ABSTRACT
The effect of germination of soybean and sesame seeds on the bioavailability of minerals of complementary foods was investigated using rats. Formulations of complementary diets 1 to 4 were produced from sweet potato, soybean and sesame flours at a ratio of 60:30:10, respectively. Formulated diets were significantly higher (p<0.05) in protein, fat and moisture content and lower in carbohydrate and crude fibre than in control. Diet 4 was significantly higher (p<0.05) in minerals (Ca, Zn and Fe) content than in control. The formulated diets were generally low in phytate and oxalate contents, while phytate content was much lower in the diets from germinated flour blends. Anti-nutritional factor (ANF): mineral molar ratio suggested good mineral absorption from the formulated diets. Mineral content of the tissues of rats fed with the formulated diets indicated good absorption within the rats; rats fed with the germinated flour blends had significantly higher (p<0.05) zinc, iron and calcium contents of tissues when compared. Diet 4 consistently received better rating among the samples.
Key words: Complementary food, sweet-potato, germination, anti-nutrient, micronutrient.
Complementary feeding is said to be the process starting from when breast milk alone is no longer sufficient to meet the nutritional requirements of infants and therefore other foods and liquids are needed, along with breast milk (Dewey, 2001). The target age range for complementary feeding is generally taken to be 6 to 24 months of age, even though breastfeeding may continue beyond two years (Cohen et al., 1994; WHO, 2005). Due to their high demand and rapid growth, malnutrition starts in many infants, contributing to the high prevalence of malnutrition in children less than two years of age (FAO, 2011). In many developing countries, complementary diets are derived mainly from local staples such as cereals and tubers, with animal proteins as supplements. However, since animal protein is expensive, attempts have been made to identify alternative sources of protein especially from plants (Ikujenlola et al., 2013).
The high content of anti-nutritional factors and poor availability of minerals in plant-based foods as well as losses during processing play a vital role in micronutrient deficiency (Solomon, 2005). Meanwhile, minerals such as iron, zinc and calcium play a vital role in growth, health and development of infants (Tizazu et al., 2011). The commercially standardized foods are generally very good and can help meet the nutritional requirements of young children in both developed and developing countries (Obiakor-Okeke et al., 2014). But as in most other developing countries, the high cost of nutritious proprietary complementary foods is always, if not prohibitive, beyond the reach of low income families. Such families often depend on inadequately processed traditional foods consisting of non-supplemented cereal porridges made from maize, sorghum and millet (Solomon, 2005). According to Gibson et al. (2010), complementary foods based on either root or tuber crops have been shown to be significantly lower in phytate (by 3 to 20%) than cereal and legume based foods. Also, cereal-based complementary foods form a relatively high viscous porridge which requires excessive dilution usually with water to reduce viscosity to be appropriate for infant feeding which leads to ‘energy and nutrient thinning’ (Amagloh et al., 2013). Therefore, at the household level sweet potato based complementary food has been suggested as a more suitable complementary food as it would be lower in starch, resulting in less viscous porridge as compared with a maize-based infant product in Ghana (Amagloh et al., 2012).
The nutrient potential of soybean and sesame seeds informed the supplementation with a sweet potato based complementary food. Several reports have been documented on sweet potato complementary foods (Sanoussi et al., 2013; Oyareku, 2013; Obiakor-Okeke et al., 2014; Amagloh et al., 2012, 2013), however, bioavailability and absorption of calcium, zinc and iron from a sweet potato-based complementary food blended with soybean and sesame seeds flours has not been reported. It was envisaged that germination would help to breakdown complex polysaccharides and reduce anti-nutrients present in soybean and sesame seeds, and thus improving digestibility and nutrient availability, while the defatting would help to reduce the incidence of rancidity, and therefore improve the keeping quality of the diets. The objective of this study, therefore, was to evaluate the effects of germination and defatting of the soybean and sesame on the proximate and mineral contents of sweet potato based complementary diets.
Yellow fleshed sweet potato, soybeans and sesame seeds were purchased from Bodija Market in Ibadan. A commonly used proprietary infant cereal (Nestle Cerelac, South Africa) or children (6 to 24 months) was used as reference sample, and was purchased at a supermarket in Ibadan. The proximate composition of Nestle Cerelac was protein (15%), fat (10%), fibre (2%), ash (3%), and carbohydrate (68%). Male and female Wistar weanling rats of about 4 weeks old weighing between 40 and 55 g, purchased from the Faculty of Veterinary Medicine, University of Ibadan, were used in the animal experimentation aspects of the study.
