ABSTRACT
Gallic acid (GA) is a functional ingredient abundant in Chinese pu-erh tea. The aim of this study was to increase the GA content in green tea extracts using acid hydrolysis. (-)-Epigallocatechin gallate treated with 1 M hydrochloric acid at 110°C for 1 h resulted in a GA yield of 45.6%. However, under these conditions, (-)-epigallocatechin was easily oxidized and rendered undetectable. On applying the same treatment to green tea extracts of Korea-cultivated Chamnok, a native species, and Yabukita, the GA contents increased from 0.17 to 4.87, 0.28 to 5.33 and 0.17 to 4.44 mM, respectively. In Chamnok extracts prepared following harvesting at three different time points, the GA contents increased from 0.17 to 4.48, 0.12 to 5.16 and 0.06 to 5.71 mM. Therefore, it is possible to produce green tea extracts with high GA concentrations using simple acid hydrolysis. This will greatly benefit the production of functional ingredients and will be useful in the beverage industry.
Key words: Domestic cultivar, EGCG, EGC, tannase.
Abbreviation:
EC, (-)-epicatechin; ECG, (-)-epicatechin gallate; EGC, (-) -epigallocatechin; EGCG, (-)-epigallocatechin gallate; GA, gallic acid.
Green tea is consumed globally and has been reported to have beneficial effects on fat oxidation and energy expenditure; it also favors weight loss (Kim et al., 2020). Green tea contains catechins, which represent a major component that can comprise up to approximately 30% of dry tea leaves (Chacko et al., 2010). The most abundant green tea catechins are (-)-epigallocatechin gallate (EGCG), which account for approximately 68–69% of all green tea catechins, followed by (-)-epigallocatechin (EGC, approximately 15–18% of all green tea catechins), (-)-epicatechin gallate (ECG, approximately 5–6% of all green tea catechins), and (-)-epicatechin (EC, approximately 2–5% of all green tea catechins) (Wang et al., 2019). The health benefits of green tea have been highly attributed to these polyphenolic compounds (Shang et al., 2020). In vivo, green tea catechins, especially EGCG, reduce the incidence of cancers, demonstrate antiviral activity, and reportedly ameliorate collagen-induced arthritis, oxidative stress-induced neurodegenerative diseases, and streptozotocin-induced diabetes (An et al., 2020; Hayakawa et al., 2020; Izzo et al., 2020; Mohan et al., 2020). In addition, EGCG tends to reduce body weight and fat.
During the fermentation process, gallate-type catechins such as EGCG or ECG are degraded, and gallic acid (GA, 3,4,5-trihydroxylbenzoic acid) levels are markedly increased (Ge et al., 2019). GA is a characteristic component of fermented tea. It not only exerts antioxidant, anti-obesity, antibacterial, and anti-inflammatory effects, but also promotes the loss of body fat (Ge et al., 2019; Zhou et al., 2020). Furthermore, the Ministry of Food and Drug Safety in Korea has recognized the tea extract of pu-erh tea (containing GA as the standard component) as a functional health food ingredient. Green tea, a raw material of fermented tea, originally contained a small amount of GA, but also contained other types of catechins, such as EGCG, ECG, EGC, and EC (Kongpichichoke et al., 2016).
The three predominant green tea species cultivated in Korea are the native species, and the cultivars Yabukita and Chamnok. Native species accounted for the highest percentage of cultivation area, and Yabukita is typically processed mechanically, mostly employing the Jeju Island as its center. The production area of Chamnok, a domestic breed in Korea, is currently expanding. Both tannins and catechins are major contributors to the bitter taste of green tea, which are important indicators of tea quality, and their levels increased with an increased delay in harvesting. Conversely, the levels of free amino acids, theanine, and total nitrogen (all of which contributed to the pleasant taste of green tea) decreased with an increased delay in harvesting, resulting in a deterioration of quality (Jo et al., 2011). Isolated catechins appeared as a brown powder, which is extremely bitter and easily oxidizable; this hindered their application as a pharmaceutical agent (Dai and Mumper, 2010). Consequently, green tea leaves at the third harvest (collected in August) are less desirable for beverage production (Cao et al., 2019). More recently, GA was found to contribute to the sweet aftertaste of a green tea infusion at the third harvest, following its biotransformation using tannase (Cao et al., 2019). Additionally, Kim et al. (2019, 2020) reported that EGC and GA complexes from EGCG or green tea infusion by tannase biotransformation exert an anti-obesity effect in brown adipose tissues.
