Full Length Research Paper
ABSTRACT
The increasing presence of food and feed products derived from genetically modified organisms (GMO) has led to the development of detection methods that distinguish between foods derived from biotechnology and conventional foods. Many countries have implemented the Regulations for GM products labelling, therefore the need of establish reliable and accurate detection methods for GMO in raw materials and food products. The aim of the present study was to screen foods sold in the selected Mozambican markets for the presence of GMOs. Out of 47 samples analysed, 22 (46%) were positive either for 35S promoter or t-NOS terminator. Results of the event-specific analysis indicated the presence of RRS, Mon863 and TC1507 in 8, 6 and 1 sample respectively. None of the positive samples had a GM label. This study demonstrates for the first time, as far as we know, the presence of GM food products circulating in Mozambican markets, therefore strengthening the need for establish of labelling system and quantitative methods in routine analyses, to ensure compliance with existing regulations.
Key words: Genetically modified organisms, roundup ready soybean, P35S, food.
INTRODUCTION
Maize, soy cotton and canola are the most cultivated genetically modified organisms (GMOs) and they constitute the essential ingredient of many foods (Datukishvili et al., 2015; Turkec et al., 2015; Erkan and Dastan, 2017; Soylu et al., 2020; Ashrafi-Dehkordi et al., 2021). In the mid 90’s, GMOs foods, mainly derived from varieties of Roundup herbicide tolerant soybeans (Roundup Ready) and maize (Bt 176) began to be marketed and many more others are in the process of being approved for commercialization (Zhang et al., 2016; Giraldo et al., 2019; Yu, 2021). The resistance of many consumers to these foods which leads to restrictions on consumption and introduction of barriers for commercialization of products derived from GMOs (Smyth, 2017). With the establishment of specific legislation, which may vary by countries or group of countries, it was imposed labelling of products that may contain or are produced from GMOs, assess the possible impact on public and environmental health and to give consumers the opportunity of choice (Fraiture et al., 2015; Safaei et al., 2019).
Few African countries have released transgenic crops for farmers use and have access to the genetic modification technology. This scenario is due to the absence or inefficiency of the biosafety legislation and political will. African countries such as South Africa, Zimbabwe, Egypt, Kenya, Tunisia, Algeria, Burkina Faso, Mali, Togo, Ghana Uganda, Sudan and Mauritius has so far implemented this technology and have functional National Biosafety Framework (Gbashi et al., 2021). However, only South Africa, Egypt and Burkina Faso have already advanced to the commercial release crops (Akinbo et al., 2021). While in the European Union, Korea, Japan and Australia the labelling of food and derived products is compulsory (Aburumman et al., 2020; Li et al., 2020; Ashrafi-Dehkordi et al., 2021), most African countries still lack such regulations (Gbashi et al., 2021).
In Mozambique, through Decree no.6/2007, the Council of Ministers approved the Biosafety Regulation on GMOs. The approved document outlines the rules for all activities with GMOs and their products (Boletim da República, 2014). Despite the existence of this legal instrument, little is known about the situation of GMOs in Mozambique, including production, transformation or even trade or use by the public.
The detection of genetically modified (GM) components in compound samples is a challenging task (Turkec et al., 2015). Analytical methods are necessary for detection of GMOs in raw materials, as well as processed products. One of the most commonly applied methods for detection of GMOs is the polymerase chain reaction (PCR) due to its high sensitivity and specificity for DNA detection (Fraiture et al., 2015; Bak and Emerson, 2019; Giraldo et al., 2019; Leão-Buchir et al., 2018; Safaei et al., 2019; Aburumman et al., 2020; Li et al., 2020; Ashrafi-Dehkordi et al., 2021; Park et al., 2021). However quick tests have been also adopted for GMO testing depending on their accuracy, speed and quality. Lateral flow strips, also known as immunochromatographic assays, are the simplest mechanism used to identify the protein expressed by a GMO. They use antibodies to specifically bind and therefore detect the genetically expressed protein by a GM crop (Akiyama et al., 2006; Malik et al., 2018). On the other hand, another type of GMO testing based on protein is the Enzyme-Linked Immunosorbent Assay (ELISA) used to detect the protein expressed by a GM culture (Malik et al., 2018). To date, there are no studies carried out in Mozambique for the detection of GMOs using the PCR method. Therefore, this is the first study that aims to detect the presence of GMOs in food products commercialized in Mozambican markets.