Preparation of sweet potato flour
The sweet potato flour was prepared using the method of Obiakor-Okeke et al. (2014) and sesame seed flour was produced using the method of Makinde and Akinoso (2013). Sweet potato roots were washed thoroughly with water to remove adhering soil particles, the washed roots were drained, peeled using sharp knives and uniformly chipped into slices of about 2 mm thickness to facilitate drying using kitchen plantain slicer. The sweet potato chips were thinly spread on aluminium foil lined oven cabinet (Genlab, QE/200, Widnes, Cheshire, United Kingdom) and dried at 65°C to a constant weight, and milled using the locally fabricated attrition mill, the obtained sweet potato flour was sieved using a 0.25 mm British standard sieve (Model BS 410) and kept in an airtight polyethylene bag until further processing.
Preparation of soybean flour
Soybean flour was prepared using the method of Malomo et al. (2012). The soybeans were cleaned, picked manually and winnowing to remove all forms of foreign particles and defects. The soybeans were divided into two portions; the first portion was not subjected to any treatment. The second portion of soybeans where the soybean was washed with 0.7% sodium hypochlorite solution, drained and soaked in water for 6 h. After soaking, soybean was spread thinly in muslin-lined trays and allowed to germinate for 72 h, followed by drying in the oven at 65°C to 10% moisture content. During the period of germination, the seeds were intermittently sprinkled with water to facilitate germination. The dried germinated soybeans and non-germinated soybeans were milled using the locally fabricated attrition mill, the obtained flour samples were sieved using a 0.25 mm British standard sieve (Model BS 410) and kept in separate airtight polyethylene bag until further use.
Preparation of sesame flour
The sesame seeds were cleaned, manually picked and winnowed to remove all forms of foreign particles and defective seeds before processing commence; the sesame seeds were also divided into two portions, the first was not subjected to any treatment. The second portion of the sesame seeds were soaked in water for 6 h, drained and spread thinly in a muslin-lined tray, allowed to germinate for 72 h and sprinkled with water intermittently to facilitate germination. The germinated seeds were collected, dried in the oven (Genlab, QE/200, Widnes, Cheshire, United Kingdom) at about 40°C to a constant weight. The non-germinated seeds and the germinated seeds were milled using locally fabricated attrition mill, the obtained meal was kept in separate airtight polyethylene bags until further processing. The obtained meal from non-germinated sesame seeds and germinated sesame seeds were divided into two portions each, the first portions was kept as full-fat non-germinated sesame and full-fat germinated sesame seeds in airtight bags until further processing. While, the second portions were defatted by cold maceration using Unal and Yalcsn (2008) method, the meals from non-germinated sesame seeds and germinated sesame seeds were separately soaked in n-hexane at room temperature for 72 h, during which they were stirred intermittently. The supernatant was collected and the process was repeated three times using fresh solvent each time. The resulting defatted flours were air dried in hot air oven at 45°C (Genlab, QE/200, Widnes, Cheshire, United Kingdom) for 24 h and kept in airtight polyethylene bags for further use.
Formulation of experimental diets
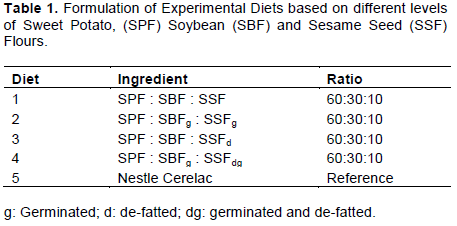
The experimental diets were formulated from sweet potato flour (SPF), non-germinated soybean flour (SBF), germinated soybean flour (SBFg), non-germinated sesame seeds flour (full-fat) (SSF), non-germinated sesame seeds flour (defatted) (SSFd), germinated sesame seeds flour (full-fat) (SSFg) and germinated sesame seeds flour (defatted) (SSFdg) using the methods of Adenuga (2010) with some modifications which are shown in Table 1.
Chemical analysis of flours and formulated diets
Moisture content was determined using the method described by AOAC (2006 Method No 934.01). One gram of sample in pre-weighed crucible was placed in oven (100 ±5°C) for 24 h, cooled and reweighed. The percentage moisture was calculated as:
where W1 = weight of crucible, W2 = weight of crucible after drying at 105°C and sample, and W3 = weight of crucible and sample after cooling in an airtight desiccator.