Therefore, this study attempts to improve the functionality and aftertaste of EGCG in pu-erh tea using a simple acid-based chemical reaction by replacing biotransformation with tannase or long-time fermentation. In this study the aim was to produce a green tea extract with an increased GA content through the acid hydrolysis of EGCG or a green tea infusion.
Materials
The green tea leaves were supplied by Jeonnam Agricultural Research & Extension Services Centre (Bosung, Korea) and by the Agricultural Research Centre for Climate Change (Jeju, Korea). For use as standards, EC, ECG, EGC, EGCG, and catechin (C) were purchased from Aktin Chemicals Inc. (Cheng-du, China), and GA was purchased from Tokyo Chemical Industry Co. (Chuo-ku, Tokyo, Japan). All standards had a purity of >95%.
Preparation of EGCG solution and green tea extracts
The EGCG solution (at a concentration of 0.2 M) was prepared by shaking at 25oC for 2 h in the dark. Green tea powder (5 g) was dissolved in 250 ml of 60% (v/v) ethanol, followed by homogenization and incubation at 25°C for 1 h in the dark (Kim et al., 2019). After filtration through a No. 2 Whatman filter paper (GE Healthcare, Buckinghamshire, UK), the remaining insoluble residue was added to 250 ml of 100% (v/v) ethanol, followed by incubation under the same conditions. The total filtrate was concentrated to a volume of 30 ml using 60% (v/v) ethanol. This green tea extract was used for hydrolysis experiments.
Acid hydrolysis of green tea extracts and identification of catechins by high-performance liquid chromatography (HPLC)
EGCG was acid-hydrolyzed using different catalysts, treatment times, and temperature conditions. Both the EGCG solution and green tea extract were subjected to hydrolysis using HCl (0.025–1 M) for 5, 15, 30, 45, or 60 min at 110°C, as previously described at various temperatures (Zhang et al., 2014). After cooling on ice, EGCG solution-acid mixtures were neutralized using 1 M sodium hydroxide, prior to lyophilization and resuspension, for HPLC analysis. Dried samples were dissolved in 100 μl of solvent A (20 mM phosphoric acid in water); they were diluted, filtered through a 0.20-μm, filtered (DISMIC-13HP, Advantec, Tokyo, Japan), and injected onto a HPLC column (Shimadzu, Tokyo, Japan) equipped with a Shodex Silica C18P 4D column (5 μ , 4.6×150 mm, Shodex, Tokyo, Japan) pumps A and B (LC-20AD, Shimadzu, Kyoto, Japan), and a UV-VIS detector (SPD-20A, Shimadzu, Kyoto, Japan). Catechins and GA were eluted using a mobile phase composed of a mixture of 20 mM phosphoric acid in water (phase A) and acetonitrile (phase B), at a flow rate of 1 ml/min. The linear gradient dynamics were as follows: phase B increased from 0 to 15% (v/v) over 5 min, maintained at 15% (v/v) for 10 min, increased from 15 to 40 % (v/v) over 15of a mixture of 20 mM phosphoric acid in water (phase A) and a (v/v) over 25–30 min, and then maintained at 0% (v/v) for an additional 5 min. The UV-VIS detector wavelength was set to 280 nm, and the identification of individual catechins and GA was based on a comparison of peak retention times of the samples with those of the reference standards. The concentrations of green tea constituents were estimated from the area under the curve (Baik et al., 2015).