MATERIALS AND METHODS
Samples
This study was carried out in Maputo, Sofala and Nampula, three different sampling areas located in the South, Center and North of Mozambique, respectively. A total number of 47 processed food samples were purchased randomly from different markets and included 6 maize flour, 17 baby food, 5 biscuits, 5 chips, 5 breakfast cereal, 6 sweet corn, 2 soy milk and 1 popcorn (Table 1). Samples were collected in 2009 and 2011. The Certified Reference Materials included 1% RRS, 0 and 10% MON863, 0% and 1% TC1507, and were used in this study for quality controls.
DNA extraction and quality analysis
Genomic DNA was isolated from samples in duplicate using the cetyltrimethylammonium bromide (CTAB) method described by Van den Eede et al. (2000) with some modifications. 1000 µl of pre-heated (65°C) extraction buffer and 10 µl of RNAse (10 mg/ml) were added to 200 mg of each sample and mixed properly. After homogenization, the mixture was incubated at 65°C for 30 min. 10 µl of Proteinase K (20 mg/ml) was added, mixed, and incubated at 65°C for 30 min and centrifuged at 12000 × g for 10 min). The supernatant was transferred to a new 1.5 ml tube containing 500 µl of chloroform and mixed. The material was centrifuged (12000×g, 15 min) and the upper phase was transferred into a fresh 1,5 ml tube containing 500 µl of chloroform and mixed. The material was centrifuged (12000 × g, 5 min) and the upper phase transferred to a new 2 ml tube. To the upper phase (aqueous) were added 2 volumes of CTAB, mixed and then incubated for 1 hour at room temperature and the supernatant was carefully discarded after centrifugation at 13 000 x rpm for 5 min. 350 µl of NaCl (1.2 M) and equal volume of chloroform were added to the pellet, mixed carefully and centrifuged at 10 000 × g for 10 min. The upper aqueous phase was transferred into a new tube and 0.6 volumes of isopropanol were added, mixed and incubated at room temperature. The samples was concentrated by centrifugation at 12 000 × g for 10 min before discard the supernatant. Afterwards, the pellet was washed with 70% ethanol and centrifuged at 12 000× g for 10 min, then the pellet air dried at 37°C for 10 min. The pellet was therefore dissolved in 150 µl of TE buffer (pH 7.5) stored at -20°C. To ensure the quality control and reduce false positive or false negative due to the contamination, an environmental control was included on each lote during DNA extraction procedure. Isolated DNA concentration was determined by UV-spectroscopy (Nanodrop 1000, Thermo Scientific) and the absorbance was measured by the ratio of 260 and 280 nm. The DNA quality was assessed on an agarose gel. To ensure that there is no contamination during DNA extraction process, an environmental control (tube with no sample) was included and processed in parallel with the samples.
PCR amplification
All PCR reactions were performed using a thermocycler (Eppendorf Mastercycler Gradient). For quality control of the extracted DNA, primer sets Agh-F3/R4 and QPCR-LecF/GM1R were used for maize and soy endogenous genes. To screen for GM soy and maize products P35SF/R and t-NOS F/R primer sets were used. For the identification of the event-specific Mon 883, TC1507 and RRS, primers Mon863-F/R, MaiY- F1/R3 and RRS were used. The PCR reactions mix contained 1 × PCR buffer, 3 mM MgCl2, 0.2 mM dNTP, 0.4 μM of each primer, 1U of Taq and 5 µl of genomic DNA in a total reaction volume of 50 μl. Primer sets used are listed on Table 2. In order to validate the results, positive and nnegative control (certified reference materials), environmental control and control of the mix (MQ water) were included for each PCR reaction. The PCR cycling condition consists of initial denaturation at 95°C for 10 min, followed by 50 cycles of denaturation at 95°C for 30 s, annealing at 60°C for 30 s and extension at 72°C for 30 s, and a final extension at 72°C for 8 min.
