05) as storage proceeded from 5.30 to 23.97 (6th Wk) (Table 6). TBA (mg MDA/g) significantly increased during storage from 0.25 mg/g (Wk 0) to 1.42 mg/g (6th Wk) (Table 6). pH decreased significantly during storage from 7.60 (Wk 0) to 6.52 (6th Wk). However, Wk 0 was significantly different (p<0.05) from 7.60 (Wk 0) to 6.52(6th Wk) (Table 6).
Rate of change in the storage indices of the fish
The percentage of increase or decrease and the rates of increase (slopes of regression) in these storage indices plots of the increase in PV, FFA, TVN, TBA, pH, protein and lipids contents of fish stored as FFF, SFF and SFH for six weeks are presented in Table 7.
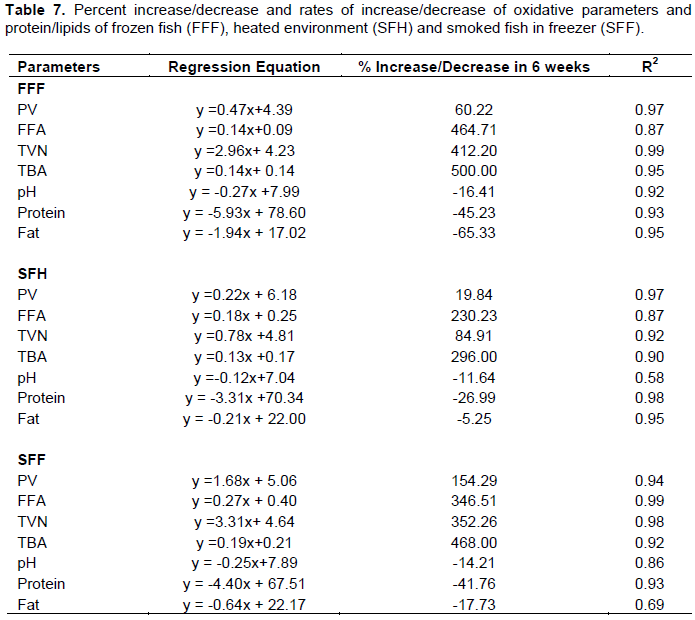
In general, there was increment in all the parameters. The percentage of increase were higher in the FFF which increased by 464.71% (0.14) in FFA, 412.20% (2.96) in TVN and 500% (0.14) in TBA. This was followed by smoked fish SFF with a percentage increase of 346.51% (0.27) in FFA, 352.26% (3.31) in TVN and 468.00% (0.19) in TBA and 16.56% (0.22) in pH, while SFH has the lowest increase of 230.23% (0.18) in FFA, 84.91% (0.78) in TVN and 296.00% (0.13) in TBA and 13.18% (0.12) in pH.
The PV increased fastest in the sample SFF than the other two treatments. This was also confirmed by the SFF which showed the highest % increase of 154.29% while FFF and SFH were 60.22% and 19.22% respectively. SFF also had the highest rate of increase in PV (1.69) while FFF and SFH had 0.47 and 0.23,
respectively.
However, pH, protein and lipids contents decreased. The percentage of decrease in pH -16.41% was higher in FFF, followed by -14.21% in SFF and -11.64% in SFH. The rate of decrease in protein was faster in FFF kept in the freezer which decreased by 45.23% followed by smoked fish kept in the freezer (41.76%) while it was slowest in smoked fish kept in the heated environment (26.99%).
This observation was confirmed by the rates of decrease in pH which was highest in FFF (-16.41) followed by SFF (-14.21) while SFH was the slowest (-11.64), protein was highest in FFF (-45.23) followed by SFF (-41.76) while SFH was the slowest (-26.99). The lipid contents decreased by 65.33% in FFF, 17.73% in SFF and 5.25% in SFH.
The slopes of the plots also follow the same trend. The rate of reduction was faster in FFF (-1.94), followed by SFF (-0.64) and SFH (-0.21). This shows that there was a higher decrease in pH, lipid content of the fresh fish than the smoked fish.