Statistical analysis
All experiments were performed in triplicate and the data were analyzed for statistical significance using a one-way analysis of variance (ANOVA), followed by Duncan’s multiple range test [SPSS version 18.0 (IBM Co., Armonk, NY, USA)]. The significance level was set at p<0.05. Mean values obtained from each group were compared using ANOVA.
Catechin and GA contents in green tea extracts
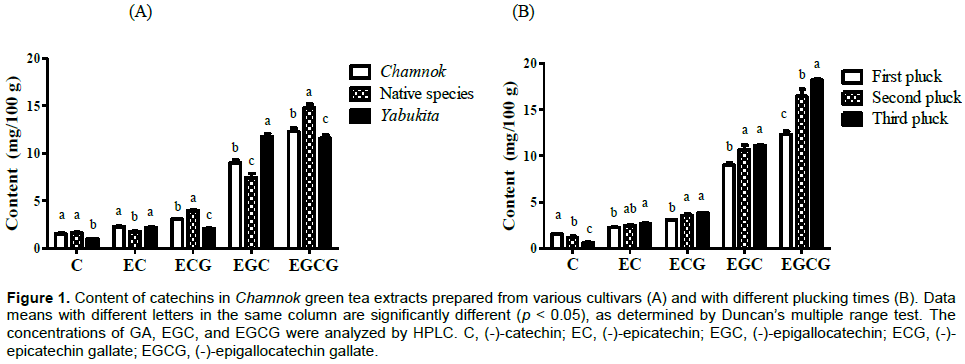
Polyphenols, especially catechins and phenolic acids are considered the major mediators of the health effects presented by green tea. The EGCG, ECG, or GA contents varied depending on the Korean domestic cultivar. As shown in Figure 1, phenolic compounds constituted approximately 30–40% of the dry weight of green tea leaves. Consistent with findings reported in a previous study (Wang et al., 2019), assessment of the catechin content in green tea extracts from various cultivars revealed that EGCG was the most abundant, followed by EGC, ECG, EC, and C. Furthermore, although EGCG and EGC were the most abundant among the native species and in the cultivar Yabukita, respectively, EGCG, GA, and C levels did not differ significantly among the three cultivars evaluated. The levels of catechins, phenolic acids, and caffeine in commercial teas varied with the species, season, horticultural conditions, and particularly, the degree of fermentation (Yang et al., 2018). In the Chamnok green tea extract, we observed that the content of catechins (except C) increased when the harvesting (plucking) period was delayed (Figure 1B). Accordingly, for Chamnok, the EGCG content gradually increased based on the plucking time (from April to August).
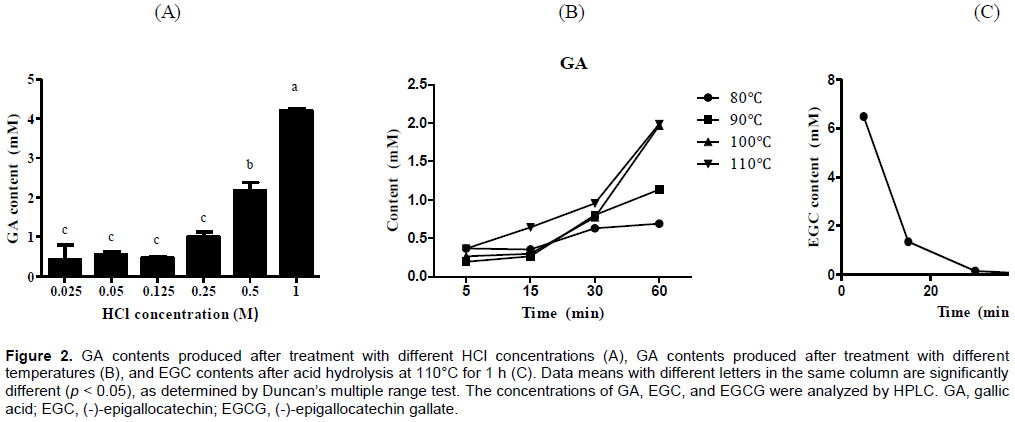
Investigation of the acid hydrolysis conditions for EGCG
Depending on the HCl concentration, EGCG was graduallyhydrolyzed up to a concentration of 1 M HCl (Figure 2A). Consequently, the GA content was highest at 110°C for 1 h, revealing a GA yield of 45.6% of the initially added EGCG (Figure 2B). Additionally, EGCG was not hydrolyzed following exposure to mild acids such as acetic, lactic, or citric acid, but it was degraded into GA and EGC in an HCl concentration-dependent manner under heating conditions (Figure 2A). Lee et al. (2012) have demonstrated that radical-mediated oxidation of EGCG can result in a low EGCG yield. Furthermore, acid hydrolysis did not increase the EGC content in the EGCG solution over time, and ECG was undetectable in the green tea infusion after acid treatment, indicating that it is unstable under acidic heating conditions (Figure 2C). Additionally, GA was not produced at a yield exceeding 50%, possibly due to a radical reaction.