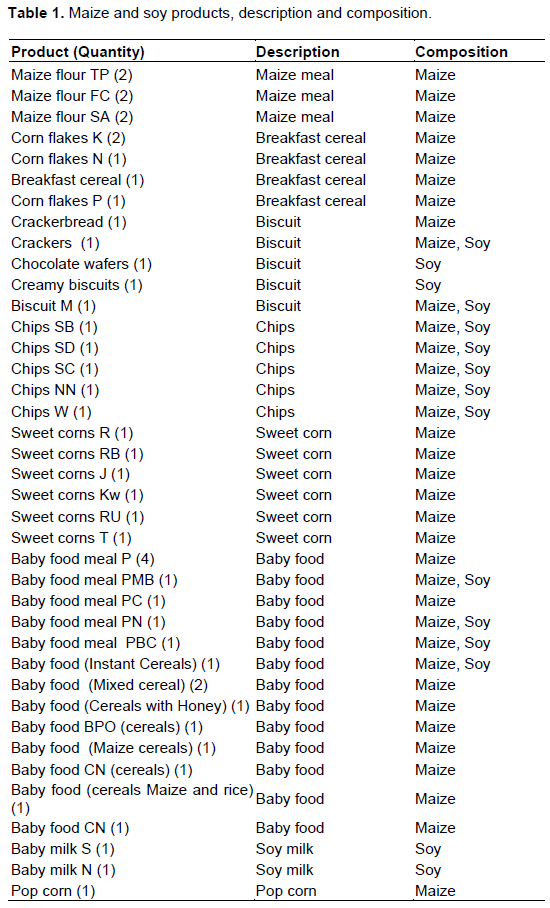
Agarose gel electrophoresis
The PCR products were analysed on 1.5% agarose gel with DNA safe view stain and visualized by a UV light.
RESULTS AND DISCUSSION
DNA extraction and amplification
The extraction of high-quality DNA is very important for any molecular analysis (Turkec et al., 2015; Soylu et al., 2020; Ashrafi-Dehkordi et al., 2021). The CTAB method for extracting DNA from food products and reference materials yielded DNA of good quality for further analysis however in low quantity. Our results are in line with studies conducted by Pinto et al. (2011), Turkec et al. (2015) and Ashrafi-Dehkordi et al. (2021). The physical grinding process to which the samples were subjected before DNA extraction together with the high level of food processing might be related to the low amount of DNA obtained (Turkec et al., 2015; Coello et al., 2017; Soylu et al., 2020). Arun et al. (2013) and Li et al. (2020) also showed that food processing methods such as heat, may affect the integrity of the nucleic acid. According to Xiang et al. (2015), the methods used to process food, involving physical treatments, chemical changes and biological reactions affect in different ways the integrity of endogenous and exogenous genes. Zhang et al. (2014) evaluated the effects of food processing methods on the degradation of endogenous and exogenous genes on GM rice, where frying was the toughest process for rice crackers while fermentation impacted more on degradation for sweet rice wine. According to Al-Salameen et al. (2012), DNA extracted from processed food is often of low quality, may be absent, present in very low concentrations or even severely damaged, making it not adequate for detection and quantification with molecular analysis. This statement corroborates the finding in the present study.
Screening and event specific detection
DNA extracted from all samples was subjected to soybean and maize-specific PCR to determine if the DNA is amplifiable to prevent false negatives due to non-amplifiable DNA (Alasaad et al., 2016). According to Aburumman et al. (2020), house-keeping genes provides internal control to optimize DNA quantity for PCR reaction with good amplification. The specific primers set targeting Adh and Lectin genes for maize and soybean respectively (Table 2) were used. Expected amplified fragments of 136 bp for Adh (Figure 1) and 74 bp for Lectin genes were detected confirming that tested samples contain either maize or soybean as shown in Table 3. Similar results were found in a study conducted with processed food sold commercially in Iran (Rabiei et al., 2013).