Mineral content of fresh frozen fish (FFF)
The calcium content (mgkg-1) of Wk 0 sample was significantly (p<0.05) higher than stored samples and
decreased during storage in the range of 0.15 (Wk 0) to 0.03 (6th Wk) (Table 8). There was no significant difference (p>0.05) within 1st Wk (0.07) too 6th Wk (0.03). Week 0 sample had the highest iron content. During storage, iron level (mgkg-1) decreased significantly ranging from 0.97 (Wk 0) to 0.28 (6th Wk). However, 5th Wk was not significantly different (p>0.05) from 6th Wk. A high level of zinc was 0.57 (zero time). During storage, the zinc level significantly decreased (p<0.05) from 0.57 (0 Wk) to 0.19 (6th k). These result showed that frozen storage reduced the mineral content of fresh C. gariepinus.
Mineral content of smoked fish stored in heated environment (SFH)
Table 9 revealed that freshly smoked fish had calcium level (mgkg-1) increased significantly (p<0.05) during storage where 0.40 (Wk 0) to 1.62 (6th Wk). Iron content in increased significantly (p<0.05) from zero time Wk 0 (1.13) to Wk 6 (2.27). Also, zinc level (mgkg-1) increased significantly (p<0.05) from 0.90 (0 Wk) to 2.14 (6th Wk).
Mineral content of smoked fish stored in a freezer (SFF)
Calcium level (mgkg-1) decreased significantly (p<0.05) from 0.40 (0 Wk) to 0.04 (6th Wk). The same trend for iron level (mgkg-1) from 1.13 (0 Wk) to 0.55 (6th Wk). Zinc level (mgkg-1) increased slightly from Wk 0 0.90 (0 Wk) to 2.59 (1st Wk) (Table 10).
The crude protein content of the fresh catfish 74.00% was higher than smoked catfish 70.91%. However, this value was higher than the value of 53.10% recorded by Ogbonna and Ibrahim (2009), 68.17% by Agbabiaka et al. (2012) and 68.40% reported by Olayemi et al. (2011).
Smoking and frozen storage reduced the percentage protein content of the samples. In support of present findings, Beklevik et al. (2005) in sea bass (Dicentrarchus labrex) and Siddique et al. (2011) in Puntius sp. reported significant decrease in protein content during frozen storage. They attributed this protein loss due to the leaching effect of amino acids and water soluble protein leaching out with melting ice. Arannilewa et al. (2005) noted that protein decreased with increasing duration of frozen storage with fresh samples not frozen having higher protein content. Disadvantages such as product dehydration, rancidity, drip loss and product bleaching have an overall effect on the quality of frozen food. According to Saeed and Howell (2002), proteins exposed to oxidizing environments are very susceptible to chemical modification, such as amino acid destruction, peptide scission and formation of protein-lipid complexes that results in decrease in protein content. Eyo (2001) attributed this loss to gradual degradation of the initial crude protein to more volatile products such as Total Volatile Bases (TVB), Hydrogen sulphide and Ammonia. Saliu (2008) reported that frozen storage reduced the percentage protein content in Malapterurus electricus, Synodontis clarias, Chrysichthys nigrodigitatus, C. gariepinus and sarotherodon melanotheron species. Audrey et al. (2006) observed that smoking cause nutrient loss due to associated heat flow of gases and interaction of the smoke components with protein. During storage, reduction in protein was corroborated with increase in TVN. However, SFH was higher in protein than SFF and FFF at the end of six weeks storage period. The values of 15.7 and 21.7% were obtained for lipids in the fresh and smoked samples were similar to those earlier reported by Ogbonna and Ibrahim (2009), Olayemi et al. (2011) and Agbabiaka et al. (2012). FFF and SFF showed a greater decrease in lipid content during storage when compared with the SFH. This result was supported by Arannilewa et al. (2005) in Tilapia; Žoldoš, et al. (2011) in Alaska Pollack (Theragra chalcogramma); Siddique et al. (2011) in Puntius sp; Roopma et al. (2012) in Labeo rohita and Saliu (2008) who found a significant loss in total lipid content during frozen storage. These workers attributed this loss due to oxidation of lipid. Reduction in lipid content could be attributed to oxidation of poly-unsaturated fatty acids (PUFA) contained in the fish tissue to products such as peroxides, aldehydes, ketones and free fatty acids (Gueraud et al., 2010). There might be high risks of rancidity during prolonged storage due to the fatty nature of fish (Horner 1992). This was confirmed by higher peroxide value and free fatty acid profiles of the stored products. Daramola et al. (2007) also reported that reduction in lipid is associated with higher PV and FFA in five different species of smoked freshwater fish: Bony tongue, Heterotis niloticus, African carp, Labeo coubie, Snake fish, Parachanna obscura, Nile Tilapia, Oreochromis niloticus and African mud catfish, C. gariepinus during storage at ambient temperature for 8 weeks.