Similar results were obtained in the experiment investigating the impact of different cultivars (Figure 3A) and harvesting (plucking) time points on the content of EGCG in the green tea extract (Figure 3B). However, the GA yield was high owing to the presence of multiple gallated catechins (ECG, GCG, and EGCG). Indeed, in the green tea extract, the contents of EGCG and ECG were significantly reduced, whereas the GA content was enhanced following acid hydrolysis at 110°C. The GA content increased from 0.17 to 4.87 mM (28.64-fold), 0.28 to 5.33 mM (19.04-fold), and 0.17 to 4.44 mM (26.12-fold) in the green tea extracts prepared from Chamnok, a native species, and Yabukita, respectively (Table 1).
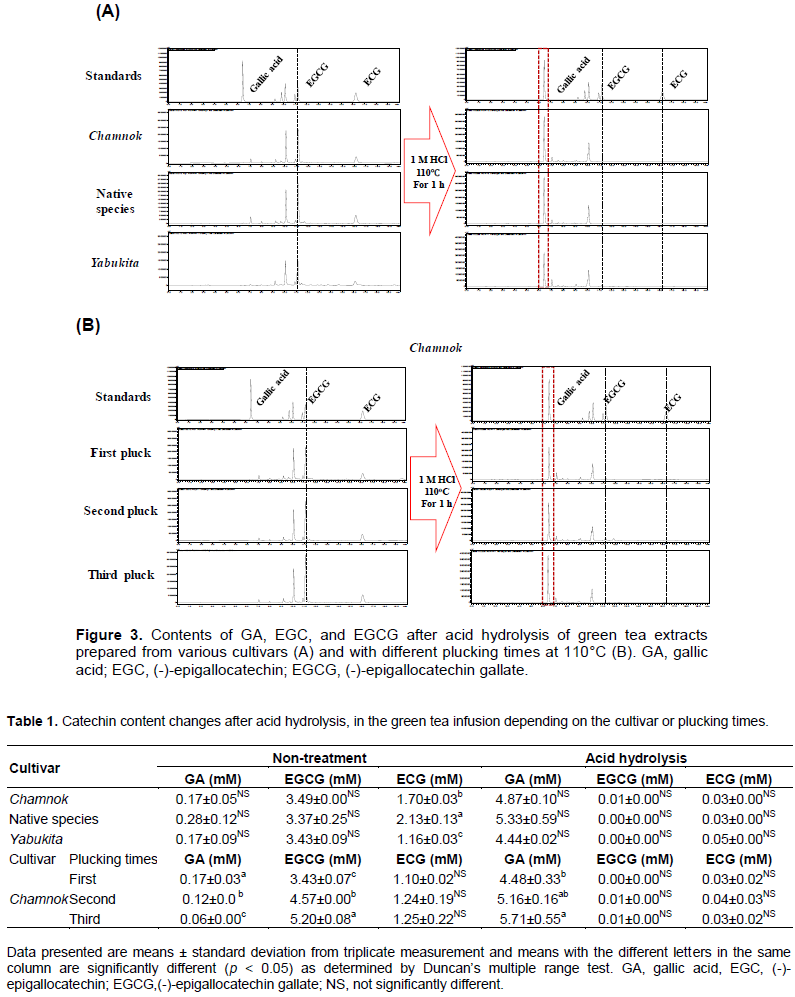
The content of catechins increased with an increasing delay in harvesting (plucking) time, and subsequent acid hydrolysis proportionately increased the GA levels in relevant green tea extracts. In case of the Korea-cultivated Chamnok, the GA content increased from 0.17 to 4.48 mM (22.35-fold), 0.12 to 5.16 mM (43-fold), and 0.06 to 5.71 mM (95.17-fold) in green tea extracts prepared from the first (April), second (June), and third (August) harvests (plucks), respectively (Table 1).