The results were in accordance with the composition of the sample as all maize-based samples were Adh-positive and all soybean-based samples were lectin-positive. Additionally, to determine the specificity of the maize and soybean-specific primers, DNA from tomato, sesame, wheat, peanut, coconut, banana and rice were included in PCR. As expected, the primers did not amplify in the no-maize and soy samples due to the lack of Adh and lectin gene in tomato, coconut, banana and rice.
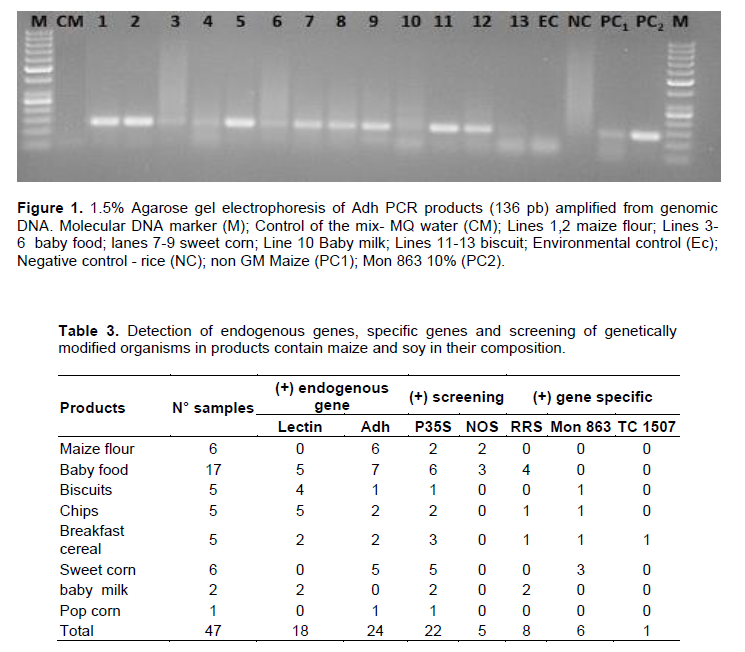
To assess the presence of genetic modification in food samples, P-35S promoter and NOS terminator were analyzed. The P-35S promoter from CaMV and NOS are the most favorable candidates screening methods and are most frequent promoter and terminator sequences inserted in most GM crops as regulatory genes respectively (Safaei et al., 2019; Aburumman et al., 2020; Ashrafi-Dehkordi et al., 2021). Oraby et al. (2021) suggested that primers P-35S from CAMV can be used in parallel with the primer GT88 targeting the new region of the CAMV-35P promoter to strengthen the results. Out of 47 tested samples, 22 showed the presence of CAMV-35S promoter and 5 for the terminator NOS confirmed by the PCR fragment of 80 bp (Figure 2) and 118 bp respectively. The results indicate that soybean and maize positive samples for these two genes are genetically modified. Most of the samples showed low intensity bands, and this may be related with the low amount of DNA yielded or the sensitivity of the conventional PCR. Investigating the efficiency of conventional PCR, Ahatovic et al. (2021) reported 12.3% of tested samples with low band intensity, and the sensitivity of the agarose gel was mentioned as one of the factors affecting the PCR efficiency. Positive result for CaMV 35S promoter in processed foods may indicates a probability of presence of the GM material (Arun et al., 2013; Bak and Emerson, 2019). Although some samples in our study did not amplify with NOS, they have shown an exogenous gene introduced. A similar results were found by Safaei et al. (2019) in a study conducted with genetically modified rice where no sample was positive for the presence of NOS.
In other study, detection of P35S and NOS in maize and soy processed foods samples revealed that 13 of 23 samples were GM positive (Park et al., 2021).
On the other side, none of the environmental control (corresponding to the extraction control) and Blank reference material (Mon 863 0%) included in the study were positive for the 35S promoter and NOS terminator amplification (Figure 2). As expected, these results demonstrated that there were no cross contamination. Almost 90% of the GM samples detected in our study did not carry any GMO-label in their package. Our results are in line with many other food products studies in which it has been shown that more than 70% of GM food products marketed are not labelled (Ujhelyi et al., 2008; Kaur et al., 2010).