Ash values 7.4 and 6.34% recorded for fresh and smoked samples were also similar to those earlier reported by Ogbonna and Ibrahim (2009), Olayemi et al. (2011) and Agbabiaka et al. (2012), respectively. Salán et al. (2006) reported that the increase in ash content when fish are smoked is due to loss of moisture. The decrease in ash content during FFF and SFF during storage is in agreement with Beklevik et al. (2005) who working on sea bass fillets and Okoyo et al. (2009) on Nile perch reported a decrease in total ash content during frozen storage. But Arannilewa et al. (2005) observed that the ash content remained almost the same throughout the 60 days of frozen storage of tilapia. The decrease in ash content was attributed to the drip loss (Beklevik et al., 2005).
Akinneye et al. (2010) did not detect fibre in the three fish species (H. niloticus, Sardinella spp. and Bonga spp.) that were subjected to oven and sun drying. Ogbonnaya (2009) recorded 1.91, 1.06 and 1.45% in fresh, kiln-dried and electric-dried respectively in O. niloticus. Fibre helps and speeds up the excretion of waste and potentially carcinogenic substances from the human body, preventing them from sitting in the intestine or bowel for too long, rid of wastes and potentially carcinogenic substances such as hormones and cholesterol, both of which can contribute to disease (Monroe et al., 2007; Gann et al., 2003).
Peroxide value is a primary indicator of oxidation of fat (rancidity) (Adeyemi et al., 2013). Results presented here indicate that preservation method increased peroxide values meq O2/kg to 7.37 in FFF, 7.55 in SFH and 16.02 in SFF. The peroxide values corresponding to incipient spoilage are usually in the order of 20-40 meq O2/kg ml/kg. However, Connell (1995) reported that when peroxide value is above 10-20 meq O2/kg, fish develop rancid taste and smell. Thus, it can be concluded that the values from this study are still within acceptable limit of spoilage, although SFF was fastest in deterioration.
Accumulation of secondary oxidation products was measured by determining the thiobarbituric acid value (TBA) (Pegg, 2004). The initial value of TBA 0.18 mg MDA/g (fresh fish Wk 0), suggesting that limited lipid oxidation occur during post-mortem handling. From this result, TBA slightly increased during smoking (0.25 mgMDA/g in smoked fish Wk 0) and also during subsequent storage periods in SFF and in SFH. The highest increase in SFF than in FFF and SFH are in agreement with Goulas and Kontominas (2005), who reported that the initial TBA value of 0.23 mg MDA/g in Chub mackerel (Scomber japonicus). The increase in TBA value during the smoking procedure may be attributed to the partial dehydration of fish and to the increased oxidation of unsaturated fatty acids as a result of smoking. This result was also in agreement with results reported by Mohamed and Atef (2012) who observed increase in TBA value of Grass Carp (Ctenopharyngodon idella) fillets after smoking. The combined effects of smoking and frozen storage could be attributed to the high values of TBA in smoked fish stored in freezer. However, the results from this study contrast with Bugueno et al. (2003) where no changes in TBA value of smoke brined salmon under vacuum until 25 days of smoke brined; and that of Gómez-Estaca et al. (2007) on cold smoked dolphin fish.
Free Fatty Acid (FFA), a tertiary product of rancidity, increased during storage. FFA is a measure of hydrolytic rancidity- the extent of lipid hydrolysis by lipase action (Farzana et al., 2014). Hydrolysis of glycerol-fatty acid esters is one of the important changes that occur in fish muscle lipids during post-harvest with the release of free fatty acids (Chaijan et al., 2006). Generally, the formation of FFA in fish oil during storage is related to the initial lipid content, the lipolytic activity and temperature. It was noted that FFA increased in (FFF, SFH and SFF). Eyo (1993) reported that in most fish oils, rancidity is noticeable when the FFA (calculated as oleic acid) is between 0.5-1.5%. Total volatile nitrogen TVN increased in the limit of acceptability of fish is reported to be 30 mg N/100 g by Connell (1995) while Kirk and Sawyer (1991), suggested that a value of 30-40 mg N/100 g as the upper limit. Beyond this level, white fish and prawns are regarded as unacceptable. However, result from this study shows that the three methods of storage still have their final TVN within acceptable limits, since they all have values less than 30 mg N/100 g.