In this study an attempt was made to improve the GA contents in EGCG solutions or green tea infusions using a simple acid-based chemical reaction, and expanded the same process using domestically cultivated cultivars or different harvesting times of green tea. There was GA yield of 45.6% after treatment with 1 M HCl at 110°C for 1 h. Following acid hydrolysis, GA levels improved by 19-, 26-, and 28-fold in green tea infusions of the native species, Yabukita, and Chamnok, respectively, compared with those observed before treatment. Compared with tannase treatment, acid hydrolysis did not lead to the production of EGC or EC from EGCG or ECG, owing to their instability under conditions of strong acid hydrolysis. Although, EGC or EC was not obtained, acid hydrolysis is a simple and cost-effective process to increase the GA content in green tea infusions. Based on the present results, the high concentration of GA in green tea infusions can be replaced with several processes in pu-erh tea that require more than six months to over 10 years to ferment. Thus, the production of green tea solutions with high GA contents will have potential applications in manufacturing functional ingredients and beverages at an industrial level.
This research was supported by the "Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ012565)", Rural Development Administration, Republic of Korea.
The authors declare that there are no conflicts of interest.
EC, (-)-epicatechin; ECG, (-)-epicatechin gallate; EGC, (-) -epigallocatechin; EGCG, (-)-epigallocatechin gallate; GA, gallic acid.
REFERENCES
|
An L, Zhang W, Ma G, Wang K, Ji Y, Ren H, Wang Y (2020). Neuroprotective effects of Camellia nitidissima Chi leaf extract in hydrogen peroxide-treated human neuroblastoma cells and its molecule mechanism. Food Science and Nutrition 8(9):4782-4793.
Crossref
|
|
|
|
Baik JH, Shin KS, Park Y, Yu KW, Suh HJ, Choi HS (2015). Biotransformation of catechin and extraction of active polysaccharide from green tea leaves via simultaneous treatment with tannase and pectinase. Journal of the Science of Food and Agriculture 95:2337-2344.
Crossref
|
|
|
|
|
Cao QQ, Zou CH, Zhang YH, Du QZ, Yin QZ, Yin JF, Shi J, Xue S, Xu YQ (2019). Improving the taste of autumn green tea with tannase. Food Chemistry 277:432-437.
Crossref
|
|
|
|
|
Chacko SM, Thambi PT, Kuttan R, Nishigaki I (2010) Beneficial effects of green tea: a literature review. Chinese Medicine 5:13.
Crossref
|
|
|
|
|
Dai J, Mumper RJ (2010) Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 15:7313-7352.
Crossref
|
|
|
|
|
Ge Y, Bian X, Sun B, Zhao M, Ma Y, Tang Y, Li N, Wu JL (2019). Dynamic profiling of phenolic acids during pu-erh tea fermentation using derivatization liquid chromatography-mass spectrometry approach. Journal of Agricultural and Food Chemistry 67:4568-4577.