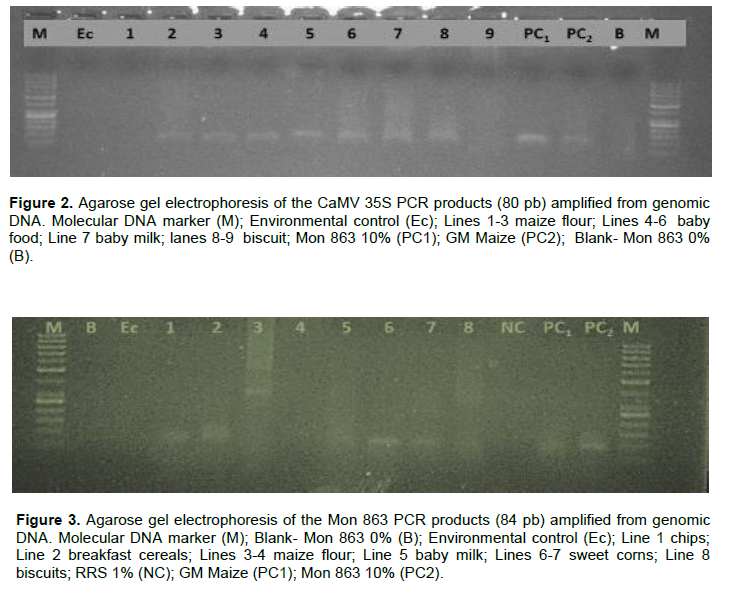
Positive soy and maize samples for P-35S or NOS genes were analysed for specific transgenic events RRS, MON 863 and TC 1507. In total, 22 positive samples where at least one regulatory gene was detected, the RRS gene was detected in 8 samples, MON 863 in 6 samples and TC 1507 in 1 sample indicated that all samples contained GM event specific fragments (Figure 3). From the analyzed samples, baby food meal and baby milk containing soy protein showed more positive results for RRS gene and sweet corns for Mon 863. Similar findings were reported by Erkan and Dastan (2017) in their study, where soy protein and cereals constituted the common GMO-containing products observed. The presence of the RRS, MON 863 and TC 1507 events in samples from Mozambique means that the samples were modified genetically with herbicide tolerance and insect resistance genes introduced, the two most frequent transgenic sequences used in the construction of transgenic soy and maize crops (Datukishvili et al., 2015; Rosculete et al., 2018; Ashrafi-Dehkordi et al., 2021). Safaei et al. (2019) reported similar findings in food products marketed in Iran where it was found that 57 soy food products were GM positive for RRS event and for maize food samples, 40% were positive for Bt11 and 13.3% positive for MON 810 event, proving the presence of GM sequences in their genome. In other study carried out in Brazil, 14 maize flour samples were positive for MON 810, Bt11 and TC 1507 and 10 of the 14 samples also tested positive for NK 603 event (Branquinho et al., 2013).
CONCLUSION
The results of the present study showed that the DNA extraction method and the conventional PCR used were efficient for isolation and detection of GMOs in food products. None of the labels on samples of processed foods collected in the Mozambican market reported the presence of GM corn or soy. However, these samples indicated the presence of GM materials in their composition. The results of this study will assist in the implementation of the existing regulation in the country regarding labelling of GMOs in food products and ensure the free choice and protection of the consumers in Mozambique. Although these results are encouraging, the need for real-time quantification (RT-PCR) of current events in the country was clearly demonstrated.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGEMENTS
The authors are grateful to the Eduardo Mondlane University Research Fund and Biotechnology Center -UEM for financial and technical support. This study was supported by the Research Fund of Eduardo Mondlane University.