pH decreased during storage in all the three samples. Eyo (1993) stated that pH is an indicator of the extent of microbial spoilage in fish and that some proteolytic microbes produce acid after decomposition of carbohydrate, thereby increasing the acid level of the medium. The pH value is a reliable indicator of the degree of freshness or spoilage. Decrease in the pH level is due to the fact that carbohydrate of the fish was fermented to acids. Daramola et al. (2007) observed that a decrease in the pH level of smoke-dried fish species: Bony tongue, H. niloticus, African carp, L. coubie, Snake fish, P. obscura, Nile Tilapia, O. niloticus and African mud catfish, C. gariepinus stored at ambient temperature after 8 weeks. This showed that fresh frozen fish has the tendency to spoil faster than smoked fish as indicated by its higher percentage increase of -16.41%.
Smoking was a better preservative technique for fish than freezing of fresh fish. However, when the two samples of smoked fish were compared, the rate of increase was faster for PV, FFA, TVN and TBA in the smoked fish kept in the freezer than the one kept in heated environment. This was contrary to recommendation that smoked fish should be kept under frozen condition (Flick, 2010). Granata (2012) recommended that smoked fish should be handled, packaged and stored much like fresh fish. It should be kept frozen or under refrigeration just above freezing temperatures. If storage temperatures rise above 3°C, there is a risk that Clostridium botulinum may grow and produce toxins in some types of smoked fish. Mold growth on smoked fish can be retarded if it is package in a porous material such as cloth or paper towelling. This prevents “sweating,” a process in which moisture moves from the fish to the inside of the bag, causing a wet spot where mould can grow. This can be a problem if warm, plastic wrapped fish is put in a refrigerator.
The initial calcium (Ca), iron (Fe) and zinc (Zn) values of the smoked fish were significantly (P≤0.05) higher than fresh fish. This result is in accordance with Beyza and Ozeren (2009) who observed significant increase in mineral contents of cooked African catfish using different cooking treatments (baking, grilling, microwaving and frying). The changes with respect to frozen period in all the minerals evaluated could be attributed to drip loss and dehydration that is associated with frozen storage (Arannilewa et al., 2005). Calcium is good for growth and maintenance of bones, teeth and muscles (Turan et al., 2003). Normal extra cellular calcium concentrations are necessary for blood coagulation and for the integrity, intracellular cement substances (Okaka and Okaka, 2001). Iron is an important constituent of haemoglobin (Onwordi et al., 2009). The presence of zinc in the fishes could mean that the fishes can play valuable roles in the management of diabetes, which result from insulin malfunction (Okaka and Okaka, 2001). Iron and Zinc (micro elements) are important in trace amounts, but they tend to become harmful when their concentrations if the tissues exceed the metabolic demands (Ako and Salihu, 2004).
This study has shown that at any of the storage conditions- heated environment and frozen storage, there were significant differences in the quality of fresh and smoked C. gariepinus. There was reduction in the proximate, mineral while chemical quality changes increased during the six weeks storage period. However smoked fish stored in heated environment has less reduction in quality. There is need for more attention and necessity for microbiological study as well as safety.
The authors have not declared any conflict of interest
REFERENCES
|
Adeyemi OT, Osilesi1 OO, Onajobi F, Adebawo O, Afolayan AJ (2013). Stability study of smoked fish, horse mackerel (Trachurus trachurus) by different methods and storage at room temperature. Afri. J. Biochem. Res. 7(6): 98-106.
|
|
|
|
Adesehinwa AOK, Ayanda JO, Bolorunduro PI (2005). Adoption of improved fish preservation technologies in Northwestern Nigeria. Tropicultural, 23: 117-123.
|
|
|
|
Agbabiaka LA, Amadi AS, Eke LO, Madubuko CU, Ojukannaiye AS (2012).Nutritional and storage qualities of Catfish (Clarias gariepinus) smoked with Anthonatha macrophylla. Sci. Res. Rep. 2(2):142-145.