Crude protein was determined using micro-Kjeldahl method as described by Pearson (1976); percentage protein was determined using nitrogen protein conversion factor, 6.25. Ash and fibre content were determined according to AOAC (2006 Method Nos. 942.05 and 978.10), respectively. Two grams of sample was added into pre-weighed crucible and incinerated in muffle furnace at 600°C for 18 h to light grey ash. Thereafter, they were removed and placed immediately in a desiccator to cool and weighed.
where W1 = weight of cleaned, dried, ignited and cooled crucible; W2 = weight of crucible and sample after incinerating at 600°C; W3 = weight of crucible and sample after cooling in an airtight desiccator.
The method of Pearson (1976) was employed in determining crude lipid content, where 3 g of the sample was extracted using petroleum ether, dried and weighed to obtain percentage fat extracted. The total crude carbohydrate content of the diet samples was obtained by difference (AOAC, 2006, Item 85). Minerals (Ca, Zn and Fe) contents were determined using Atomic Absorption Spectrophotometer (PerkinElmer AAnalyst 200, Shelton, Connecticut, USA). The standard curve for each mineral was prepared from known standards and the mineral value of samples estimated against that of standard curve (AOAC, 1990).
Phytate and total oxalate contents of the diets were determined using the method of AOAC (1990), standard curve was prepared for each of the anti-nutrient and their concentration in the diet samples was extrapolated from the generated standard curve and expressed as mg/100 g sample.
Rat feeding programme
Twenty male and female Wistar-strain weanling rats weighing between 40 and 55 g were randomly distributed into 5 groups of 4 rats each during the feeding experimentation (2 males and 2 females per group). They were kept in well ventilated plastic cages with wire net lid. The rats were allowed to acclimatize on the animal pellets for 7 days before feeding with the experimental diets commenced. The animals were fed a known amount of each of the diet daily, fresh clean water was also served on a daily basis. Daily records of feed intake and weights of rats as well as mortality rate were kept. The experiment lasted 21 days after which the animals were sacrificed. The rats were anaesthetized and sacrificed by cervical dislocation. The liver, heart and kidneys were removed, drained of blood and weighed. The thigh muscle and femur was also harvested as a whole. The thigh muscle and femur was subjected to ashing in a furnace (Carbolite Gero, BWF 12/13, Derbyshire, UK) and dissolved in 10% HCl and made up to 100 mL standard flask with distilled water. The mineral (Ca, Zn and Fe) content of the tissue was analyzed using Atomic Absorption Spectrophotometer (PerkinElmer, AAnalyst 200, Shelton, Connecticut, USA) (AOAC, 1990).
Statistical analysis
Data obtained from chemical analysis, mineral content assay, were statistically analyzed using the analysis of variance (ANOVA), SPSS 20.0 version. Means were separated using Duncan (1955) multiple range test (p<0.05).
The proximate composition of the experimental diets is shown in Table 2. The formulated diets were significantly higher (p<0.05) in protein, fat and moisture contents than the reference sample (Nestlé’s Cerelac). Crude protein was significantly higher (p<0.05) in diets 1 to 4 (21.37 to 21.94%) than in reference sample (14.70%). The moisture content of the experimental diet was significantly higher (p<0.05) in diets 1 to 3 (5.35 to 6.6%) than in reference sample (4.36%) and diet 4 (4.75%) which are not significantly different (p>0.05). Fat content (11.07 to 17.09%) was statistically higher (p<0.05) in diets 1 to 4 than in reference sample (9.50%).
The fibre and total carbohydrates contents were significantly lower (p<0.05) in the experimental diets than in reference sample, while the values obtained for ash content were comparable in diets 4 and 5 (reference sample), and lower in the diets which diet numbers. Fibre content (1.10 to 1.65%) was significantly lower (p<0.05) in diets 1 to 4 than in reference sample (3.00%). Total estimated carbohydrate, obtained by difference, was significantly higher (p<0.05) in the reference sample diet (65.24%) than the other experimental diets (52.94 to 56.76%). Ash contents of the reference sample (3.20%) was not significantly different (p>0.05) from those of the other diets (2.0 to 4.0%). Protein content of the experimental diets which is considerably high could be as a result of the soybean flour included in the diet owing to the fact that the seeds of soybean are rich in protein (Sanoussi et al., 2013; Onoja et al., 2014; Abioye et al., 2011).