REFERENCES
|
Aburumman A, Migdali H, Akash M, Al-Abdallat A, Dewir YH, Farooq M (2020). Detection of genetically modified maize in Jordan. GM Crops and Food 11(3):167-170. |
|
|
Ahatovic A, Al-Momani E, Bajrovic K, Durmic-Pasic A (2021). Efficiency of end-point PCR Based detection of genetically modified organisms (GMOs) in food and feed. Journal of Agriculture Science and Technology 23(2):437-446. |
|
|
Akinbo O, Obukosia S, Sinebo W, Savadogo M, Timpo S, Ouedraogo J, Mbabazi R, Maredia K, Makinde D, Ambali A (2021). Commercial release of genetically modified crops in Africa: interface between biosafety regulatory systems and varietal release system. Frontiers in Plant Science 12(314):1-18. |
|
|
Akiyama H, Watanabe T, Kikuchi H, Sakata K, Tokishita S, Hayashi Y, Hino A, Teshima R, Sawada J, Maitani T (2006). A detection method of CryIAc protein for identifying genetically modified rice using the lateral flow strip assay. Journal of the Food Hygienic Society of Japan (Shokuhin Eiseigaku Zasshi) 47(3):11-114. |
|
|
Alasaad N, Alzubi H, Kader AA (2016). Data in support of the detection of genetically modified organisms (GMOs) in food and feed samples. Data in Brief pp. 243-252. |
|
|
Al-Salameen F, Kumar V, Al-Aqeel H, Al-Hashash H, Hejji AB (2012). Detection of genetically modified DNA in fresh and processed foods sold in Kuwait. GM Crops and Food 3(4):283-288. |
|
|
Arun OO, Yilmaz F, Muratoglu K (2013). PCR detection of genetically modified maize and soy in mildly and highly processed foods. Food Control 32(2):525-531. |
|
|
Ashrafi-Dehkordi E, Mazloomi SM, Hemmati F (2021). A comparison of DNA extraction methods and PCR-based detection of GMO in textured soy protein. Journal of Consumer Protection and Food Safety 16(1):51-57. |
|
|
Bak A, Emerson JB (2019). Multiplex quantitative PCR for single-reaction genetically modified (GM) plant detection and identification of false-positive GM plants linked to Cauliflower mosaic virus (CaMV) infection. BMC Biotechnology 19(73):1-12. |
|
|
Boletim da República (2014). Regulamento de Biossegurança Relativa à Gestão de Organismos Geneticamente Modificados. Decreto n.º 71/2014. I SÉRIE - Número 96. |
|
|
Branquinho MR, Gomes, DMV, Ferreira RTB, Lawson-Ferreira R, Cardarelli-Leite P (2013). Detection of genetically modified maize events in Brazilian maize-derived food products. Food Science and Technology 33:399-403. |
|
|
Coello RP, Justo JP, Mendoza AF, Ordoñez ES (2017). Comparison of three DNA extraction methods for the detection and quantification of GMO in Ecuadorian manufactured food. BMC Research Notes 10(758):2-7. |
|
|
Datukishvili N, Kutaleladze T, Gabriadze I, Bitskinashvili K, Vishnepolsky B (2015). New multiplex PCR methods for rapid screening of genetically modified organisms in food. Frontiers in Microbiology 6:757. |
|
|
Erkan I, Dastan K (2017). Real-time PCR detection of genetically modified organisms in several food products and their environmental effects in Turkey. Fresenius Environmental Bulletin 26(4):2589-2595. |
|
|
Fraiture MA, Herman P, Taverniers I, De Loose M, Deforce D, Roosens NH (2015). Current and new approaches in GMO detection: Challenges and solutions. Biomed Research International 2015:1-22. |
|
|
Gbashi S, Adebo O, Adebiyi JA, Targuma S, Tebele S, Areo OM, Olopade B, Odukoya JO, Njobeh P (2021). Food safety, food security and genetically modified organisms in Africa: a current perspective. Biotechnology and Genetic Engineering Reviews 37(1):30-63. |
|
|
Giraldo PA, Shinozuka H, Spangenberg GC, Cogan NOI, Smith, KF (2019). Safety assessment of genetically modified feed: Is there any difference from food? Frontiers in Plant Science 10:1-17. |
|
|
Kaur J, Radu S, Ghazali FM, Kqueen CY (2010). Real-time PCR-based detection and quantification of genetically modified maize in processed feeds commercialized in Malaysia. Food Control 21(11):1536-1544. |
|
|