|
|
|
|
Akinneye J, Amoo I, Bakare O (2010). Effect of drying methods on the chemical composition of three species of fish (Bonga spp., Sardinella spp. and Heterotis niloticus). Afri. J. Biotechnol. 9(28): 4369-4373.
|
|
|
|
Akise OG, Abolagba OJ, Eyong MM (2013). Comparative study on quality attributes of three fish species smoke- dried using rubber wood (Hevea brassillensis) in Nigeria. Br. J. Appl. Sci. Technol. 3(4):1177-1186.
Crossref
|
|
|
|
Ako PA, Salihu SO (2004). Studies on some major and trace metals in smoked and oven-dried fish. J. Appl. Sci. Environ. Manag. 8(2): 5-9.
|
|
|
|
AOAC (2000). Association of Official Analytical chemist. Official methods of Analysis, 17th edition. Washington. D.C.
|
|
|
|
AOAC (2005). Association of Official Analytical Chemist, Official Methods of Analysis 18th Ed., AOAC international, suite 500, 481 north frederick avenue, Gaithersburg, Maryland 20877-2417, USA.
|
|
|
|
Arannilewa ST, Salawu SO, Sorungbe AA, Olasalawu BB (2005). Effect of frozen period on the chemical, microbiological and sensory quality of frozen tilapia fish (Sarotherodon galilaleus). Afr. J. Biotechnol. 4(8):852- 855.
|
|
|
|
Audrey M, Audia B, Olive JB (2006). Effect of processing on nutrient content of foods.Cajarticles No 3c 2004:160-164.
|
|
|
|
Bashir FA, Shuhaim-Othman M, Mazlan AG (2012). Evaluation of trace metal levels in tissues of two commercial fish species in Kapar and Mersing coastal waters, Peninsular Malaysia. J. Environ. Pub. Health. 10pp.
Crossref
|
|
|
|
Beklevik S, Visessanguan W, Thongkaew C, Tanaka M (2005). Effect of frozen storage on chemical and gel forming properties of fish commonly used for surimi production in Thailand. Food Hydrocolloids, 19:197-207
Crossref
|
|
|
|
Beyza E, Ozeren A (2009). The effect of cooking methods on mineral and vitamin contents of African Catfish. Food Chem. 115:419-422.
Crossref
|
|
|
|
Bugue-o G, Escriche I, Martinez-Navarrete N, Camacho MM, Chiralt A (2003). Influence of storage conditions on some physical and chemical properties of smoked salmon (Salmo salar) processed by vacuum impregnation techniques. Food Chem. 81:85-90.
Crossref
|
|
|
|
Chaijan M, Benjakul S, Visessanguan W, Faustman C (2006). Changes of lipids in sardine (Sardinella gibbosa) muscle during iced storage. Food Chem. 99:83-91.
Crossref
|
|
|
|
Connell JJ (Ed.) (1995). Control of fish quality. 4th Edition. Fishing News Books, Farnham. England.
|
|
|
|
Daramola JA, Fasakin EA, Adeparusi EO (2007). Changes in physicochemical and sensory characteristics of smoke-dried fish species stored at ambient temperature. Afr. J. Food. Agric. Nutr. Dev. 7(6):11-20.
|
|
|
|
Emere MC, Dibal DM (2013). A Survey of the methods of fish processing and preservation employed by artisanal fishermen in Kaduna City. Food Sci. Qual. Manag. 11:16-22.
|
|
|
|
Eyo AA (1993). Traditional and improved Fish handling, preservation and processing techniques. National workshop on fish processing storage, marketing and utilization. 91-95p.
|
|
|
|
Eyo AA (2001). Fish processing technology in the tropics. National Institute for Freshwater Fisheries Research (NIFFR), New Bussa, Nigeria. pp. 10-170.
|
|
|
|
Farzana BF, Gulshan AL, Subhash CC, Mosarrat NN, Mohajira B (2014).Comparative study on shelf life quality of brine salted Taki (Channa punctatus Bloch,1793) and Shoal (Channa striatus Bloch, 1801) at refrigerator temperature (40C). J. Agric. Vet. Sci. 7(10):63-69.