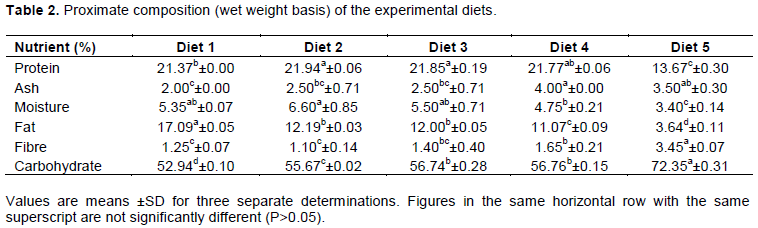
The mineral element (Ca, Zn, and Fe) composition of the experimental diets is shown in Table 3. The calcium content of the diets showed significantly higher (p<0.05) content in diet 4 (544.50 mg/100 g) than the reference sample (406.00 mg/100 g) which was not significantly different (p>0.05) from the value of diet 2 (392.00 mg/100 g), while diets 1 and 3 (254.00 and 308 mg/100 g, respectively) were significantly lower (P<0.05). Zinc content was significantly higher (p<0.05) in diet 4 (2.89 mg/100 g) than in reference sample (2.51 mg/100 g). In diet 2, the zinc content (2.64 mg/100 g) was comparable to that of the reference sample and values obtained for diets 1 and 3 (2.07 and 2.12 mg/100 g, respectively) were significantly lower (p>0.05) than the reference sample. Values obtained for Fe contents showed a significantly higher (p<0.05) content in diet 4 (14.50 mg/100 g) than in reference sample (12.25 mg/100 g), while diet 1 content (11.00 mg/100 g) was significantly lower. Mineral content of the diets was higher than what was observed by Solomon (2005). This high mineral content could be due to the inclusion of sesame seeds in the diet. According to Bamigboye et al. (2010), sesame seeds are a good source of iron, zinc and calcium as it contains about 3.83, 4.46 and 281.1 mg/100 g, respectively.
Phytate and oxalate values are shown in Table 4. Anti-nutrients were generally lower in the reference sample than in the experimental diets (Table 4). Phytate was significantly lower (p<0.05) in the reference sample (0.10 mg/100 g) than in the experimental diets (which ranges from 0.22 to 0.31 mg/100 g). Total oxalate content was not significantly different (p>0.05) between the reference sample diet (0.19 mg/100 g), and diets 1 and 2 (0.20 mg/100 g in both diets), while the content of total oxalate was observed to be significantly higher (p<0.05) in diets 3 (0.24 mg/100 g) and 4 (0.26 mg/100 g). Phytate and oxalate contents of the diets were generally low and this is comparable to data in the literature (Udensi et al., 2012), but higher as reported by Obiakor-Okeke et al. (2014) and Onoja et al. (2014). Low phytate content observed in the diets could be due to high proportion of sweet potato flour used for the formulation of the complementary diets. Both root or tuber crops have been shown to be significantly lower in phytate (by 3 to 20%) than cereal and legume based foods (Gibson et al., 2010).
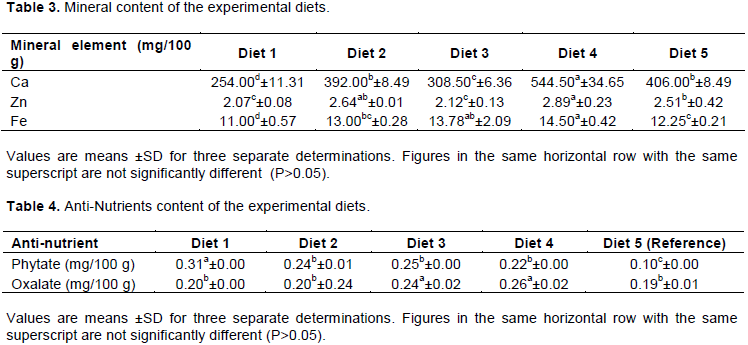
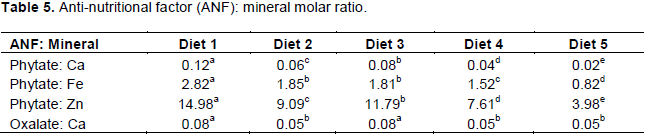
The values obtained for the ANF, mineral molar ratio showed that phytate: Ca ratio was significantly lower (p<0.05) in the reference sample (0.02) than the other experimental diets (0.04 to 0.12) with diet 1 having the highest phytate: Ca value (0.12). Phytate: Fe and phytate: Zn values were all significantly lower (p<0.05) in the reference sample diet (0.82 and 3.98 respectively) than the other experimental diet (1.52 to 2.82 and 7.61 to 14.98, respectively) (Table 5). Values obtained for oxalate: Ca show a significantly higher (p<0.05) values in diets 1 and 3 (0.08 in both). The anti-nutrient: mineral molar ratio obtained from this study is considerably low. The phytate: calcium, iron and zinc were comparable to those obtained from a sweet potato based complementary food (approximately 0.17, 1 and 15, respectively) which predicted better absorption as it contains at least half the phytate: mineral molar ratio of a maize based complementary food (Amagloh et al., 2012).