Leão-Buchir J, Pereira GVM, Silva ALL, Alban S, Rocha MC, Polettini J, Thomaz-Soccol V, Soccol CR (2018). Real-Time PCR for traceability and quantification of genetically modified seeds in lots of non-transgenic soybean. Bioscience Journal 34(1):34-41. |
|
|
Li X, Pan L, Li J, Zhang Q, Zhang S, LV, R, Yang L (2020). Establishment and application of event-specific polymerase chain reaction methods for two genetically modified soybean events, A2704-12 and A5547-127. Journal of Agricultural and Food Chemistry 59(24):13188-13194. |
|
|
Malik K, Sadia H, Basit MH (2018). Protein-Based Detection Methods for Genetically Modified Crops. In Protein-Protein Interaction Assays. IntechOpen. |
|
|
Oraby HAS, Aboul-Maaty NAF, Al-Sharawi HA (2021). Exploratory and confirmatory molecular approaches to determine genetically modified status in different crops. Bulletin of the National Research Centre 45(1):1-11. |
|
|
Park SB, Kim JY, Lee DG, Kim JH, Shin MK, Kim HY (2021). Development of a systematic qPCR array for screening GM soybeans. Foods 10(3):2-11. |
|
|
Pinto GBA, Silva M, Greiner R, Konietzny U, Soccol CR, Spier MR, Filho MASC, Pandey A, Thomaz-Soccol V (2011). Application of Polymerase Chain Reaction for high sensitivity detection of roundup readyTM soybean seeds and grains in varietal mixtures. Food Technology and Biotechnology 49(3):277-285. |
|
|
Rabiei M, Mehdizadeh M, Rastegar H, Vahidi H, Alebouyeh M (2013). Detection of genetically modified maize in processed foods sold commercially in Iran by qualitative PCR. Iranian Journal of Pharmaceutical Research 12(1):25-30. |
|
|
Rosculete E, Bonciu E, Rosculete CA, Teleanu E (2018). Detection and quantification of genetically modified soybean in some food and feed products. A case study on products available on Romanian Market. Sustainability 10(1325):3-13. |
|
|
Safaei P, Aghaee EM, Khaniki GJ, Afshari SAK, Rezaie S (2019). A simple and accurate PCR method for detection of genetically modified rice. Journal of Environmental Health Science and Engineering 17(2):847-851. |
|
|
Smyth SJ (2017). Genetically modified crops, regulatory delays, and international trade. Food and Energy Security 6(2):78-86. |
|
|
Soylu BB, Erkan I, Yukselo?lu EH (2020). Searching of the genetically modified organisms and their products' status and evaluation of food safety and regulations in Turkey in terms of the Forensic Sciences. Commagene Journal of Biology 4(2):104-109. |
|
|
Turkec A, Kazan H, Karacanli B, Lucas SJ (2015). DNA extraction techniques compared for accurate detection of genetically modified organisms (GMOs) in maize food and feed products. Journal of Food Science and Technology 52(8):5164-5171. |
|
|
Ujhelyi G, Vajda B, Béki E, Neszlényi JJ, Jánosi A, Némedi E, Gelencsér É (2008). Surveying the RR soy content of commercially available food products in Hungary. Food Control 19(10):967-973. |
|
|
Van den Eede G, Lipp M, Eyquem F, Anklam E (2000). Validation of an analytical method for the detection of GMO - derived DNA in processed foodstuffs. European Commission Joint Research Centre, Institute for Health and Consumer Protection, Food Products and Consumer Goods Unit 19677. Ispra, Italy. |
|
|
Xing F, Zhang W, Selvaraj JN, Liu Y (2015). DNA degradation in genetically modified rice with Cry1Ab by food processing methods: Implications for the quantification of genetically modified organisms. Food Chemistry 174:132-138. |
|
|
Yu YT (2021). The study of the impact of genetically modified soybean imports on China's food safety management. International Journal of Metrology and Quality Engineering 12(18):1-7. |
|
|
Zhang C, Wohlhueter R, Zhang H (2016). Genetically modified foods: A critical review of their promise and problems. Food Science and Human Wellness 5(3):116-123. |
|
|
Zhang W, Xing F, Selvaraj JN, Liu Y (2014). Degradation of endogenous and exogenous genes of genetically modified rice with Cry1Ab during food processing. Journal of Food Science 79(5):T1055-T1065. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0