|
|
|
|
Fawole OO, Ogundiran MA, Ayandiran TA, Olagunju OF (2007). Mineral composition in some selected fresh water fishes in Nigeria. J. Food Safety, 9:52-55.
|
|
|
|
Flick GJ (Ed.) (2010). Smoked Fish Part II. Smoking, storage, microbiology global aquaculture advocate. pp. 31-32.
|
|
|
|
Foline OF, Adedayo MR, Bamishaiye EI, Awagu EF (2011). Proximate composition of catfish (Clarias gariepinus) smoked in Nigerian Stored Products Research Institute (NSPRI): Developed kiln. Int. J. Fish. Aquacult. 3(5): 96-98.
|
|
|
|
Gann PH, Chatterton RT, Gapstur SM, Liu K, Garside D, Giovanazzi S, Thedford K, Van Horn L (2003). The effects of a low-fat/high-fiber diet on sex hormone levels and menstrual cycling in premenopausal women: a 12-month randomized trial (the diet and hormone study). Cancer 98:1870-1879.
Crossref
|
|
|
|
Granata LA, Flick GJ, Martin RE (2012). The Seafood industry: Species, products, processing and safety. Wiley-Blackwell (2nd Ed.). 468pp.
Crossref
|
|
|
|
Guéraud F, Atalay M, Bresgen N, Cipak A, Eckl PM, Huc L, Jouanin I, Siems W, Uchida K. Gueraud (2010). Chemistry and biochemistry of lipid peroxidation products. Free Radic. Res. 44(10):1098-1124.
Crossref
|
|
|
|
Gómez-Estaca, J, Gómez-Guillén MC, Montero P (2007). High pressure effects on the quality and preservation of cold smoked dolphin fish (Coryphaena hippurus) fillets. Food Chem. 102(4):1250-1259.
Crossref
|
|
|
|
Goulas AE, Kontominas MG (2005). Effect of salting and smoking method on the keeping quality of chub mackerel (Scomber japonicus): Biochemical and sensory attributes. Food Chem. 93:511-520.
Crossref
|
|
|
|
Horner WFA (1992). Preservation of Fish by curing: Fish processing technology. Chapman and Hall, London. pp. 254-257.
|
|
|
|
Kiin-kabari DB, Barimalaa IS, Achinewhu SC, Adeniyi TA (2011). Effect of extracts from three indigenous spices on the chemical stability of smoke-dried Catfish (Clarias Lezera) during storage. Afr. J. Food Agric. Nutr. Dev. 11(6): 5335-5343.
|
|
|
|
Kumolu-Johnson CA, Aladetohun NF, Ndimele, PE (2010). The effects of smoking on the nutritional qualities and shelf-life of Clarias gariepinus (BURCHELL 1822). Afr. J. Biotechnol. 9(1):73-76.
|
|
|
|
Kirk RS, Sawyer R (Eds.)(1991). Nitrogen determination. Pearson's Composition and Analysis of Foods. Longman Scientific Publisher, London. pp. 29-36.
|
|
|
|
Louka N, Juhel F, Fazilleau V, Loonis P (2004). A novel colorimetry analysis used to compare different drying fish processes. Food Control 15:327-334.
Crossref
|
|
|
|
Mohamed IS, Atef EEI (2012). Changes in the Quality Properties of Grass Carp (Ctenopharyngodon idella) Fillets smoked during chilled storage. J. Arab. Aquat. Soc. 7(2):165-184.
|
|
|
|
Monroe KR, Murphy SP, Henderson BE, Kolonel LN, Stanczyk FZ, Adlercreutz H, Pike MC (2007). Dietary fiber intake and endogenous serum hormone levels in naturally postmenopausal Mexican American women: the multiethnic cohort study. Nutr. Cancer 58(2):127-135.