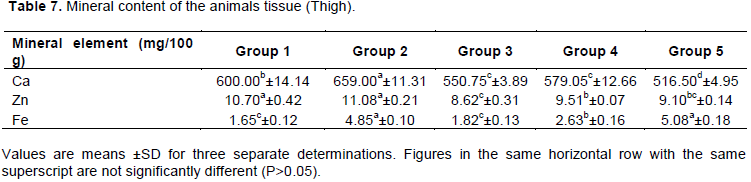
Food intake and growth rate from the animal experimentation is shown in Figures 1 and 2 respectively, while Table 6 shows the food intake, protein intake, weight gain, protein efficiency ratio (PER) and food efficiency ratio (FER) per rat.
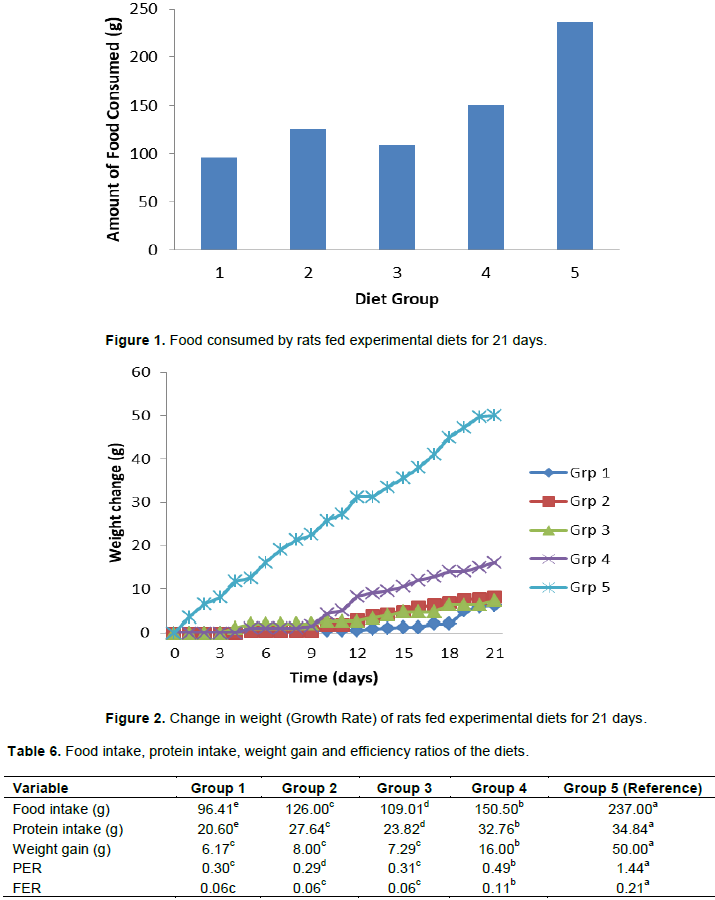
Table 6 shows that the group of rats fed the reference sample diet has a significantly higher (p<0.05) food intake (237.00 g), protein intake (34.84 g), weight gain (50.00 g), PER (1.44) and also FER (0.21) than the group of rats fed the other experimental diets. The growth rate of rats fed the experimental diets was low as compared to that of proprietary formula. As observed in literature, proprietary formula promotes optimum growth amidst the rats (Ibironke, 2014). Low PER and FER were observed in the experimental diets. The values obtained were similar to PER of 0.66 to 0.99 obtained by Ijarotimi and Olopade (2009), and lower than 1.77 to 1.90 reported by Ibironke (2014) and Shiriki et al. (2015). This low PER was expected as protein of plant origin have low biological value (Solomon, 2005).