Crossref
|
|
|
|
|
Hayakawa S, Ohishi T, Miyoshi N, Oishi Y, Nakamura Y, Isemura M (2020). Anti-cancer effects of green tea epigallocatechin-3-gallate and coffee chlorogenic acid. Molecules 25: 4553.
Crossref
|
|
|
|
|
Izzo S, Naponelli V, Bettuzzi S (2020). Flavonoids as epigenetic modulators for prostate cancer prevention. Nutrients 12:1010.
Crossref
|
|
|
|
|
Jo JS, Kim JC, Cho KH, Kim RM, Han JY (2011). Chemical constituent variabilities of the green tea leaves by harvest periods. Journal of the Korean Wood Science and Technology 39:370-380.
Crossref
|
|
|
|
|
Kim HS, Jeon DY, Javid HMA, Sahar NE, Lee HN, Hong SJ, Huh JY, Kim YM (2020). Biotransformation of green tea infusion with tannase and its improvement on adipocyte metabolism. Enzyme Microbial Technology 135:109496.
Crossref
|
|
|
|
|
Kim HS, Moon JH, Kim YM, Huh JY (2019). Epigallocatechin exerts anti-obesity effect in brown adipose tissue. Chemistry and Biodiversity 16:e1900347.
Crossref
|
|
|
|
|
Kongpichichoke T, Chiu MT, Huang TC, Hsu JL (2016). Gallic acid content in Taiwanese teas at different degrees of fermentation and its antioxidant activity by inhibiting PKCδ activation: In vitro and in silico studies. Molecules 21:1346.
Crossref
|
|
|
|
|
Lee HJ, Cho JY, Moon JH (2012). Chemical conversions of salvianolic acid B by decoction in aqueous solution. Fitoterapia 83:1196-1204.
Crossref
|
|
|
|
|
Mohan T, Narasimhan KKS, Ravi DB, Velusamy P, Chandrasekar N, Chakrapani LN, Srinivasan A, Karthikeyan P, Kannan P, Tamilarasan B, Johnson T, Kalaiselvan P, Periandavan K (2020). Role of Nrfs dysfunction in the pathogenesis of diabetic nephrophathy: Therapeutic prospect of epigallocatechin-3-gallate. Free Radical Biology and Medicine 160:227-238.
Crossref
|
|
|
|
|
Shang A, Liu HY, Luo M, Xia Y, Yang X, Li HY, Wu DT, Sun Q, Geng F, Li HB, Gan RY (2020). Sweet tea (Lithocarpus polystachyus rehd.) as a new natural source of bioactive dihydrochalcones with multiple health benefits. Critical Reviews in Food Science and Nutrition, pp. 1-18.
Crossref
|
|
|
|
|
Wang Y, Kan Z, Wang D, Zhang L, McGinley JN, Thompson HJ (2019). Differences in chemical composition among commercially important cultivar of genus Camellia. Journal of Agricultural and Food Chemistry 67:5457-5464.
Crossref
|
|
|
|
|
Yang C, Hu Z, Lu M, Li P, Tan J, Chen M, Lv H, Zhu Y, Zhang Y, Guo L, Peng Q, Dai W, Lin Z (2018). Application of metabolomics profiling in the analysis of metabolites and taste quality in different subtype of white tea. Food Research International 106:909-919.
Crossref
|
|
|
|
|
Zhang L, Wang Y, Xu M (2014). Acid hydrolysis of crude tannins from infructescence of Platycarya strobilacea Sieb. et Zucc to produce ellagic acid. Natural Product Research 28:1637-1640.
Crossref
|
|
|
|
|
Zhou B, Ma C, Ren X, Xia T, Zheng C, Liu X (2020). Correlation analysis between filamentous fungi and chemical compositions in a pu-erh type tea after a long-term storage. Food Science and Nutrition 8:2501-2511.
Crossref
|
|