Crossref
|
|
|
|
Ogbonnaya C, Ibrahim MS (2009). Effect of drying methods on proximate composition of catfish (Clarias gariepinus). World J. Agric. Sci. 5(1):114-116.
|
|
|
|
Ogbonnaya C (2009). Influences of drying methods on nutritional properties of Tilapia fish (Oreochromis niloticus). World J. Agric. Sci. 5(2):256-258.
|
|
|
|
Okaka JC, Okaka ANO (2001). Food composition, spoilage and shelf life Extension. Cjarco Academic Publishers, Enugu, Nig. pp. 54-56.
|
|
|
|
Okoyo GO, Lokuruka MNI, Matofari JW (2009). Nutritional composition and shelf life of the Lake Victoria Nile Perch (Lates niloticus) stored in ice. Afr. J. Food Agric. Nutr. Dev. 9(3):901-919.
|
|
|
|
Olayemi FF, Adedayo MR, Bamishaiye EI, Awagu EF (2011). Proximate composition of catfish (Clarias gariepinus) smoked in Nigerian stored products research institute (NSPRI). Developed kiln. Int. J. Fish Aquac. 3(5):96-98.
|
|
|
|
Onwordi CT, Ogungbade AM, Wusu AD (2009). The proximate and mineral composition of three leafy vegetables commonly consumed in Lagos, Nigeria. Afr. J. Pure Appl. Chem. 3:102-107.
|
|
|
|
Pegg RB, Wrolstad RE, Acree TE, Decker EA, Penner MH, Reid DS, Schwartz SJ, Shoemaker CF, Smith DM, Sporns P (2004). (Eds.), Spectrophotometric measurement of secondary lipid oxidation products. Handbook of Food Analytical Chemistry. John Wiley & sons Inc.; Hoboken, NJ, USA: pp. 547-564.
|
|
|
|
Roopma G, Meenakshi K, Sweta G, Shallini S (2012). Change in proximate composition and microbial count by low temperature preservation in fish muscle of Labeo Rohita (Ham- Buch). J. Pharm. Biol. Sci. 2(1):13-17.
|
|
|
|
Saeed S, Howell NK (2002). High-performance liquid chromatography and spectroscopic studies on fish oil oxidation products extracted from frozen Atlantic mackerel. J. Amer. Oil Chem. Soc. 76(3):391 397.
Crossref
|
|
|
|
Salán OE, Juliana AG, Marilia O (2006). Use of smoking to add value to Salmoned trout. Braz. Arch. Biol. Technol. 49(1):57-62.
Crossref
|
|
|
|
Saliu JK (2008). Effect of Smoking and frozen storage on the nutrient composition of some African fishes. Adv. Nat. Appl. Sci. 2(1): 16-20.
|
|
|
|
Siddique MN, Hasan MJ, Reza MZ, Islam MR, Boduruzaman M, Forhadur M, Reza S (2011). Effect of freezing time on nutritional value of Jatpunti (Puntius sophore), Sarpunti (P. sarana) and Thaisarpunti (P. gonionotus). Bangl. Res. Publ. J. 5(4):387-392.
|
|
|
|
Serkan K, Sevim K, Bekir T (2010). The Effect of storage temperature on the chemical and sensorial quality of hot smoked Atlantic Bonito (Sarda sarda, Bloch, 1838) packed in aluminum foil. Turk. J. Fish Aqua. Sci. 10:439-443.
|
|
|
|
Saliu JK (2008). Effect of Smoking and frozen storage on the nutrient composition of some African fishes. Adv. Nat. Appl. Sci. 2(1):16-20.
|
|
|
|
Tairu HM, Adedokun MA, Adelodun OB, Badmus BD (2017). Microbial examination of some selected natural preservatives on the shelf Life and safety of smoked Tilapia fish (Oreochromis niloticus). Res. Rev: J. Microbiol. Biotechnol. 6(1): 28-35.
|
|
|
|
Turan M, Kordali S, Zengin H, Dursun A, Sezen, Y (2003). Macro and micro- mineral content of some wild edible leaves consumed in Eastern Anatolia. Acta Agric. Scand. Sect. B, Plant. Soil. Sci. 53:129-137.
|
|
|
|
Ward AR, Jeffries DJ (Eds.) (2000). A manual for assessing post-harvest fisheries losses. Natural Resources Institute, Chatham, UK.
|
|
|
|
Žoldoš P, Popelka P, MarcinÄák S, Nagy J, MesarÄová L, Pipová M, Jevinová P, Nagyová A, Maľa P (2011). The effect of glaze on the quality of frozen stored Alaska pollack (Theragra chalcogramma) fillets under stable and unstable conditions. Acta Vet. Brno. 80:299-304.
Crossref
|