Table 7 shows the mineral content of the tissue collected from the rats fed the experimental diets. The Ca content of the tissue from group 2 rats (659.00 mg/100 g) was significantly higher (p<0.05) than that of rats fed with the reference sample (516.50 mg/100 g). The Zn content of the tissue was significantly higher (p<0.05) in groups 1 and 2 rats (10.70 and 11.08 mg/100 g, respectively) than in those fed with the reference sample (9.10 mg/100 g) and lower in group 3 rats (8.62 mg/100 g). The iron (Fe) content of the tissue obtained from the rats, showed no significant difference (p>0.05) in the reference sample group rats (5.08 mg/100 g) and group 2 (4.85 mg/100 g) which were both significantly higher (p<0.05) than those of the other groups (1.65 to 2.63 mg/100 g). Non-heme iron which was present in plant food is poorly absorbed (Lean, 2006), this explains the higher iron absorption in the proprietary formula fed rat. Generally, absorption of mineral was high which made the mineral available for body metabolism and physiological functions.
Nutritious complementary food could be produced from sweet potato based diet supplemented with germinated and defatted soybean and sesame seed flours which had low anti-nutrients, increased bio-availability of minerals and absorption rate. The low anti-nutrient content and increased bioavailability of the complementary food make the products suitable for infants and children. These products could be produced at the cottage level to alleviate the prevalent malnutrition problems.
The authors have not declared any conflict of interests.
REFERENCES
|
Abioye VF, Ade-Omowaye BI, Babarinde GO, Adesigbin MK (2011). Chemical, Physico-Chemical and Sensory Properties of Soy-Plantain Flour. African Journal of Food Science 5(4):176-180.
|
|
|
|
Adenuga W (2010). Nutritional and Sensory Profiles of Sweet Potato-Based Infant Weaning Food Fortified with Cowpea and Peanut. Journal of Food Technology 8(5):223-228.
Crossref
|
|
|
|
|
Amagloh FK, Brough L, Weber JL, Mutukumira AN, Hardacre A, Coad J (2012). Sweet Potato-based Complementary Foods Would Be Less Inhibitory on Mineral Absorption Than a Maize-based Infant Food Assessed by Compositional Analysis. International Journal of Food Science and Nutrition 63(8):957-963.
Crossref
|
|
|
|
|
Amagloh FK, Hardacre A, Mutukumira AN, Weber JL, Brough L, Coad J (2013). Carbohydrate Composition, Viscosity, Solubility, and Sensory Acceptance of Sweetpotato-based and Maize-based Complementary Foods. Food Nutrition Research 57:18717.
Crossref
|
|
|
|
|
Association of Official Analytical Chemists (AOAC) (1990). Official Methods of Analysis 15th Ed. Association of Official Analytical Chemists, Washington, D.C., USA P 145.
|
|
|
|
|
Association of Official Analytical Chemists (AOAC) (2006) Official Methods of Analysis of the Association of Analytical Communities, Gaithersburg, MD, 17th Ed., 2006 P 148.
|
|
|
|
|
Bamigboye AY, Okafor AC, Adepoju OT (2010). Proximate and Mineral Composition of Whole and Dehulled Nigerian Sesame Seed. African Journal of Food Science and Technology 1(3):071-075.
|
|
|
|
|
Cohen RJ, Brown KH, Dewey KG, Canahuati J, Rivera L (1994). Effect of age of introduction of complementary foods on infant breast milk intake, total energy intake, and growth: a randomised intervention study in Honduras. The Lancet 344(89180: 288-293,
|
|
|
|
|
Dewey K (2001). Guiding Principles for Complementary Feeding of Breast-fed Child. Pan American Health Organisation (PAHO), World Health Organisation (WHO) pp. 18-40
|
|
|
|
|
Food and Agriculture Organization (FAO) (2011). Complementary Feeding for Children Aged 6-23 Months: A recipe for mothers and caregivers. Food and Agriculture Organization of United Nations (FAO) pp. 1-46.
|
|
|
|
|
Gibson RS, Bailey KB, Gibbs M, Ferguson ML (2010). A review of phytate, iron, zinc, and calcium concentrations in plant-based complementary foods used in low-income countries and implications for bioavailability. Food Nutrition Bulletin 31:S134-S146.
Crossref
|
|
|
|
|
Ibironke SI (2014). Formulation of Infant weaning Foods from Vegetable Proteins and Cereals. American Journal of Food Technology 9(2):104-110.
Crossref
|
|
|
|
|
Ijarotimi OS, Olopade AJ (2009). Determination of Amino Acid Content and Protein Quality of Complementary Food Produced from Locally Available Food Materials in Ondo State, Nigeria. Malaysian Journal of Nutrition 15(1):87-95.
|
|
|
|
|
Ikujenlola AV, Oguntuase SO, Omosuli SV (2013). Physic-chemical Properties of Complementary Food from Malted Quality Protein Maize (Zea mays L.) and Defatted Fluted Pumpkin (Telfairia occidentalis Hook F.). Food and Public Health 3(6): 323-328.
|
|
|
|
|
Lean ME (2006). Food Science, Nutrition and Health (7th Ed). Edward Arnold (publishers) Ltd. pp. 190-192.
|
|
|
|
|
Makinde FM, Akinoso R (2013). Nutrient Composition and Effect of Processing Treatments on Anti Nutritional Factors of Nigerian Sesame (Sesamum indicum Linn) Cultivars. International Food Research Journal 20(5):2293-2300.
|
|
|
|
|
Malomo O, Ogunmoyela OAB, Oluwajoba SO, Kukoyi I (2012). Effect of Germinated Soy Flour on the Sensory Acceptability of Soy-Wheat Composite Bread. Asian Journal of Natural and Applied Sciences 1(3):91-105.
|
|
|
|
|
Obiakor-Okeke PN, Amadi JA, Chikwendu JN (2014). Development and Evaluation of Complementary Foods Based on Soybean, Sorghum and Sweet Potato Flour Blends. Food Science and Quality Management 33:77-86.
|
|
|
|
|
Onoja US, Akubor PI, Gernar DI, Chinmma CE (2014). Evaluation of Complementary Food Formulated from Local Staples and Fortified with Calcium, Iron and Zinc. Journal of Nutrition and Food Science 4:326.
|
|
|
|
|
Oyareku MA (2013). Effect of Co-fermentation on Nutritive Quality and Pasting Properties of Maize/Cowpea/Sweet potato as Complementary Food. African Journal of Food, Agriculture, Nutrition and development 13(1):7171-7191.
Crossref
|
|
|
|
|
Pearson D (1976) Chemical Analysis of Foods. 7th Edition. Churchill London pp. 7-11.
|
|
|
|
|
Sanoussi AF, Dansi A, Bokossa-yaou I, Dansi M, Egounlety M, Sanni LO, Sanni A (2013). Formulation and Biochemical Characterization of Sweet Potato (Ipomoea batatas) Based Infant flours Fortified with Soybean and Sorghum Flours. International Journal of Current Microbiology and Applied Science 2(7):22-34.
|
|
|
|
|
Shiriki D, Igyor MA, Gernah DI (2015). Nutritional Evaluation of Complementary Food Formulations from Maize, Soybean and Peanut Fortified with Moringa oleifera Leaf Powder Food and Nutrition Sciences 6:494-500.
Crossref
|
|
|
|
|
Solomon M (2005). Nutritional Evaluation of Cereal and Legume-Based Complementary Diets Used in Jos, Plateau State. A PhD thesis in the Faculty of Medical Sciences, University of Jos. pp. 93-147.
|
|
|
|
|
Tizazu S, Urga K, Belay A, Abuye C, Retta N (2011). Effect of Germination on Mineral Bioavailability of Sorghum-based Complementary Foods. African Journal of Food Agriculture Nutrition and Development 11:5.
Crossref
|
|
|
|
|
Udensi EA, Odom TC, Nwaorgu OJ, Emecheta RO, Ihemanma CA (2012). Production and Evaluation of the Nutritional Quality of Weaning Food Formulation from Roasted Millet and Mucuna cochinchinesis. Sky Journal of Food Science 1(1):1-5.
|
|
|
|
|
Unal MK, Yalcsn H (2008). Proximate Composition of Turkish Sesame Seeds and Characterization of their Oils. Grasas Aceites 59:23-26.
|
|
|
|
|
World Health Organization (WHO) (2005). Guiding Principles for feeding Non-breastfed Children 6-24 months of age. Available at
View
|
|